Western blot - alpha-synuclein
Michael J Hurley
Abstract
This protocol describes how to detect alpha-synuclein in protein derived from STC-1 cells by western blot using DAB/peroxidase or ECL to visualise the bands.
Steps
Sample preparation
Sample preparation using either protein pellet from an extraction (step 1.1) or direct lysis (step 1.2) or direct lysis (step 1.2)
Dissolve protein pellets from the TRI Reagent® extraction in 2% SDS containing 8 M urea.
Vortex 0h 0m 30s twice, then orbital shaker 260rpm 0h 30m 0s
Dissolve cells in RIPA buffer containing protease inhibitors (see Materials) On ice 0h 30m 0s
Mix samples with 4x NuPAGE™ LDS sample buffer containing 50 mM dithiothreitol
Vortex 0h 0m 20s twice, then orbital shaker 260rpm 0h 15m 0s
Heat samples 70°C 0h 10m 0s
Centrifuge samples 0h 0m 30s to collect condensation from tube lid. Vortex 0h 0m 20s then centrifuge again 0h 1m 0s
Electrophoresis and transfer
Separate samples (~ 10 ug total protein) by polyacrylamide gel electrophoresis using precast Bolt™ or NuPAGE™ 12%, Bis-Tris, 1.0 mm, Mini Protein Gels at 180 V 0h 45m 0s or until dye front reaches the bottom of the gel. Run with pre-stained or biotinylated size markers
Wet PVDF membrane with methanol and then equilibrate in Towbin buffer (see materials)
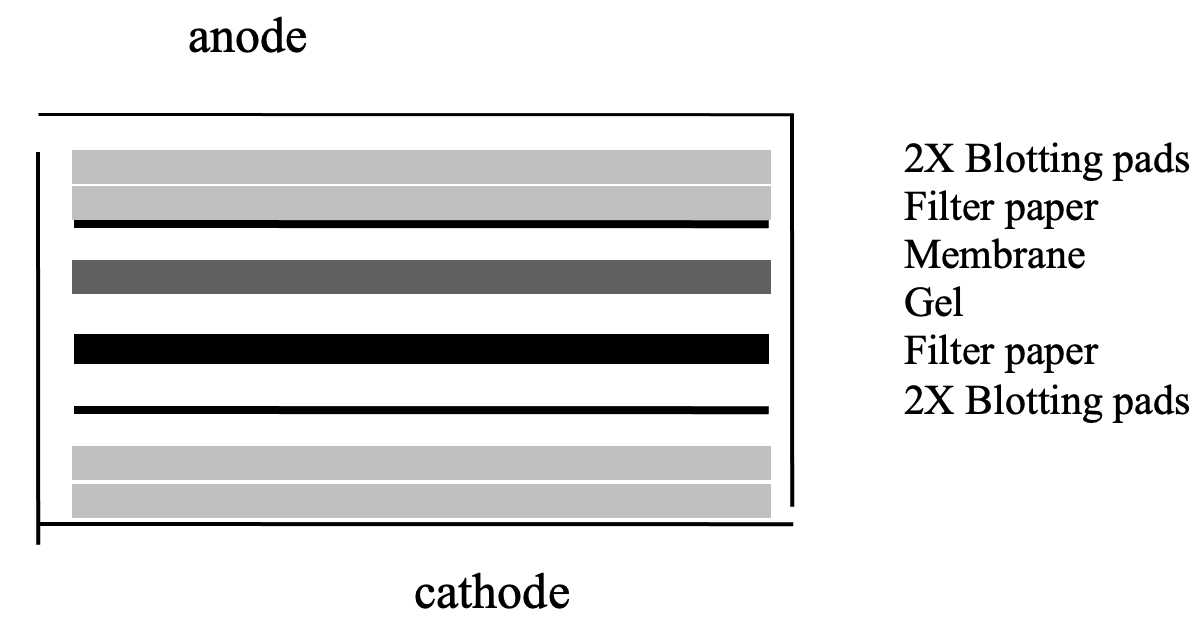
Soak transfer sandwich components (4 sheets of filter paper and 5 blotting pads) in Towbin transfer buffer and assemble in the transfer cassette in the following order:
Starting with the cathode plate
2 x blotting pads
2 x filter paper
gel
PVDF
2 x filter paper
3 x pad (use 3 because there is less chance of the gel slipping)
Use a roller to remove any air bubbles
Place cassette in transfer tank and transfer protein onto 0.2 mm PVDF (polyvinylidene difluoride) membranes (30 V, 1h 0m 0s in Towbin buffer
Rinse membranes with PBS 0h 0m 30s
Immunodectection of protein bands
Fix proteins to membrane for 0h 20m 0swith 4% paraformaldehyde
Wash with PBST (PBS containing 0.01% (v/v) Tween™-20) 4 x 0h 5m 0s
Block non-specific binding sites with block solution (PBST containing 2% (w/v) BSA and 0.005 % (w/v) thiomersal) for 1h 0m 0s
Incubate membranes in block solution containing rabbit monoclonal antibody against α-synuclein (1:1250) (ab212184) and β-actin (1:5000) (ab241153) 20h 0m 0s Room temperature
Wash with PBST 4 x 0h 5m 0s
Incubate membrane with secondary antibody in block solution (step 15.1 or step 15.2) or step 15.2)
Incubate membrane with biotinylated goat anti-rabbit IgG (1:1000) (Stratech) 1h 15m 0s and go to step 16
OR incubate membrane with goat anti-rabbit conjugated to peroxidase (1:25,000) (Stratech) 1h 15m 0s and go to step 18
Wash with PBST 4 x 0h 5m 0s
incubated with peroxidase conjugated streptavidin (1:1000) (Roche) 0h 45m 0s
Wash with PBST 4 x 0h 5m 0s
Wash with PBS 2 x 0h 5m 0s
Visualise bands with DAB if biotinylated secondary and streptavidin-peroxidase were used (step 20.1) or use ECL plus (Amersham) if anti-rabbit peroxidase secondary was used (step 20.4) or use ECL plus (Amersham) if anti-rabbit peroxidase secondary was used (step 20.4)
For DAB detection incubate in PBS containing 0.05 % (w/v) 3,3′-diaminobenzidine, 0.015 % (v/v) H2O2 and 0.05 % (w/v) nickel ammonium sulphate for ≤ 0h 0m 30s
Rinse in running tap water > 0h 5m 0s
Dry membrane 60ºC 1 hour
For chemiluminescent detection use an Amersham ECL plus kit according to the manufacturer's instructions
Visualize ECL or digitize DAB stained membrane using a ChemiDoc™ MP imaging system (Biorad)
ECL reagents can be washed off the membrane and the membrane then processed for DAB staining if desired

