Synthesis of 2-Acetamido-1,3,4-Tri-O-Acetyl-2-Deoxy-D-Mannopyranose -6-Phosphate Prodrugs as Potential Therapeutic Agents
Fabrizio Pertusati, Fabrizio Pertusati, James Morewood, James Morewood
Abstract
Sugar phosphates are emerging as potential therapeutic candidates for certain diseases. However, their high polarity makes them poorly absorbed by the body and their penetration inside the cell is even more difficult without a proper transporter. Amino sugar phosphates (n-amino-n-deoxy-sugars, carbohydrates in which a hydroxyl group has been replaced with an amine group), such as N -acetyl-D-mannosamine (ManNac)-6-phosphate have shown potential as a treatment for a muscular disease called GNE myopathy caused by a deficiency in the production of sialic acid. However, its high polarity leads to poor absorption and consequent high dosage in humans, causing unwanted side effects. Herein, we describe the application of phosphoramidate prodrug chemistry to 1,3,4-O-acetylated N -acetylmannosamine (Ac3ManNAc) to deliver ManNAc-6-phosphate (ManNAc-6-P), a critical intermediate in sialic acid biosynthesis. Sialic acid deficiency is a hallmark of GNE myopathy, a rare congenital disorder of glycosylation (CDG), caused by mutations in the gene “GNE,” that limit the production of ManNAc-6-P. Synthetic methods were developed to provide a library of Ac3ManNAc-6-phosphoramidates that were evaluated in a series of studies for their potential as a treatment for GNE myopathy. © 2022 The Authors. Current Protocols published by Wiley Periodicals LLC.
Basic Protocol 1 : Synthesis of 2-Acetamido-1,3,4-tri-O-acetyl-2-deoxy-D-mannopyranose.
Basic Protocol 2 : Preparation of 3-acetamido-6-((((((S)-1-ethoxy-4-methyl-1-oxo-pentan-2-yl) amino) (phenoxy)phosphoryl) oxy) methyl) tetrahydro-2H-pyran-2,4,5-triyl triacetate (5).
Support Protocol : Preparation of ethyl (chloro(phenoxy)phosphoryl)-l-leucinate.
INTRODUCTION
Sugar phosphates are important biological intermediates in living systems, as a backbone for nucleic acids but more importantly for their major role in metabolism due to their task of storing and transferring energy. Key to this protocol is N -acetyl Mannosamine-6-phosphate (ManNac-6P) a key player in the biosynthesis of sialic acid. Deficiency in this compound, generated by a deficiency of an enzyme called UDP-GlcNAc 2-Epimerase/ManNAc 6-Kinase is responsible for the development of a muscular disease called GNE myopathy. We have demonstrated that ManNAc-6-phosphoramidate prodrugs can restore the level of sialic acid by providing GNE cells with ManNac-6P upon enzymatic activation. Currently, the methods available to prepare phosphoramidate prodrugs of carbohydrates at the 6-position are very few and mostly restricted to simple structural motifs (Ashmus & Lowary, 2014) or with symmetrical groups around the phosphorus as reported by (Kannan, Vinodhkumar, Varghese, & Loganathan, 2001) and by Jalsa (Subratti, Ramkissoon, Lalgee, & Jalsa, 2021) for complex carbohydrates. Following a report from Serpi (McGuigan et al., 2008; Serpi et al., 2012) for N -acetyl glucosamine. No application to other carbohydrates has been reported today although prodrugs of Mannose-1-phosphate have been prepared by Meier (Muus, Kranz, Marquardt, & Meier, 2004). The proposed protocol is generically applicable to all carbohydrates. Acetylation of the 1,3 and 4-hydroxy groups is necessary to avoid multiple phosphorylation of the sugar hydroxyl groups. This would generate a mixture of mono-, di-, and tri phosphorylated species that would require tedious chromatographic separations to isolate the pure compounds. To avoid this problem, we opted for the preparation of the 1,3,4-O-triacetyl mannosamine as a suitable substrate. In Basic Protocol 1, we address the selective protection of the 6-hydroxy group with an acid-labile protecting group (triphenylmethyl chloride, trityl chloride). This will allow further protection of 1,3,4 hydroxy groups with acetic anhydride (acid resistant protecting group). Selective removal of the 6-trityl group under mild acidic conditions affords 2-Acetamido-1,3,4-tri-O-acetyl-2-deoxy-D-mannopyranose (4), which is the main substrate for the prodrug synthesis. Basic Protocol 2 presents the synthesis of the ManNac-6P prodrugs via reaction of compound 4 with the phosphoramidating agent (prepared according to our previous protocol (Serpi, Madela, Pertusati, & Slusarczyk, 2013). The relevance of this protocol is quite substantial for scientists involved in carbohydrate chemistry in the field of congenital disorder of glycosylation. GNE myopathy patients may gain benefit from a compound that delivers the product of the defective enzyme, which could improve the outcome of this muscular disease. This protocol might be applicable to other sugar as well.
