Analysis of Tomato Post-Harvest Properties: Fruit Color, Shelf Life, and Fungal Susceptibility
Vera Thole, Vera Thole, Philippe Vain, Philippe Vain, Ray-Yu Yang, Ray-Yu Yang, Juliana Almeida Barros da Silva, Juliana Almeida Barros da Silva, Eugenia M. A. Enfissi, Eugenia M. A. Enfissi, Marilise Nogueira, Marilise Nogueira, Elliott J. Price, Elliott J. Price, Saleh Alseekh, Saleh Alseekh, Alisdair R. Fernie, Alisdair R. Fernie, Paul D. Fraser, Paul D. Fraser, Peter Hanson, Cathie Martin
Botrytis
fruit color
fruit quality
fruit storage
fungal susceptibility
post-harvest analysis
shelf life
Abstract
A wide variety of fresh market and processing tomatoes (Solanum lycopersicum) is grown and consumed worldwide. Post-harvest losses are a major contributing factor to losses in crop productivity and can account for up to 50% of the harvest. To select and breed elite tomato varieties, it is important to characterize fruit quality and evaluate the post-harvest properties of tomato fruits. This includes the analysis of shelf life (the period during which a fruit remains suitable for consumption without qualitative deterioration), color, and pathogen susceptibility. Tomato shelf life depends upon the rate of fruit softening which accompanies fruit ripening and exacerbates damage during transport and handling. Furthermore, the susceptibility of tomatoes to fruit pathogens is also often linked to fruit ripening, especially for necrotrophic fungi such as Botrytis cinerea , also known as gray mold. The methods described here are critical for determining fruit quality and fungal susceptibility during storage. © 2020 The Authors.
Basic Protocol 1 : Fruit color as a determinant of fruit quality
Basic Protocol 2 : Shelf life test of tomato fruits
Basic Protocol 3 : Botrytis cinerea pathogen test of tomato fruits
Support Protocol : Preparation of Botrytis spore inoculum
INTRODUCTION
Tomato (Solanum lycopersicum L.) is one of the most important horticultural crop plants and a well-established model system for fleshy fruits, fruit development, and ripening. Tomatoes are a great source of phytonutrients, including vitamins, carotenoids, and polyphenols, and are consumed fresh as well as in many cooked and processed products.
In a climacteric fruit such as tomato, ethylene triggers the onset of the ripening process, and ethylene is also required for normal, full fruit ripening (Alexander & Grierson, 2002). During fruit ripening, tomatoes undergo dramatic changes in color, texture, flavor, and aroma. The change in fruit color is accompanied by the degradation of chlorophyll that unmasks pre-existing pigments, as well as the synthesis and accumulation of different types of compounds such as carotenoids.
Commercially, tomatoes destined for the fresh market are picked at different ripening stages, depending on the proximity between production and market place, and vary from green and firm to almost ripe for local markets. Long-distance transportation of tomatoes involves storage at low temperatures to limit post-harvest losses and to extend shelf life. Fruits often also undergo ethylene treatment to induce color and ripening before reaching the supermarket shelf. Artificial ripening and cold storage have, however, detrimental effects on tomato flavor, aroma, and texture (Baldwin, Plotto, Narciso, & Bai, 2011; Zhang et al., 2016).
Tomatoes, like other fleshy fruits, are particularly prone to post-harvest losses. The shelf life of food is defined as the period during which a stored product remains suitable for consumption and is normally determined by the degree of softening, shriveling, and rotting of fruit. Post-harvest shelf life is one of the most important traits for commercially grown tomatoes and can be shortened as a result of rapid over-ripening induced by various factors such as changes in temperature and pathogen exposure. Fruit softening and over-ripening leading to cell breakdown favors pathogen takeover and increases susceptibility to pathogens such as the necrotroph Botrytis cinerea , commonly known as gray mold, the second-most-important fungal plant pathogen (Cantu et al., 2008, 2009; Dean et al., 2012).
Important challenges for tomato production and commercialization include post-harvest losses and reduced quality due to fruit senescence and pathogen infection. Here, we describe three effective and robust protocols that represent standard operating procedures for assessing color, shelf life, and susceptibility to gray mold (B. cinerea) to define tomato fruit quality parameters important for consumers. The protocols can be applied to all types of tomato genotypes from wild tomato species to commercially grown tomato varieties.
Basic Protocol 1: FRUIT COLOR AS A DETERMINANT OF FRUIT QUALITY
Tomato fruit color and uniformity play a pivotal role in consumer perception of fruit quality. Tomato fruits display various colors depending on the fruit ripening stage, the dominant carotenoids, and other phytochemicals produced in the fruits, as well as environmental factors. It is an esthetic parameter whereby the consumer associates color with a better tasting, more nutritious, and fresher product. The color value is also an important fruit quality indicator for breeders and industry for the selection of varieties and the monitoring of quality.
In general, fruit color can be measured by visual analysis or different instrumental methods such as colorimetry, spectrophotometry, or a computer vision system (for reviews see Pathare, Opara, & Al-Said, 2013; Wu & Sun, 2013). Most color measuring instruments use mathematically defined color spaces standardized by the International Commission on Illumination (Commission Internationale de l'Éclairage; CIE). The two-color space system named CIELAB (also known as CIE Lab*, Lab*, or Lab) is commonly used commercially for food color measurement as it closely approximates the color perception by humans. CIELAB, which is related to the alternative Lab color space Hunter Lab, mathematically describes all perceivable colors in three dimensions and measures three values: the achromatic component L* (light vs. dark) and two color descriptors, the a* (red vs. green) and b* (yellow vs. blue) values (Fig. 1A).
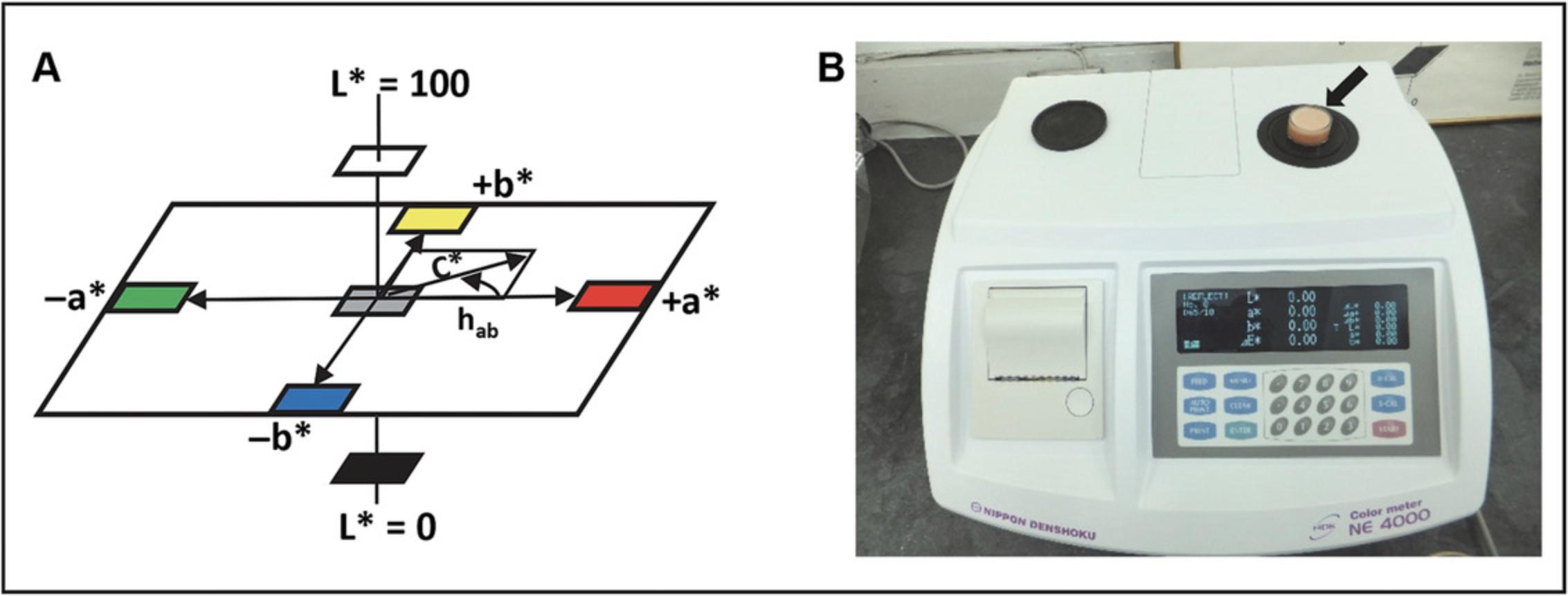
This protocol is intended for researchers to rapidly become familiar with the method routinely used by industry and breeders to determine tomato fruit color.
