Wholemount immunolabeling of mouse gut tissue
Marthe Howard, Andrea Kalinoski
Abstract
Wholemount Immunolabeling-Gut
The application was developed for large pieces of mouse gut tissue.
Animal care, breeding procedures, and experimental protocols were approved by the UTHSC animal care and use committee. Animals were housed in an AAALAC-approved facility with a 12-hour light cycle with food (standard chow) and water ad libitum. Male and female mice aged 3 to 9 months were used in the reported studies. All reporter mice, except where indicated, were generated by crossing either R26REYFP/EGFP mice or RCL-tdTomato mice with one of the Cre lines which express the reporter in a promoter-specific manner following Cre-mediated recombination. When applicable mice were purchased from the Jackson Laboratories, Bar Harbor, Maine. All genotyping was done using primers recommended by the Jackson Laboratories according to their protocols.
Steps
Harvesting and fixing the mouse colon for determination of shrinkage with PFA fixation
Mouse dissection protocol for wholemount immunolabeling-gut
Using the angled probes and forceps, gently unravel the intestines and choose which end you will begin your dissection from. Cut with scissors and using forceps move to small petri dish trying not to spill the contents.
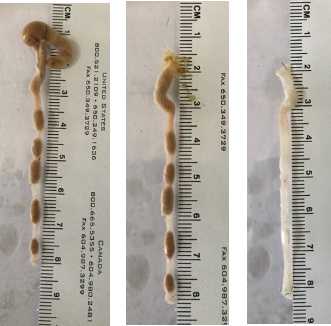
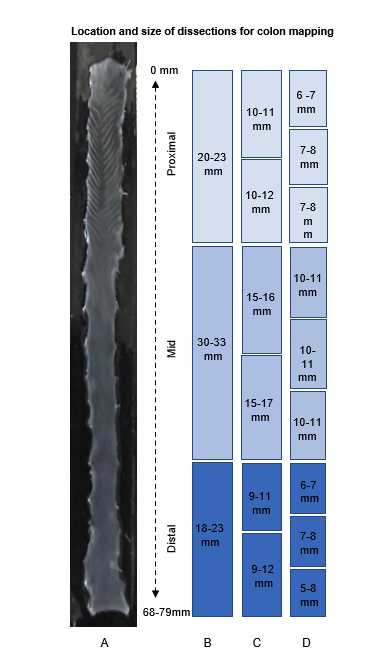
Cover tissue with just enough water to cover the bottom of the dish. Tissues should be well washed prior to fixation. Take the guts to the microscope for dissection. Cut the colon segments according to diagram below.
Fixation in 4% PFA/PBS pH 7.0-7.4 @ RT for 2-4 hours depending on the tissue size or 40C overnight, followed by washing in PBS over 4-6hrs and overnight in 30% sucrose in PBS if you want to cut sections.
We store in PBS if only for immunostaining.
Wash tissue in 0.1M Tris/1.5% Nacl/TX-100 (0.5%) 3 X 30 minutes
Block in Tris/NaCL/TX-100 (0.5%) 20% HS @RT 2 x 1 hour with rotation
Wash in Tris/NaCl/TX-100 (0.5%) 2 times….quick wash
Incubate in primary antibody(s) in Tris/NaCl/TX-100 (0.5%) + 20% HS @ 40C on the rotating table 2 days (minimum volume 1.5 ml)
Wash in Tris/NaCl/ TX-100 (0.5%) with 10% HS 3 X 1 hr. on the rotating table
Wash in Tris/NaCl/ TX-100 (0.5%) 3 X 10 min. on rotating table
Incubate in secondary antibody in Tris/NaCl/Tx-100 (0.5%) 2 days @ 40C on the rotating table (minimum volume 2.5 ml)
Wash in Tris/NaCl/Tx-100 (0.5%) 6 X 10 min. with rotation
Visualize in PBS preferably as soon as it is ready, i.e. make a reservation for the confocal when you start
Image intact or flat-mount colon samples using a Leica TCS SP5 laser scanning confocal microscope (Leica Microsystems, Bannockburn, IL) equipped with continuous-wave solid-state lasers (458, 488, 514, 561, 633 nm) and a titanium-sapphire tunable (705-980nm) multiphoton laser (Coherent, Santa Clara, CA). Acquire images at 512 X 512 in the XYZ planes as a single field of view or as tile scans (XYZS) in 1 µm steps with 20x (NA 0.70), 40x (NA 1.25) or 63x (NA 1.4) objectives using an automated scanning stage.