U54 SCENT Intracellular Staining (ICS) Senescence Flow Cytometry Panel
Zachary R Healy, David Murdoch, Alicia Cooper-Volkheimer
Abstract
This protocol describes the Intracellular Staining (ICS) Senescence Flow Cytometry Panel
Steps
FBS Aliquot Prep
FBS (hiFBS): Gemini Bio Products Cat #100-106 1L
Prepare FBS Aliquots:
Thaw heat-inactivated FBS (hiFBS):
- Thaw a 500mL Bottle of FBS at 4°C. This may require more than overnight so the 500mL Bottle may be removed 2 or 3 days prior to use. Do not leave the FBS at room temperature overnight.
Aliquot the 500mL FBS Bottle in 50mL aliquots (a total of ten 50mL hi-FBS Aliquots)
Label the aliquots with Batch#, Expiration Date, Aliquot Date
Store 50mL aliquots @ -20ºC until expiration date or for up to 2 freeze/thaw cycles
FACS Wash
FACS Wash w/ EDTA (D-PBS with 0.5% FBS + 2 mM EDTA): Invitrogen Cat # 41190-250
Remove 4.5mL PBS from a 500mL bottle
Add 2.5mL of thawed FBS
Add 2mL of 0.5M EDTA solution (Catalog #: E7889-100ML)
Label the bottle with preparer's initials and expiration date (one month from preparation)
Store @ 4°C
Pen-Strep-Glut (PSG)
Pen-Strep-Glut (PSG) (L-Glutamine-Penicillin-Streptomycin Soln): Sigma Cat#: G6784-100ML
Thaw 100mL bottle
Aliquot into 10mL into 15mL conicals (total of ten 15mL conicals)
Label the aliquots with Batch#, Expiration Date, Aliquot Date
Store @ -20°C
R10FBS Media Preparation
R10FBS Media Preparation (“R10”)
Remove 55mL RPMI from a 500mL bottle of RPMI
Add 50mL aliquot of thawed, hiFBS
Add 5mL aliquot of thawed Pen-Strep-Glut
Label the bottle with preparer's initials and expiration date (one month from preparation)
Cytofix/CytopermSolution (10X) with GolgiStop (monensin)
Cytofix/Cytoperm Solution (10X) with GolgiStop (monensin): BD Biosciences Cat # 554715
Kit Contents :
- 125mL 10X Perm/Wash Buffer
- 100mL Cytofix Solution
- 0.7mL GolgiStop (monensin)
To prepare 1X Perm Wash h:
- Dilute 10X BD Perm/Wash buffer in distilled H2O to make a 1X solution prior to use
Formaldehyde Solution
1% Formaldehyde Solution (“1% Fix”) (“PFA”)
Reagents:
- 10% Formalin
- PBS
Add 5 mL 10% Formalin to a sterile 50 mL centrifuge conical tube
Add 45 mL PBS
Label the bottle with reagent name, initials & expiration date (one month from preparation)
Store 1% Fix at Room Temperature (18-25°C) for up 1 month
PepMixes
PepMixes:
- PepMix CEFX Ultra SuperStim Pool MHC-II Subset, JPT Technologies Cat #:PM-CEFX-3
- PepMix CEF Pool (extended), JPT Technologies Cat #: PM-CEF-E-1
Resuspend peptide pellet [25ug] in 50uL of DMSO [500ug/mL]
- Add 10uL DMSO at a time, with vortexing to resuspend
Aliquot and store at -20°C until day of use
On day of use, use 0.45uL per test
Protocol
Overview
Day 1: : Thaw cells and distribute to 2 plates. Rest plates at 37C/5% CO2 for >6 hours
Overnight: Stim for 6 hours
Day 2: 1 Stim + 1 Unstim plate stain for surface and ICS then acquire flow data
Time experiment steps according to lab and anticipated acquisition schedule
Day 1 – Thawing
-
Prepare 20ml of R10 in 50mL conical tube per sample (1-4 vials per tube)
-
Warm the R10 for 30 min at 37C prior to use
-
Place cryovials in a 37C bath for 3-5 sec at a time. Withdraw, examine and repeat (usually 3-4 rounds) until small, pea-sized amount of ice remains
-
Spray with 70% EtOH and wipe off before returning to the hood
-
To each cryovial, add 1ml of R10 dropwise to each cryovial
-
Transfer the 2ml PBMC sample from the cryovials into the 50ml conicals
-
Invert 3x to mix
-
Centrifuge at 350g for 10 min
-
Pour off the supernatant, do not shake to allow some volume to remain
-
Gently swirl the 50mL conical in remaining volume to loosen pellet
-
Add 10mL pre-warmed R10 and resuspend by pipetting 10 times. Mix sample carefully but
thoroughly to break up any cell clumps.