Materials
- 6 to 12 mature tomato fruits per genotype or tomato products (e.g., tomato puree or juice)
- Cloth, to clean tomatoes
- Knife or razor blades, to cut tomatoes
- Blender (e.g., Panasonic MX-188) or homogenizer
- Gauze fabric, 100% cotton, 20 S, non-sterile, unbleached without fluorescent chemicals (e.g., Charry Co. Ltd.)
- 50-ml centrifuge tubes or glassware, to store tomato puree
- Benchtop colorimeter and accessories (e.g., Nippon Denshoku Industries Color Meter NE 4000 or ZE6000) or portable handheld colorimeter (e.g., Hunter Lab MiniScan EZ 4500)
- Computer and software for color data analysis and management (e.g., ColorMate 5)
Sample preparation
1.Harvest tomato fruits at mature ripening stage, and transfer to the laboratory.
2.Select 6 to 12 fruits per tomato variety, depending on fruit size and sampling procedure of the study design.
3.Remove fruit calyx and remaining stem parts. Clean fruits with a cloth to remove dirt.
4.Cut each fruit into two to four pieces, and avoid juice loss as much as possible.
5.Put cut tomatoes in a blender, and blend for 1 to 2 min until pureed.
6.Filter puree through a two-layered gauze cloth into 50-ml centrifuge tubes to remove seeds and skin debris.
7.Stir tomato puree before color measurement (step 13).
Color measurement
The specific procedure depends on the type and model of the instrument used. Steps 8 to 13 relate to the use of a desktop colorimeter (e.g., Color Meter NE 4000 or ZE 6000; Fig. 1B) and the measurement of L*, a*, and b* values for tomato fruit puree. For other colorimeter models follow the manufacture's guidelines for setting up the instrument, standardization, and reading samples.
8.Preparing for measurements : Power on and set operating functions using the display menu. Set color space system to Lab* (six other color space options are available; e.g., Lab, XYZ, and RGB). Select light source mode/viewing angle for the CIE Standard Daylight Illuminant D65/10° (alternatives are CIE illuminant/observer angle C/2° or D65/2°), and set measurement mode to reflectance (the other selectable option is transmittance).
9.Select sample type (i.e., powder/paste if using tomato puree or solid sample if using whole tomato fruits) from the drop-down menu.
10.Select measuring area (6, 10, or 30 mm) and aperture (6, 10, or 30 Φ). Choose appropriate sample stage (holding/supporting the sample cell) corresponding to the measuring area (6, 10, or 30 mm) and sample type (solids, powder, or paste). For analyzing color of tomato puree, choose a wide aperture of 30 Φ, a 30-mm sample stage, and a round cell (i.e., sample cell/cuvette for powder and paste samples).
11.Zero calibration : Place 30-Φ aperture, corresponding sample stage (30 mm) for standard calibration, and 30-Φ glass plate into the instrument. Cover with “ZERO box” (black box) to prevent light exposure, and press the “zero-calibration (0-CAL)” key (Table 1).
| Setup | Zero calibration | Standard calibration | Sample measurement |
|---|---|---|---|
| Upper | ZERO box | Standard White Plate | ZERO box |
| 30-Φ glass plate | 30-Φ glass plate | 30-Φ sample cell | |
| 30-mm sample stage for standard calibration | 30-mm sample stage for standard calibration | 30-mm sample stage | |
| Lower | 30-Φ aperture | 30-Φ aperture | 30-Φ aperture |
- a
As described in Basic Protocol 1 steps 11 to 13.
12.Standard calibration : Remove “ZERO box,” and replace with the “Standard White Plate.” Press the “standard-calibration (S-CAL)” key (Table 1).
13.Sample measurement : Remove “Standard White Plate,” 30-Φ glass plate, and 30-mm sample stage used for calibration, and replace with 30-mm sample stage for sample measurement. Fill sample cell with tomato puree (from step 7) to the top, and place sample cell on top of the sample stage. Cover sample cell with “ZERO box” (Table 1), and read L*, a*, and b* values.
Data presentation
14.Present color value as L*, a*, b*, and a*/b*.
Basic Protocol 2: SHELF LIFE TEST OF TOMATO FRUITS
Here we provide a detailed description of the analysis of shelf life properties of tomato fruits, including fruit texture (firmness/softness), weight, and rotting. We describe the scoring of fruit quality before and during storage, tomato fruit harvest, preparation of fruits for storage, and storage conditions, as adapted from Zhang et al. (2013).
Materials
-
10 to 15 tomato fruits per tomato genotype
-
Sterile water
-
10% (v/v) bleach (e.g., Parozone Thick Bleach Original)
-
70% (v/v) ethanol (e.g., VWR, cat. no. 20821.330)
-
Labels (e.g., colored paper clips, small paper pricing tags)
-
Clipboard, paper, and writing instrument
-
Computer running spreadsheet analysis software
-
15-cell and 40-cell plant potting trays, ∼6.5 cm × 6.5 cm per cell and ∼3.5 cm × 3.5 cm per cell, respectively (e.g., Desch Plantpak)
-
Plastic plant propagator trays (e.g., Desch Plantpak)
-
Masking tape
-
Scale (e.g., Sartorius Practum 612-1S)
-
Lint-free paper towel sheets (e.g., Kimberly-Clark WypALL or Kimwipes)
-
500-ml containers (e.g., Phytatray II; Sigma-Aldrich, cat. no. P5929)
-
50-ml centrifuge tubes, disposable, sterile (e.g., Corning, cat. no. 430290)
-
Zip-lock polythene bag, ∼33 cm × 45 cm (e.g., Allpack Packaging Supplies, cat. no. SS16)
-
18°C, 70% humidity growth cabinet with 24-hr dark cycle (e.g., Sanyo, Fitotron)
1.Monitor development and ripening of tomato fruits daily or every other day. When fruits have reached breaker stage (i.e., when skin color changes from green to pink, red, or yellow on ∼10% of the fruit surface), label and record breaker date. Label individual fruits with a paper tag annotated with the breaker date. For large-scale experiments (∼100 tomatoes per day), use colored paper clips or unique tags to represent a specific date. Log breaker date of each fruit in a spreadsheet to facilitate subsequent scheduling of fruit harvest for experiments and to monitor the number of fruits already available for a given genotype.
