Tracing DNA paths and RNA profiles in cultured cells and tissues with ORCA
Leslie J. Mateo, Nasa Sinnott-Armstrong, Alistair N. Boettiger












































Extended
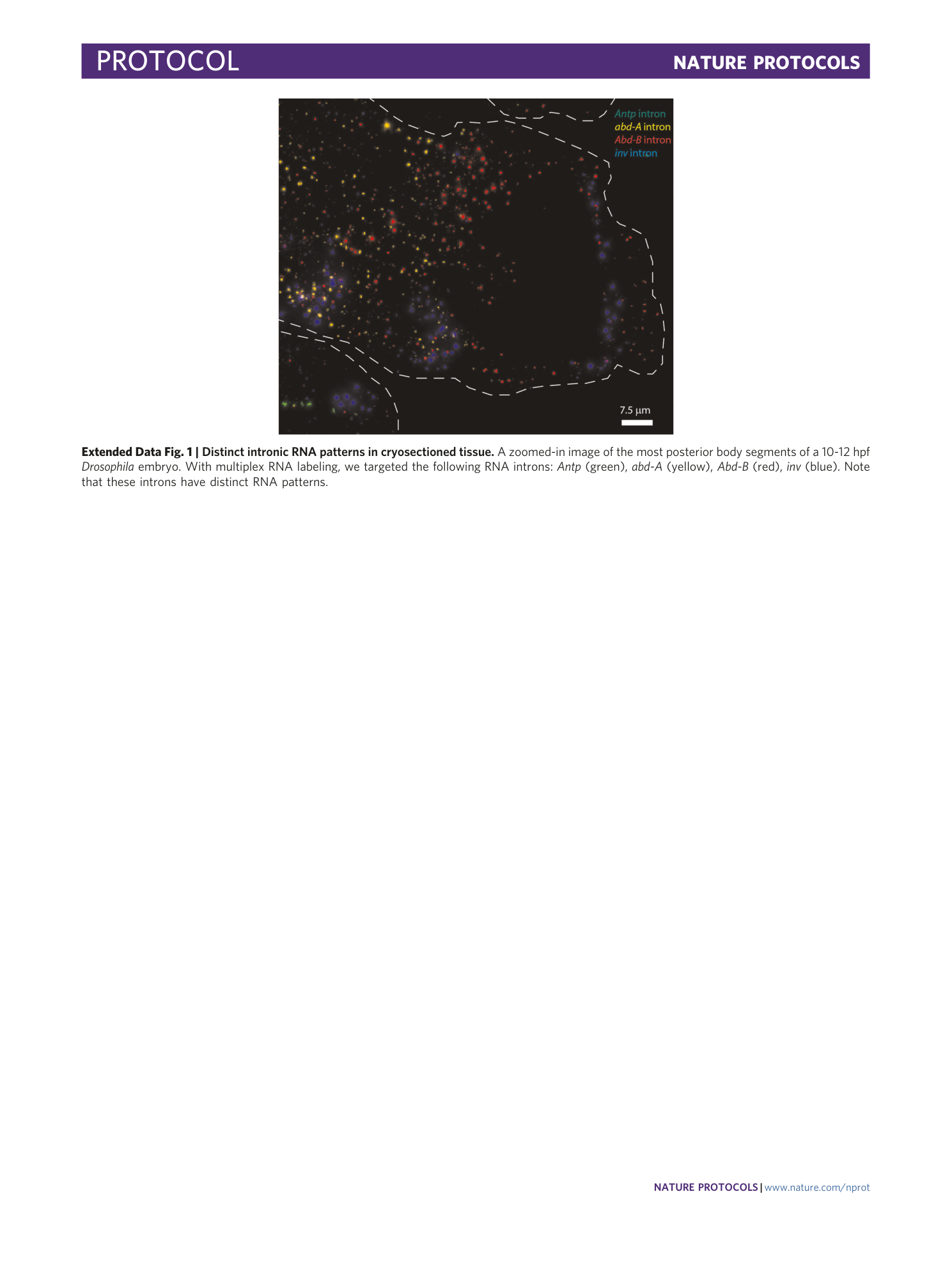
Extended Data Fig. 1 Distinct intronic RNA patterns in cryosectioned tissue.
A zoomed-in image of the most posterior body segments of a 10-12 hpf Drosophila embryo. With multiplex RNA labeling, we targeted the following RNA introns: Antp (green), abd-A (yellow), Abd-B (red), inv (blue). Note that these introns have distinct RNA patterns.