-
Centrifuge at 350g for 10 min
-
Pour off the supernatant, do not shake to allow some volume to remain
-
Gently swirl the 50mL conical in remaining volume to loosen pellet
-
Add 10mL pre-warmed R10 and resuspend by pipetting 10 times. Mix sample carefully but
thoroughly to break up any cell clumps
-
**COUNTING & VIABILITY:**
a) Perform a cell count using the Countess II to determine PBMC viability & recovery
b) Add 10uL Trypan Blue to well of mixing plate
c) Add 10uL Cells, pipette up and down
d) Remove 10uL of cell mix and dispense into Countess slide
e) Wait 30 sec
f) Calculate total cells and viability
g) Insert into Countess II to calculate total cells and viability
Overnight – Stim
-
Cells have been plated at 2x106 cells/well in 200uL R10 and rested for >6hours
-
For all wells (Stim and Unstim), add BFA/Monensin at
a. BFA: 0.23 uL per test
b. Monensin: 0.16 uL per test
c. CD107a antibody: 0.313uL per test
- For Stim plates, add CEF PepMix at
a. PepMix CEFX Ultra SuperStim Pool MHC-II Subset: 0.45uL per test
b. PepMix CEF Pool (extended) : 0.45uL per test
-
Gently plate by vortexing at medium speed for 3 sec
-
Incubate cells at 37º C, 5% CO2 for 6 hours
-
At 6 hours (9am), wrap plate in Parafilm and place in 4C refrigerator and proceed with staining
| A | B | C | D |
|---|---|---|---|
| Stim Mixes | # Tubes: | 16 | 16 |
| uL per test | Stim | UnStim | |
| BFA | 0.23 | 4.232 | 4.232 |
| Monensin | 0.16 | 2.944 | 2.944 |
| CEFX ultra MHC II | 0.45 | 8.28 | - |
| CEF Pool Ext (MHC I) | 0.45 | 8.28 | - |
| CD107a | 0.313 | 5.7592 | 5.7592 |
| R10 | 23.5 | 432.4 | 432.4 |
| Total | 25.103 | ||
| Add 25uL Mix to 200uL Cells for total 225uL |
Day 2 – Stain
- Keep everything as cold as much as possible
- Keep everything covered as much as possible; work in dark or incandescent light
Surface staining: Surface staining ng:
-
Remove plates from refrigerator
-
Centrifuge 400g x 3 min
-
Flick off supernatant and vortex gently
-
Add 47.5uL FACS wash to each well
-
Add 2.5uL TruStain FCX blocking to each well
-
Incubate 4C for 15 min
-
Prepare Surface Stain Mix in PBS with 10uL BSB Plus, set aside
-
Add 50uL of Surface Stain Mix in PBS to each well and gently mix
-
Incubate in 4C fridge for 30 min n, covered in foil
-
Add 100uL cold FACS wash & mix by pipetting up and down x3
-
Centrifuge 400g x 3 min
-
Flick off supernatant and vortex gently
-
Add 200uL cold FACS wash to each well
-
Centrifuge 400g x 3 min
-
Flick off supernatant and vortex gently
Intracellular cytokine staining (ICS): Intracellular cytokine staining (ICS) :
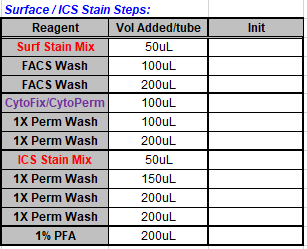
- Add 100uL BD Cytofix/Cytoperm solution to each well.