2.Harvest tomato fruits 2 weeks after breaker stage into labeled, clean containers, such as disposable 15-cell plant potting trays inserted into a plastic plant propagator tray; label individual cells using strips of masking tape. Collect only fruits that are in perfect condition. Avoid using wrinkled or damaged tomatoes, fruits with a dry calyx, or fruits grown on damaged or diseased branches or plants.
3.Remove any remaining calyx, measure individual fruit weight, and record color. Assess fruit color according to Basic Protocol 1 or using visual annotation (e.g., purple, dark red, red, yellow-red). Score fruit firmness using a scale of 1 to 5 (i.e., 1 = very firm, 2 = firm, 3 = medium, 4 = soft, 5 = very soft). Score fruit firmness by holding the fruit with both hands and moving the fingers around the whole equator of each fruit.
4.Surface clean tomato fruits by rinsing twice with sterile water directly in the 15-cell potting tray previously used for harvest (step 2). To avoid handling fruits, pour water over fruits in the tray, and then remove carefully by tilting the tray. After rinsing, wipe dry the surface of each fruit with a lint-free towel sheet, and place fruits back into the tray on top of a clean paper towel pushed into the bottom of the cell. From this point onward, handle fruits with gloves to maintain semi-sterile conditions. Next, dip each fruit for 10 to 15 s into ∼400 ml of 10% (v/v) bleach, and briefly rinse in 400 ml sterile water placed in 500-ml containers. Replace bleach and water after ∼10 to 12 dips. Following rinsing, surface dry fruits with a clean paper towel. Finally, carefully wipe each fruit using a paper towel sheet sprayed with 70% ethanol.
5.Surface clean (e.g., spray) a 40-cell plant potting tray or any equivalent container with 70% ethanol. Divide tray into two parts that will be placed on top of each other to increase rigidity. Label each fruit with a permanent marker near the calyx attachment zone (optional), and place, top up, in a cell. Adjust the number of tomatoes per tray according to size (up to 12 tomatoes per tray). When using small fruits (like cherry tomatoes), add plastic caps from 50-ml centrifuge tubes (or equivalent support) on the bottom of the cell. Place each tray in a new, clean zip-lock polythene bag to prevent extensive water loss during fruit storage. Add one or two 50-ml centrifuge tubes into empty cell(s) as a “spacer” between the tomatoes and the bag to avoid direct contact between them (Fig. 2A). Record the position of individual tomatoes in each tray (Fig. 2B).
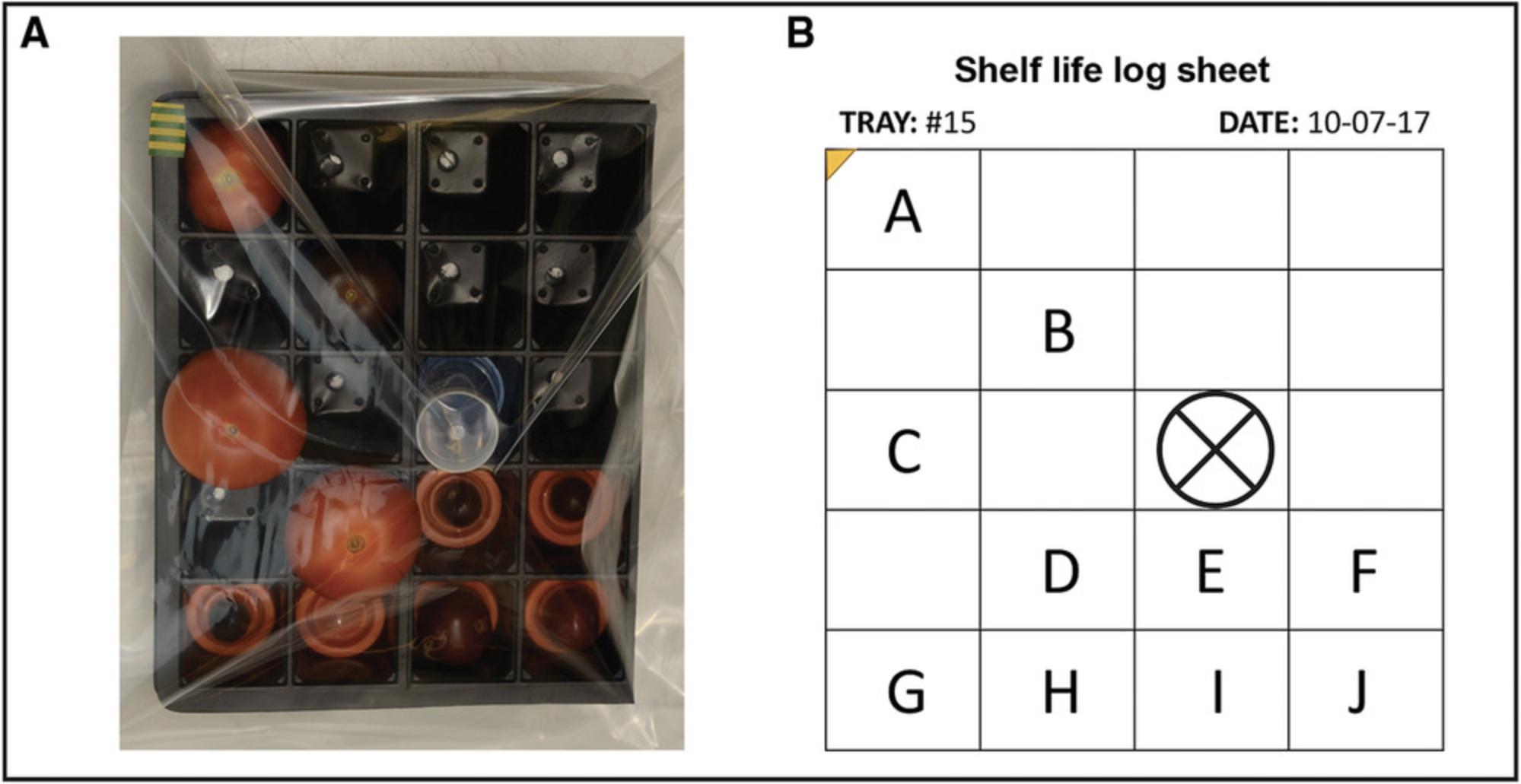
6.Incubate tomatoes at 18°C in the dark with 70% humidity in a controlled environment room or cabinet (Fig. 3) until the end of the storage test (step 7).

7.Each week, evaluate fruit weight and quality by scoring fruit softness on a scale of 1 (very firm) to 5 (very soft; see step 3) and by rating fruit surface integrity from 1 to 4 (D1 = little, D2 = medium, D3 = severe fruit shriveling or skin damage, D4 = split fruits or tomatoes presenting major necrosis or wounds). Wear clean gloves when handling or weighting fruits. Compile all data (i.e., genotype name, breaker date, date of fruit harvest [number of days after breaker]) and weekly fruit weight, softness, and surface integrity into a spreadsheet to enable data analysis. Collect data for at least 10 biological replicates (scattered in different trays according to their date of maturity), and compare to well-characterized, control genotypes.
Basic Protocol 3: BOTRYTIS CINEREA PATHOGEN TEST OF TOMATO FRUITS
Tomato fruit quality and shelf life properties are also greatly influenced by the susceptibility to diseases, especially fungal infections. B. cinerea is one of the most important post-harvest pathogens in tomato, although it can also cause significant damage and losses during plant growth and development. Here, we describe the analysis of fruit pathogen susceptibility with respect to B. cinerea , from harvest to wound inoculation of tomato fruits, which was adapted from Zhang et al. (2013). We also present a method for isolation of Botrytis spores used as an inoculum for wound inoculation (see Support Protocol).