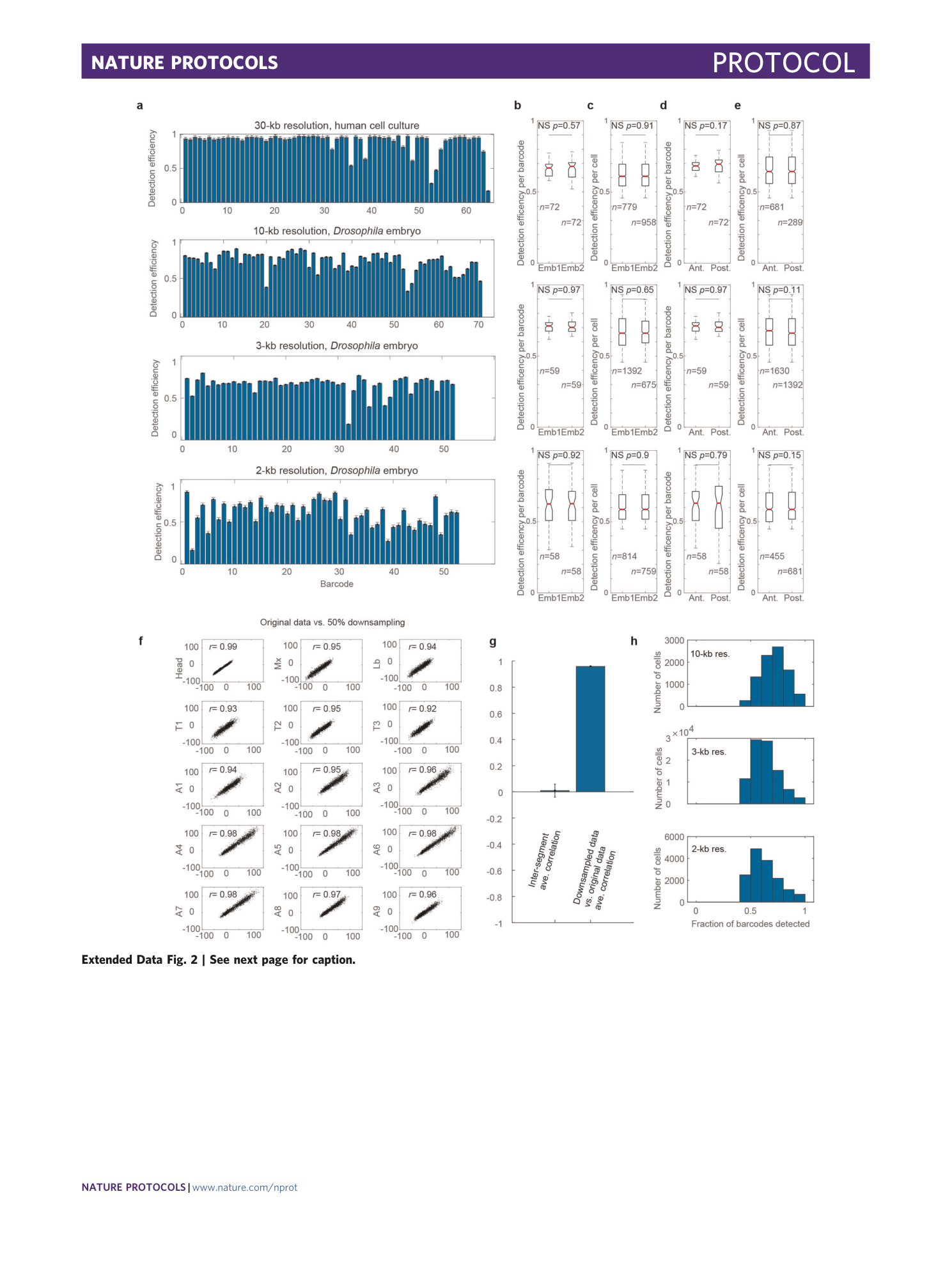
Extended Data Fig. 2 Barcode detection efficiency.
Figure modified from previous publication 25 . a , The average detection efficiency of each barcode is shown for probe sets with the resolution and cell type indicated. Error bars represent standard error of the mean. n = 1,979 cells, n = 8,801 cells, n = 94,300 cells, and n = 15,230 cells for the 30-kb, 10-kb, 3-kb, and 2-kb probesets, respectively. b , Box-and-whisker plots comparing the distribution of detection efficiencies across barcodes between two different embryos. N.S. indicates not statistically significant (two-sided Wilcoxon test, p-values indicated). For the boxplots in (b-e), the red line marks the median, the upper and lower limits of the box mark the interquartile range, and the whiskers extend to the furthest datum within 1.5 times the interquartile range. Notches denote the 95% confidence interval around the median. Sample size (n) is indicated. c , Comparison of the distribution of detection efficiencies between cells of different embryos. d , Comparison of the detection efficiency across barcodes between anterior and posterior cells. e , Comparisons of the detection efficiency across all cells between anterior and posterior cells. Results are shown for the 10-kb, 3-kb and 2-kb probesets as indicated. f , Correlation between normalized distance data from different cell types and a modified dataset in which 50% of detected barcodes were removed at random to simulate a high missed detection rate. The correlation coefficients are indicated (Pearson), computed using all unique combinations of 52 barcodes. g , Bar graph comparing the mean correlation between matched segments in the original and downsampled data shown in (f) compared to the mean inter-segment correlation. The correlation coefficients are indicated (Pearson), computed using all unique combinations of 52 barcodes. All 15 matched segment pairs shown in f are plotted compared to all 210 inter-segment pairs. This illustrates that the difference between the substantially downsampled data and the original measurements are much more similar than the typical differences between segments, demonstrating the robustness of the segment-specific findings to missing data. Error bars denote standard error of the mean. h , Histogram showing the detection efficiency per cell for each probeset.