a. NOTE: mix well with cells
- Incubate on ice for 20 min, covered in foil
a. NOTE: do not over-incubate (Cytofix is toxic to cells)
-
Prepare ICS Stain Mix in 1X Perm Wash with 10uL BSB Plus, set aside
-
Add 100uL cold 1X Perm Wash solution
-
Centrifuge 400g x 3 min
-
Flick off supernatant and vortex gently
-
Add 200uL cold 1X Perm Wash solution
-
Centrifuge 400g x 3 min
-
Flick off supernatant and vortex gently
-
Add 50uL of the ICS Mix in Perm Wash to each well and gently mix
-
Incubate at 4° in refrigerator x 30 min, covered in foil
-
Add 150uL cold 1X Perm Wash solution
-
Centrifuge 400g x 3 min
-
Flick off supernatant and vortex gently
-
Add 200uL cold 1X Perm Wash solution
-
Centrifuge 400g x 3 min
-
Flick off supernatant and vortex gently
-
Add 200uL cold 1X Perm Wash solution
-
Centrifuge 400g x 3 min
-
Flick off supernatant and vortex gently
-
Add 200uL 1% PFA
-
Transfer samples to bullet tubes, cover with aluminum foil, store at 4°C, & acquire within 6 hours
| A | B | C | D | E | F | G | H | I | J |
|---|---|---|---|---|---|---|---|---|---|
| Staining Step | Specificity | Fluor | Vendor | Cat# | Clone | Isotype | Conc ug/mL | MRA uL | Titered uL |
| Stim | CD107a | PE | BL | 328608 | H4A3 | IgG1 k | 400 | 5 | 0.313 |
| Surface | KLRG1 | BV421 | BL | 367706 | SA231A2 | IgG2a k | 100 | 5 | 1.25 |
| Surface | CD45RA | Pacific Blue | BL | 304118 | H100 | IgG2b k | 500 | 1 | 0.5 |
| Surface | CD8 | BV570 | BL | 301038 | RPA-T8 | IgG1 k | 100 | 5 | 2.5 |
| Surface | CD127 | BV605 | BL | 351334 | A019D5 | IgG1 k | 100 | 5 | 2.5 |
| Surface | CD56 | BV650 | BL | 362532 | 5.1H11 | IgG1 k | 100 | 5 | 1.25 |
| Surface | CCR7 | BV711 | BL | 353228 | G043H7 | IgG2a k | 100 | 5 | 5 |
| Surface | CD27 | BV750 | BL | 302850 | O323 | IgG1 k | 100 | 5 | 2.5 |
| Surface | PD1 | VioBright515 | Miltenyi | 130-120-386 | REA1165 | rHu IgG1 | 2 | 2 | |
| Surface | NKG2A | PE-Vio615 | Miltenyi | 130-120-035 | REA110 | rHu IgG1 | 2 | 1 | |
| Surface | CD16 | PerCP-Cy5.5 | BL | 302028 | 3G8 | IgG1 k | 200 | 5 | 2.5 |
| Surface | CD38 | PerCp-eFluor710 | TF | 46-0388-42 | HB7 | IgG1 k | 120 | 5 | 5 |
| Surface | CD19 | SparkNIR685 | BL | 302270 | HIB19 | IgG1 k | 100 | 5 | 1.25 |
| Surface | CD14 | SparkNIR685 | BL | 367150 | 63D3 | IgG1 k | 200 | 5 | 0.156 |
| Surface | Zombie nIR | Zombie nIR | BL | 423105 | - | - | - | 1 | 0.4 |
| Surface | HLA-DR | APC-Fire810 | BL | 307674 | L243 | IgG2a k | 50 | 5 | 2.5 |
| ICS | CD3 | BV480 | BD | 566105 | UCHT1 | IgG1 k | 200 | 5 | 2.5 |
| ICS | CD4 | PerCP | BD | 550631 | L200 | IgG1 k | 6.3 | 20 | 5 |
| ICS | IFN-g | PE-Cy7 | BL | 502528 | 4S.B3 | IgG1 k | 50 | 5 | 0.313 |
| ICS | IL-2 | APC | BL | 500310 | MQ1-17H12 | Rat IgG2a k | 25 | 5 | 0.313 |
| ICS | TNFa | Alexa700 | BL | 502928 | MAb11 | IgG1 k | 100 | 5 | 0.63 |