Materials
-
10 to 15 tomato fruits per tomato genotype
-
Sterile water
-
70% (v/v) ethanol (e.g., VWR, cat. no. 20821.330)
-
1 × 105 spores/ml Botrytis macrospore inoculum (see Support Protocol)
-
10% (v/v) bleach (e.g., Parozone Thick Bleach Original)
-
Labels (e.g., colored paper clips, small paper pricing tags)
-
Clipboard, paper, and writing instrument
-
Computer running spreadsheet analysis software
-
15-cell and 40-cell plant potting trays, ∼6.5 cm × 6.5 cm per cell and ∼3.5 cm × 3.5 cm per cell, respectively (e.g., Desch Plantpak)
-
Plastic plant propagator trays and transparent lids (e.g., Desch Plantpak)
-
Masking tape
-
Scale (e.g., Sartorius Practum 612-1S)
-
Lint-free paper towel sheets (e.g., Kimberly-Clark WypALL or Kimwipes)
-
50-ml centrifuge tubes, disposable, sterile (e.g., Corning, cat. no. 430290)
-
200-µl pipette tips, for wounding fruit (e.g., StarLab, cat. no. S1113-1810-C)
-
Parafilm, 50 mm × 76 mm (e.g., R&L Slaughter, cat. no. 291-1214)
-
20°C, 70% humidity growth cabinet with 12-hr light/12-hr dark cycle, 70% light intensity (e.g., Sanyo, Fitotron)
-
Caliper (e.g., Draper Dual Reading Digital Vernier Caliper)
CAUTION : Perform all steps involving Botrytis macrospore inoculum in a laminar flow hood, dedicated incubator, or laboratory area suitable for working with biosafety level (BSL) 2 pathogens.
Production and preparation of tomato fruits
1.Monitor development and ripening of tomato fruits daily or every other day. When fruits have reached breaker stage (i.e., when skin color changes from green to pink, red, or yellow on ∼10% of the fruit surface), label and record breaker date. Label individual fruits with a paper tag annotated with the breaker date. For large-scale experiments (∼100 tomatoes per day), use colored paper clips or unique tags to represent a specific date. Log breaker date of each fruit in a spreadsheet to facilitate the subsequent scheduling of fruit harvest for experiments and to monitor the number of fruits already available for a given genotype.
2.Harvest tomato fruits 2 weeks after breaker stage into labeled, clean containers, such as 15-cell plant potting trays inserted into a plastic plant propagator tray; label individual cells using strips of masking tape. Collect only fruits that are in perfect condition. Avoid using wrinkled or damaged tomatoes, fruits with a dry calyx, or fruits grown on damaged or diseased branches or plants.

3.Remove any remaining calyx, measure individual fruit weight, and record color. Assess fruit color as described in Basic Protocol 1 or Basic Protocol 2 (step 3; rapid method). Score fruit firmness using a scale of 1 to 5 (see Basic Protocol 2 step 3).
4.Surface clean tomato fruits by rinsing twice with sterile water directly in the 15-cell potting tray previously used for harvest (step 2). To avoid handling fruits, pour water over fruits in the tray, and then remove carefully by tilting the tray. After rinsing, wipe dry the surface of each fruit with a lint-free towel sheet, and place fruits back into the tray on top of a clean paper towel pushed into the bottom of the cell. From this point onward, handle fruits with gloves to maintain semi-sterile conditions. Finally, carefully wipe each fruit using a paper towel sheet sprayed with 70% ethanol.
5.Surface clean (e.g., spray) with 70% ethanol 40-cell plant potting trays, propagator trays, and lids or any equivalent containers in which inoculation and incubation will be subsequently undertaken. To maintain a highly humid environment during incubation (step 8), add two towel sheets soaked with 100 to 150 ml sterile water into the propagator tray, underneath the 40-cell tray inlay. Label each fruit with a permanent marker near the calyx attachment zone, and place, top up, in a cell. When using small fruits (like cherry tomatoes), add plastic caps from 50-ml centrifuge tubes (or equivalent support) on the bottom of the cell. Label each tray, and record the position of individual tomatoes on the tray (Fig. 4B).
Pathogen test
6.Wounding surface-sterilized tomatoes : Adjust the number of wounds according to tomato fruit size, and space wounds evenly around the upper half of the fruit. Wound large tomatoes (e.g., S. lycopersicum cv. M82 fruits; ∼40 to 50 cm high × 40 to 50 cm wide × 50 to 60 cm deep) four times at 0°, 90°, 180°, and 360° positions. Wound medium-sized tomato fruits (∼30 to 40 cm × 30 to 40 cm × 40 to 50 cm) three times, and wound small tomatoes (e.g., S. pennellii or S. lycopersicum cv. MicroTom fruits; ∼20 to 30 cm × 20 to 30 cm × 30 to 40 cm) only once or twice. Use a 200-µl pipette tip to pierce and wound (1 to 2 mm deep) fruit surface (Fig. 5A). Change tip between genotypes to avoid sap cross contamination.
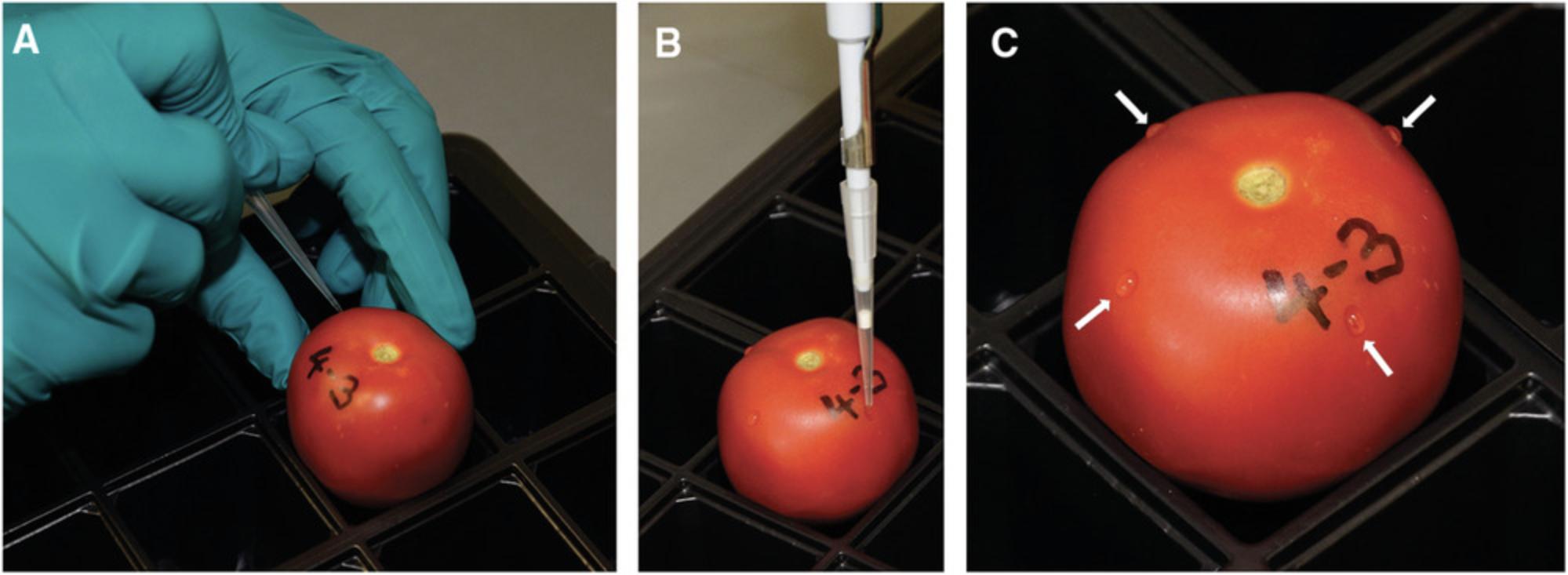
7.Inoculating wounded tomatoes : Following wounding of fruits, carefully inoculate 5 µl of 1 × 105 spores/ml pregerminated spore suspension on top of each wound (Fig. 5B). Deposit inoculum onto wound surface; do not inject inside fruit. Shake spore suspension frequently (after every third or fourth fruit inoculation) to ensure that spores do not sediment. After all fruits have been inoculated, seal lid tightly onto the propagator tray using Parafilm (Fig. 6).