Extended Data Fig. 3 Setting up the flow chamber.
a , Layout of all the components that are needed to assemble the flow chamber. Before using, clean all components with a kimwipe that is slightly damp with ultra-pure water. Note that the 400 mm coverslip is marked with an alcohol-resistant pen. The marks indicate the location of the embryos, which are cryosectioned between the two lines in the center of the coverslip. This space is determined by the middle area of gasket 2. We also used the pen to label that our sample is WT. We find that labeling the slide does not harm the sample. b , Invert the plastic chamber. Then take gasket 1 and place its two holes over the two metal aqueduct ports of the plastic chamber. c , Carefully, place gasket 2 on top of the coverslip. You can use the pen labeled markers as an indicator of where to place the gasket. Make sure to not place gasket 2 directly on top of your sample. Any sample under the gasket will not be accessible for imaging. Also, make sure to not accidentally slide the gasket on top of your sample as this may result in your sample rubbing off the coverslip. d , Place the miroaqueuct glass slide on top of gasket 2. Carefully, place the microaqueduct slide so that its wells on the side (pointed by arrows) are perpendicular to gasket 2. e , Invert the sandwiched components - including the 40-mm coverslip, gasket 2, and the microaqueduct slide - and place the two holes of the microaqueduct slide on top of the plastic chamber’s aqueduct ports. f , Place the plastic chamber on a flat surface. Invert the metal mount and place it on top of the plastic chamber. When placing it on top, it is useful to make sure that the clamps are aligned to the plastic chamber holes as shown here. g , Invert all the components. During this flip, make sure to carefully hold onto both the metal chamber and plastic chamber. We find it useful to hold the metal part with our index/middle finger(s) and to have our thumbs holding onto the tubes of the plastic chamber. h , Tighten the metal clamps by moving the chamber counterclockwise to lock it. Make sure to tighten this as hard as you can, and without breaking the glass components inside. Afterward, connect the “In” and “Out” fluidics tube to the flow chamber’s metal tubes (shown with arrows).
Supplementary information
Supplementary Information
Supplementary Data 1–8 legends.
Reporting Summary
Supplementary Data 1
Primary probe synthesis costs. An EXCEL file that contains the information of all the components - including vendor, product number, unit, etc - needed for primary probe synthesis. Importantly, we have included the total cost to make a single primary probe.
Supplementary Data 2
Cost of readout probe hybridization . An EXCEL file listing the reagents needed for each hybridization round. The user can edit this file to calculate the total cost per each experiment. The cost depends upon the number of readout probes, fluorescent oligos, and buffer used. The user can choose which fluorescent oligos to use; however, we advise that these oligos should be imaged in a different channel from the fiducial. See Supplementary Data Legends for full description.
Supplementary Data 3
Length of imaging experiment and ChrTracer3 analysis . An editable EXCEL file that is useful to estimate the amount of time it will take to complete an imaging experiment and analyze the raw data in ChrTracer3. We have listed all the parameters that would influence the length of time for both of these. Please note that in ‘Imaging Time’ we have listed the ‘Number of readouts per hybridization’ to be ‘2’, which means we are showing an example of a three-color experiment Details of a three-color experiment can be found in the legend of Supplementary Data 2.
Supplementary Data 4
Raw data storage . An editable EXCEL file to help the user calculate the amount of storage space needed for an imaging experiment. This will also calculate the cost to store the data.
Supplementary Data 5
Components for our homebuilt microscope . An EXCEL file containing detailed information - including the supplier(s), part number(s), quantity, units, and total costs - of each component used for our microscope. The total cost of the homebuilt microscope is also included in this table.
Supplementary Data 6
RNA Table . An example of a RNAtable.xlsx file that is used for downstream analysis, such as in BuildMosaicsGUI. See Supplementary Data Legends for full description.
Supplementary Data 7
DNA Table . An example of ExperimentalLayout.xlsx that is used for ChrTracer3 and downstream GUIs. The columns are the same as that of RNAtable.xlsx (see legend for Supplementary Data 6), with two exceptions. 1) For DNA labeling, we typically relabel some of the barcodes at the experiment for quantification purposes. Thus, you will see that at the bottom of the ‘DataType’ column, the last 5 rows are labeled as ‘R’, indicating that these are the barcodes that were relabeled. 2) There is no ‘rnaNames’ column as this is a DNA table.
Supplementary Data 8
ChrTracer3 output file . An example of a fov001_AllFits.csv table that is generated at the end of ChrTracer3 analysis. See Supplementary Data Legends for full description.