8.Incubate trays with tomatoes for 3 days at 20°C and 70% humidity with a 12-hr light/12-hr dark cycle at 70% light intensity (∼270 µmol) in a controlled environment room or cabinet. Cover lid of each propagator tray with a sheet of white paper to reduce light intensity (Fig. 6B) if the lighting level of the incubator cannot be adjusted to ∼270 µmol.
9.Measure and record lesion size (width and height) at 3 DPI using a caliper (Fig. 7A). Score and record lesion aspect (e.g., presence or absence of mycelium growth on fruit surface; Fig. 7B,C). Combine data with information previously collected on fruit color, texture and softness, age (e.g., 14 to 17 days after breaker), and weight (see step 3). Analyze data and combine for at least 10 biological replicates. Compare to well-characterized, control genotypes.

10.Dispose of tomato fruits inoculated with Botrytis and other contaminated consumables (i.e., 40-cell plant potting tray) according to best laboratory practice (i.e., double bagged, treated as BSL 2 waste, and autoclaved). Treat propagator trays and lids that have not been in direct contact with tomatoes using bleach, and rinse with water.
Support Protocol: PREPARATION OF BOTRYTIS SPORE INOCULUM
Here we report a protocol for the isolation of Botrytis spores used as an inoculum for fruit wound inoculations based on the methods described in Schoonbeek, van Nistelrooy, and de Waard (2003); Stefanato et al. (2009); and Zhang et al. (2013).
Materials
-
B. cinerea strain B05.10 in 15% (v/v) glycerol, stored at −80°C (see Schoonbeek et al., 2003; Stefanato et al., 2009; Zhang et al., 2013)
-
Potato dextrose agar (PDA) solid medium (see recipe)
-
Malt extract yeast agar (MEYA) solid medium (see recipe)
-
Tween 80 (e.g., Sigma-Aldrich, cat. no. P4780)
-
Sterile distilled water
-
Potato dextrose broth (PDB) liquid medium (see recipe)
-
20°C, 70% humidity incubator with 12-hr light/12-hr dark cycle, 70% light intensity
-
Scalpel with no. 11 blade (e.g., R&L Slaughter, cat. nos. 233-5454 and 233-5334)
-
Fine forceps, no. 5 (e.g., TAAB Laboratories Equipment, cat. no. T083)
-
Micropore tape (e.g., 3M, cat. no. 1530-0)
-
L-shaped spreader, sterile (e.g., StarLab, cat. no. E1412-1005)
-
5-ml pipette tip (e.g., StarLab, cat no. E1070-2596) plugged with glass wool (e.g., Sigma-Aldrich, cat. no. 18421)
-
15-ml centrifuge tube, disposable, sterile (e.g., Corning, cat. no. 430790)
-
Benchtop centrifuge (e.g., Eppendorf Centrifuge 5810)
-
1.5-ml microcentrifuge tube
-
Hemocytometer (e.g., Thoma cell counting chamber; 0.0025-mm2 mini squares, 0.1 mm-depth)
CAUTION : Perform all steps involving Botrytis macrospore inoculum in a laminar flow hood suitable for working with BSL 2 pathogens.
1.Culture a 15% glycerol stock aliquot of fungal pathogen B. cinerea strain B05.10 on PDA solid medium at 20°C with a 12-hr light/12-hr dark cycle. After 1 week, cut three small agar blocks (about 0.02 cm3) with B. cinerea mycelium from the PDA solid medium, and using fine forceps place blocks upside-down (i.e., mycelium against medium) onto MEYA solid medium. Incubate mycelium for about 10 to 14 days at 20°C with a 12-hr light/12-hr dark cycle until sporulation is observed. Plates are sealed with micropore tape.
2.Isolate spores (macroconidia):
-
FloodB. cinereamycelium developed on a MEYA plate (step 1) with 10 to 15 ml water containing 0.05% (v/v) Tween 80.
-
Carefully scrape surface of mycelium with a sterile L-shaped spreader to detach spores.
At this point, a color change from light to dark gray is observed.
-
Pipette out crude spore suspension, and filter through a 5-ml pipette tip plugged with glass wool placed into a sterile 15-ml centrifuge tube.
-
Centrifuge filtered suspension 5 min at 129 ×g, 20°C, and then carefully discard supernatant.
-
Resuspend pelleted spores in 10 ml sterile water, and count spores using a 10-fold diluted aliquot in a hemocytometer.
-
Centrifuge spores 5 min at 129 ×g, 20°C, and resuspend in sterile water to adjust the concentration to 2.5 × 107spores/ml (final stock solution) in 1.5-ml microcentrifuge tubes.
Spore stocks can be kept in water at 4°C for about 2 weeks. Minimize exposure to room temperature to prevent spore germination in a non-nutrient solution.
3.For fruit inoculum, dilute 2.5 × 107 spores/ml spore stock solution in 0.25× PDB liquid medium to 1 × 105 spores/ml (e.g., 40 µl spore stock suspension in 10 ml of 0.25× PDB).
4.Preincubate B. cinerea spores in 0.25× PDB liquid medium for 1.5 to 2 hr at room temperature with occasional shaking (i.e., tilting inoculum tube every 30 min) to stimulate and synchronize spore germination.
REAGENTS AND SOLUTIONS
MEYA solid medium
- 50 g/L malt extract agar (e.g., Thermo Fisher Scientific, cat. no. CM0059)
- 2 g/L yeast extract (e.g., Merck, cat. no. 103753)
- 5 g/L agar (e.g., Neogen, cat. no. NCM0236)
- Pour into 9-cm petri dishes (e.g., R&L Slaughter, cat. no. 101R2O)
- Store sterile medium in 200-ml aliquots at room temperature for up to 6 months
- Store solid medium poured in plates, sealed with plastic wrap, at 4°C for up to 3 months
Malt extract agar contains 30 g/L malt extract, 5 g/L mycological peptone, and 15 g/L agar.
PDA solid medium
- 20 g/L glucose
- 4 g/L potato extract
- 17 g/L agar
- Pour into 9-cm petri dishes (e.g., R&L Slaughter, cat. no. 101R2O)
- Store sterile medium in 200-ml aliquots at room temperature for up to 6 months
- Store solid medium poured in plates, sealed with plastic wrap, at 4°C for up to 3 months
PDA medium can also be purchased from commercial sources (e.g., ForMedium, cat. no. PDA0101).
PDB liquid medium
- 20 g/L glucose
- 4 g/L potato extract
- Store sterile medium in 200-ml aliquots at room temperature for up to 6 months
PDB medium can also be purchased from commercial sources (e.g., ForMedium, cat. no. PDB0101).
COMMENTARY
Background Information
The methods to assess post-harvest properties reported here could also be used by breeders and researchers to produce new or improved tomato varieties that show a prolonged shelf life and reduced susceptibility to B. cinerea by means of exploiting natural variation, plant breeding, or transgenic approaches. In the past, genetically engineered purple and indigo tomato fruits containing high levels of delphinidin-type anthocyanins have been shown to exhibit an enhanced shelf life and were less susceptible to B. cinerea infections (Zhang et al., 2013).
In our protocol for the analysis of shelf life, we adapted the storage tests commonly used by the tomato-producing industry for laboratory-based analytical studies. Mechanical tests can also be carried out using a texture analyzer to assess tomato firmness by pressure load or shear press tests. These mechanical analyses can be destructive and lead to puncturing of the fleshy fruits. Consequently, these fruits cannot be used any longer in shelf life assays, and this in turn increases the number of fruits that need to be analyzed for each genotype in a given experiment to account not only for fruit-to-fruit variability but also for loss of fruit. What is gained in precision using these numerical methods can be lost due to sampling errors as tomato fruits do not always mature uniformly, with some parts of the fruits being more mature than others.
Critical Parameters
Tomatoes should be harvested from healthy plants. To allow efficient growth of tomato plants and to enable good fruit set, yield, and quality, plants that are grown in glasshouses should be planted into large pots (minimum 10-L pots) with occasional use of fertilizers if required. When fungal susceptibility tests are performed, standard preventative spraying should be restricted as fungicides, particularly systemic fungicides, will affect fungal susceptibility tests.
Tomato fruits should be harvested at the same ripening stage (e.g., 2 weeks after breaker) to have comparable and reliable results across experiments. Fruit ripeness is generally optimal at 2 weeks (and up to 2.5 weeks) after breaker for post-harvest studies. To correctly interpret fungal susceptibility levels, it is critical to perform the pathogen test consistently at the optimal ripening stage as unripe fruits are less susceptible to fungal infections, especially when necrotrophic fungi are used. Fruit harvest for shelf life assays has also been reported at an earlier developmental or ripening stage (e.g., 7 to 12 days after breaker), which is an alternative way to analyze storage properties if performed consistently throughout an experiment.
During fungal susceptibility tests, it is critical to ensure that the inoculation droplets remain on the wounding sites. This can be achieved by careful handling and minimal transportation of wound-inoculated tomatoes, as well as by removing any fruit sap dripping out through the wound sites before inoculations. It is also important that the spore suspension is mixed frequently to maintain a homogenous inoculum. To promote fungal development, a high humidity level must be maintained during the incubation period (e.g., by placing water in the bottom of the incubation container). It is also important to measure fungal lesion width and length as lesions are not always regularly shaped; a single measurement is a source of spurious variability that may require a larger number of repetitions. The Botrytis susceptibility test should be evaluated at 3 DPI, as at later stages the fungal lesions generally overgrow and develop into each other; this would hinder accurate scoring of lesion size. Mycelium that develops on the inoculation sites can also start to sporulate around 4 DPI, and for biosafety reasons the experiments should be evaluated before any sporulation.
For shelf life analyses, fruits should be stored in single layers, not stacked, without fruit-to-fruit contact or pressure, and preferably in sealed clean containers. Judging fruit texture and firmness by using hands enables the successive scoring of the same fruit during the entire duration of storage; this is in contrast to mechanical texture analysis which is destructive and requires large quantities of fruits for weekly measurements.
For assessment of color, shelf life, and fungal susceptibility, cleaning of fruits is critical to remove surface contaminants. Each batch of fruits to be analyzed should include control fruits, enabling batch-to-batch and year-to-year comparisons, especially when large-scale experiments are performed. In general, the use of a minimum of 10 biological replicates per genotype is essential in post-harvest studies to enable meaningful statistical analysis.
Troubleshooting
No or poor fungal growth on wound sites including on control fruits could indicate that spore stock solutions were not stored or handled properly (e.g., prolonged exposure to temperatures above 4°C while resuspended in water) or that there was insufficient pregermination of spores due to a shortened pregermination period or the use of an incorrect inoculation buffer. Poor growth could also be caused by non-homogeneous, poorly mixed fungal spore suspension, unfavorable incubation conditions such as low temperatures (below 18°C to 20°C) and low humidity levels (below 70%), or not harvesting fruits at the optimal ripening stage.
Unexpected, early rotting of fruits or deterioration of fruit quality could indicate that fruits were picked from diseased plants or that fruits were not surface cleaned before experiments.
If experiencing unexpected year-to-year variation of performance for some control genotypes, check seed stocks for contamination or off-type deviation. Use only well-characterized seed stocks.
Understanding Results
In our hands, the protocols described here enabled the reliable and accurate determination of fruit color, shelf life, and fungal susceptibility levels of a collection of more than 40 different tomato accessions for researchers and breeders as part of the TomGEM project that was designed to evaluate the impact of climate change on tomato yield and quality in the context of the European Union's Horizon 2020 Programme.
Time Considerations
At ∼2 weeks after tomato seed germination (8 to 10 seeds per genotype), prick seedlings into 15-cell potting trays or 7-cm2 pots when seedlings are about 5 cm tall with well-developed cotyledons and when the first real leaves are emerging.
Transplant 4- to 4.5-week-old tomato plantlets (3 to 4 plants per genotype) about 1 to 1.5 months after sowing into 11-cm2 pots, when plants reach ∼15 to 20 cm in height. Subsequently, about 6-week-old tomato plantlets are potted an additional time into 4- to 20-L pots.
Depending on tomato genotype and cultivation conditions, about 1.5 to 2 months after sowing, flowering will start. Fruit set will be observable about 2 to 2.5 months after sowing, and fruits will start reaching breaker stage about 4 to 6 weeks later (about 3 to 4 months after sowing).
Score tomato fruit ripening every day to every other day. Label breaker stage, and ∼2 weeks after breaker perform post-harvest assays.
For storage tests, analyze fruit firmness and weight weekly until fruits are overripe and can be discarded. This is highly dependent on genotypes and can last from zero to many weeks (i.e., months).
For fungal susceptibility tests, score fungal lesion size 3 days after wound inoculation of fruits.
Acknowledgments
The authors received funding from the Horizon 2020 Research and Innovation Programme of the European Union through the TomGEM project under grant agreement no. 679796. The authors thank Dr. Henk-Jan Schoonbeek (John Innes Centre, Norwich, UK) for his help and stimulating discussions.
Conflicts of Interest
The authors declare they have no conflicts interests.
Literature Cited
- Alexander, L., & Grierson, D. (2002). Ethylene biosynthesis and action in tomato: A model for climacteric fruit ripening. Journal of Experimental Botany , 53, 2039–2055. doi: 10.1093/jxb/erf072.
- Arias, R., Lee, T. C., Logendra, L., & Janes, H. (2000). Correlation of lycopene measured by HPLC with the L*, a*, b* color readings of a hydroponic tomato and the relationship of maturity with color and lycopene content. Journal of Agricultural and Food Chemistry , 48, 51697–51702. doi: 10.1021/jf990974e.
- Baldwin, E., Plotto, A., Narciso, J., & Bai, J. (2011). Effect of 1-methylcyclopropene on tomato flavour components, shelf life and decay as influenced by harvest maturity and storage temperature. Journal of the Science of Food and Agriculture , 91, 969–980. doi: 10.1002/jsfa.4281.
- Cantu, D., Blanco-Ulate, B., Yang, L., Labavitch, J. M., Bennett, A. B., & Powell, A. L. (2009). Ripening-regulated susceptibility of tomato fruit to Botrytis cinerea requires NOR but not RIN or ethylene. Plant Physiology , 150, 1434–1449. doi: 10.1104/pp.109.138701.
- Cantu, D., Vicente, A. R., Greve, L. C., Dewey, F. M., Bennett, A. B., Labavitch, J. M., & Powell, A. L. (2008). The intersection between cell wall disassembly, ripening, and fruit susceptibility to Botrytis cinerea. Proceedings of the National Academy of Sciences of the United States of America , 105, 859–864. doi: 10.1073/pnas.0709813105.
- Dean, R., Van Kan, J. A. L., Pretorius, Z. A., Hammond-Kosack, K. E., Di Pietro, A., Spanu, P. D., … Foster, G. D. (2012). The Top 10 fungal pathogens in molecular plant pathology. Molecular Plant Biology , 13, 414–430. doi: 10.1111/j.1364-3703.2011.00783.x.
- Hanson, P. M., Yang, R.-y., Wu, J., Chen, J.-t., Ledesma, D., Tsou, S. C. S., & Lee, T.-C. (2004). Variation for antioxidant activity and antioxidants in tomato. Journal of the American Society for Horticultural Science , 129, 704–711. doi: 10.21273/JASHS.129.5.0704.
- López Camelo, A. F., & Gómez, P. A. (2004). Comparison of color indexes for tomato ripening. Horticultura Brasileira , 22, 534–537. doi: 10.1590/S0102-05362004000300006.
- Pathare, P. B., Opara, U. L., & Al-Said, F. A.-J. (2013). Colour measurement and analysis in fresh and processed foods: A review. Food and Bioprocess Technology , 6, 36–60. doi: 10.1007/s11947-012-0867-9.
- Schoonbeek, H.-j., van Nistelrooy, J. G. M., & de Waard, M. A. (2003). Functional analysis of ABC transporter genes from Botrytis cinerea identifies BcatrB as a transporter of eugenol. European Journal of Plant Pathology , 109, 1003–1011. doi: 10.1023/B:EJPP.0000003936.61182.14.
- Stefanato, F. L., Abou-Mansour, E., Buchala, A., Kretschmer, M., Mosbach, A., Hahn, M., … Schoonbeek, H.-j. (2009). The ABC transporter BcatrB from Botrytis cinerea exports camalexin and is a virulence factor on Arabidopsis thaliana. Plant Journal , 58, 499–510. doi: 10.1111/j.1365-313X.2009.03794.x.
- United States Department of Agriculture (2017). Index of official visual aids. Washington, D.C.: U.S.D.A. Retrieved from https://www.ams.usda.gov/sites/default/files/media/Official%20Inventory%20of%20FV%20Inspection%20Aids.pdf.
- Wu, D., & Sun, D.-W. (2013). Colour measurements by computer vision for food quality control – A review. Trends in Food Science and Technology , 29, 5–20. doi: 10.1016/j.tifs.2012.08.004.
- Yildiz, H., & Baysal, T. (2007). Color and lycopene content of tomato puree affected by electroplasmolysis. International Journal of Food Properties , 10, 489–495. doi: 10.1080/10942910600909063.
- Zhang, Y., Butelli, E., De Stefano, R., Schoonbeek, H.-j., Magusin, A., Pagliarani, C., … Martin, C. (2013). Anthocyanins double the shelf life of tomatoes by delaying overripening and reducing susceptibility to gray mold. Current Biology , 23, 1094–1100. doi: 10.1016/j.cub.2013.04.072.
- Zhang, B., Tieman, D. M., Jiao, C., Xu, Y., Chen, K., Fei, Z., … Klee, H. J. (2016). Chilling-induced tomato flavor loss is associated with altered volatile synthesis and transient changes in DNA methylation. Proceedings of the National Academy of Sciences of the United States of America , 113, 12580–12585. doi: 10.1073/pnas.1613910113.
Citing Literature
Number of times cited according to CrossRef: 32
- Oussama M’hamdi, Sándor Takács, Gábor Palotás, Riadh Ilahy, Lajos Helyes, Zoltán Pék, A Comparative Analysis of XGBoost and Neural Network Models for Predicting Some Tomato Fruit Quality Traits from Environmental and Meteorological Data, Plants, 10.3390/plants13050746, 13 , 5, (746), (2024).
- Hongmei Nie, Xiu Yang, Shaowen Zheng, Leiping Hou, Gene-Based Developments in Improving Quality of Tomato: Focus on Firmness, Shelf Life, and Pre- and Post-Harvest Stress Adaptations, Horticulturae, 10.3390/horticulturae10060641, 10 , 6, (641), (2024).
- Orlando Reyes Zamora, Rosalba Troncoso-Rojas, María Elena Báez-Flores, Martín Ernesto Tiznado-Hernández, Agustín Rascón-Chu, Signaling of Plant Defense Mediated by Receptor-like Kinases, Receptor-like Cytoplasmic Protein Kinases and MAPKs Triggered by Fungal Chitin in Horticultural Crops, Horticulturae, 10.3390/horticulturae10040361, 10 , 4, (361), (2024).
- Lilia Mexicano, Tarsicio Medina, Adriana Mexicano, Jesús-Carlos Carmona, Electrolyzed Oxidizing Water in Controlling Pseudomonas syringae pv. tomato in Tomato Crops, Agronomy, 10.3390/agronomy14030597, 14 , 3, (597), (2024).
- Shiwen Zhang, Shengqing Wu, Zhiqi Jia, Junhong Zhang, Ying Li, Xingyun Ma, Bingli Fan, Panqiao Wang, Yanna Gao, Zhibiao Ye, Wei Wang, Exploring the influence of a single‐nucleotide mutation in EIN4 on tomato fruit firmness diversity through fruit pericarp microstructure, Plant Biotechnology Journal, 10.1111/pbi.14352, 22 , 9, (2379-2394), (2024).
- M. Pravitha, Murugkar Dipika Agrahar, V. Ajesh Kumar, Recent developments in tomato drying techniques: A comprehensive review, Journal of Food Process Engineering, 10.1111/jfpe.14550, 47 , 2, (2024).
- Ayehu Fekadu, Belay Andarege, Analysis of the pre-harvest factors that influence on the postharvest quality attributes of Tomatoes (Lycopersicon esculentum Mill.): A systematic review, Scientia Horticulturae, 10.1016/j.scienta.2024.113460, 337 , (113460), (2024).
- Fanyue Meng, Peiwen Wang, Fulei Mo, Haonan Qi, Rui Lv, Mozhen Cheng, Aoxue Wang, Endocellulase SlGH9-21 significantly improves drought resistance and storage capacity of tomato, Scientia Horticulturae, 10.1016/j.scienta.2023.112513, 323 , (112513), (2024).
- V. Radhalakshmi, Maya Raman, Minnu Rose Joy, Protective benefits of ethyl alcohol extract of Piper betel L. to prevent colon carcinogenesis, Pharmacological Research - Natural Products, 10.1016/j.prenap.2024.100024, 3 , (100024), (2024).
- Chenchen Qi, Haijing Zhang, Wei Chen, Weizhong Liu, Curcumin: An innovative approach for postharvest control of Alternaria alternata induced black rot in cherry tomatoes, Fungal Biology, 10.1016/j.funbio.2024.02.005, 128 , 2, (1691-1697), (2024).
- Shuchao Dong, Jingwen Zhang, Jiayi Ling, Zixin Xie, Liuxia Song, Yinlei Wang, Liping Zhao, Tongmin Zhao, Comparative analysis of physical traits, mineral compositions, antioxidant contents, and metabolite profiles in five cherry tomato cultivars, Food Research International, 10.1016/j.foodres.2024.114897, 194 , (114897), (2024).
- Rohit Kumar Kasera, Shivashish Gour, Tapodhir Acharjee, In-Store Monitoring of Harvested Tomatoes Using Internet of Things, Modeling, Simulation and Optimization, 10.1007/978-981-99-6866-4_18, (249-266), (2024).
- Enlei Chen, Shufen Chao, Bin Shi, Lu Liu, Mengli Chen, Yongli Zheng, Xiaoxiao Feng, Huiming Wu, Bacillus velezensis ZN-S10 Reforms the Rhizosphere Microbial Community and Enhances Tomato Resistance to TPN, Plants, 10.3390/plants12203636, 12 , 20, (3636), (2023).
- Jirong Zheng, Hao Chen, Tonglin Wang, Ghazala Mustafa, Lihong Liu, Qiaomei Wang, Zhiyong Shao, Quality Improvement of Tomato Fruits by Preharvest Application of Chitosan Oligosaccharide, Horticulturae, 10.3390/horticulturae9030300, 9 , 3, (300), (2023).
- Halifah Afiza Ismail, Isniti Richard, Shiamala Devi Ramaiya, Muta Harah Zakaria, Shiou Yih Lee, Browning in Relation to Enzymatic Activities and Phytochemical Content in Terap Peel (Artocarpus odoratissimus Blanco) during Postharvest Ripening, Horticulturae, 10.3390/horticulturae9010057, 9 , 1, (57), (2023).
- Na Li, Kefeng Zhai, Qin Yin, Quan Gu, Xingtao Zhang, Merced G. Melencion, Ziping Chen, Crosstalk between melatonin and reactive oxygen species in fruits and vegetables post-harvest preservation: An update, Frontiers in Nutrition, 10.3389/fnut.2023.1143511, 10 , (2023).
- Anne-Sophie Brochu, Jeanne Durrivage, Dagoberto Torres, Edel Pérez-López, Diet and Injection, Important Recommendations to Characterize Clavibacter michiganensis –Tomato Interactions , Plant Health Progress, 10.1094/PHP-04-23-0040-RS, 24 , 4, (475-481), (2023).
- M. Garcia-Marti, J. Simal-Gandara, Chemical and Biological Valorization of Tomato Waste, Agri-food Waste Valorisation, 10.1039/BK9781837670093-00147, (147-168), (2023).
- María del Carmen Damas-Job, Lluvia de Abril Alexandra Soriano-Melgar, Raúl Rodríguez-Herrera, René Darío Peralta-Rodríguez, Fernando Rivera-Cabrera, Dolores Gabriela Martínez-Vazquez, Effect of broccoli fresh residues-based extracts on the postharvest quality of cherry tomato (Solanum lycopersicum L.) fruits, Scientia Horticulturae, 10.1016/j.scienta.2023.112076, 317 , (112076), (2023).
- Francine Ngaffo Mekontso, Shuhui Wu, Ruizuo Fu, Wen Li, Lanhuan Meng, Qing Wang, Jiangkuo Li, Hongmiao Song, Xiangbin Xu, Knockdown of Sly-miR160a using short tandem target mimic (STTM) enhanced expression of auxin signaling genes and delayed postharvest ripening of tomato fruit, Postharvest Biology and Technology, 10.1016/j.postharvbio.2023.112271, 198 , (112271), (2023).
- V. Radhalakshmi, Maya Raman, Minnu Rose Joy, Development of active packaging film based on poly (lactic acid) incorporated with Piper betel leaf ethanolic extract and its application in the shelf-life extension of tuna meat, International Journal of Biological Macromolecules, 10.1016/j.ijbiomac.2023.125751, 246 , (125751), (2023).
- Kaushik Mudaliar, Vikash Sharma, Charu Agnihotri, Shekhar Agnihotri, Anupama Deora, Bhim Pratap Singh, Microbiological impact and control strategies to monitor postharvest losses in fruits and vegetables, Postharvest Management of Fresh Produce, 10.1016/B978-0-323-91132-0.00003-4, (113-147), (2023).
- Milan Kumar Lal, Rahul Kumar Tiwari, Priyanka Lal, Awadhesh Kumar, Ravinder Kumar, Regulatory Role of Melatonin in Post-harvest Management of Vegetables and Fruits, Melatonin in Plants: A Regulator for Plant Growth and Development, 10.1007/978-981-99-6745-2_10, (219-244), (2023).
- Ma. Ruiz de Azua, Álvaro Cruz-Carrión, Begoña Muguerza, Gerard Aragonès, Anna Arola-Arnal, María Romero, Francisca Bravo, Manuel Suarez, In-Season Consumption of Locally Produced Tomatoes Decreases Cardiovascular Risk Indices, Nutrients, 10.3390/nu15010043, 15 , 1, (43), (2022).
- Tuba DİLMAÇÜNAL, Berna BAYAR, Özcan DEMİRHAN, Impact of Modified Atmosphere Packaging and Controlled Atmosphere Applications on ‘Seval F1’ Tomato Fruit Quality and Marketability, Iğdır Üniversitesi Fen Bilimleri Enstitüsü Dergisi, 10.21597/jist.1037827, 12 , 2, (527-538), (2022).
- Xianzhe Zheng, Yujin Yuan, Baowen Huang, Xiaowei Hu, Yuwei Tang, Xin Xu, Mengbo Wu, Zehao Gong, Yingqing Luo, Min Gong, Xueli Gao, Guanle Wu, Qiongdan Zhang, Lu Zhang, Helen Chan, Benzhong Zhu, Zhengguo Li, Louise Ferguson, Wei Deng, Control of fruit softening and Ascorbic acid accumulation by manipulation of SlIMP3 in tomato, Plant Biotechnology Journal, 10.1111/pbi.13804, 20 , 6, (1213-1225), (2022).
- Ayman A. Mohammad, Heba M. Amer, Sameh M. El-Sawy, Dalia A. Youssef, Shaimaa A. Nour, Gaziea M. Soliman, Nematicidal activity of sweet annie and garden cress nano-formulations and their impact on the vegetative growth and fruit quality of tomato plants, Scientific Reports, 10.1038/s41598-022-26819-2, 12 , 1, (2022).
- Zhiyong Shao, Hao Chen, Songshen Hu, Haoran Liu, Fanliang Meng, Songwen Li, Bo Zhang, Changqing Zhu, Guangzu Wang, Lihong Liu, Qiaomei Wang, Chitosan oligosaccharide treatment improves quality attributes of tomato fruit stored under room temperature, Postharvest Biology and Technology, 10.1016/j.postharvbio.2022.111914, 189 , (111914), (2022).
- Vera Thole, Philippe Vain, Cathie Martin, Effect of Elevated Temperature on Tomato Post-Harvest Properties, Plants, 10.3390/plants10112359, 10 , 11, (2359), (2021).
- Joan Casals, Montserrat Martí, Aurora Rull, Clara Pons, Sustainable Transfer of Tomato Landraces to Modern Cropping Systems: The Effects of Environmental Conditions and Management Practices on Long-Shelf-Life Tomatoes, Agronomy, 10.3390/agronomy11030533, 11 , 3, (533), (2021).
- Marianela Hazel Álvarez-Hernández, Ginés Benito Martínez-Hernández, Noelia Castillejo, Juan Antonio Martínez, Francisco Artés-Hernández, Development of an antifungal active packaging containing thymol and an ethylene scavenger. Validation during storage of cherry tomatoes, Food Packaging and Shelf Life, 10.1016/j.fpsl.2021.100734, 29 , (100734), (2021).
- Francis K. Kathimba, Paul Macharia Kimani, Rama Narla, Leonard Muriithi Kiirika, Shelf-Life and Processing Quality of Five Parental Lines and Four F1 Hybrids Developed in Kenya, SSRN Electronic Journal, 10.2139/ssrn.4164482.