CAUTION : All reactions must be run in a suitable fume hood with efficient ventilation; safety glasses and reagent-impermeable protective gloves should be worn. Tertbutyl magnesium chloride is a highly flammable solution and extreme care should be used: always operate under an inert (nitrogen or argon) atmosphere.
Basic Protocol 1: SYNTHESIS OF 2-ACETAMIDO-1,3,4-TRI-O-ACETYL-2-DEOXY-D-MANNOPYRANOSE
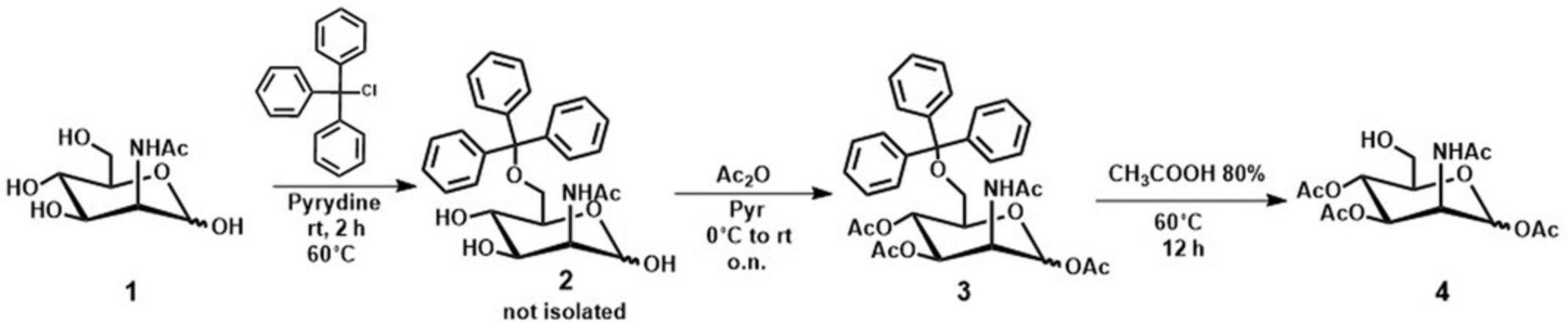
This protocol discusses the synthesis of 2-Acetamido-1,3,4-tri-O-acetyl-2-deoxy-D-mannopyranose 4 as common starting material for all prodrugs. Although the synthesis is reported (Aich et al. 2008) we have found that modification of this procedure improves the method quite substantially. The synthetic scheme is highlighted below. From ManNac (1) the 6-hydroxyl is selectively protected with triphenylmethyl chloride (trityl group, easily removed at the end of the synthetic path). The remaining hydroxyls (1,3, and 4) are acetylated with acetic anhydride in pyridine solution to yield compound 3 from which the trityl moiety is selectively removed under acidic conditions, as shown in Figure 1.The overall yield of 4 from 1 is around 30%–35%, affording 2.7 g of 4 from a starting mass of 5 g of 1.
Materials
-
N -acetyl-D-mannosamine (Carbosynth, cat. no. MA05269)
-
Argon (or nitrogen) gas
-
Sure/Seal™ Anhydrous pyridine (<50 ppm water; Aldrich)
-
Triphenylmethyl chloride, 98%, (Thermo Scientific, cat. no. 10656342)
-
Ethyl Acetate, ACS reagent grade (VWR)
-
Hexane, reagent grade ACS reagent grade (VWR)
-
Sodium chloride (Fisher Scientific)
-
Sodium sulfate (Fisher Scientific)
-
Acetic anhydride, 99% (Fisher Scientific, cat. no. AAL042950B)
-
Sure/Seal™ Toluene anhydrous (Aldrich)
-
Dichloromethane (CH2Cl2), ACS reagent grade
-
Silica gel 60 (230–400 mesh; EMD)
-
Deionized water
-
Acetic acid glacial (Fisher Scientific, cat. no. A38S-500)
-
Distilled water (produced in-house with reverse osmosis setup)
-
Chloroform-D (CDCl3; Acros)
-
Suez/Purite Select Analyst 160 ECO GP Water purifier
-
Disposable hypodermic needles (Fisher Scientific)
-
Analytical balance (Sartorius–Entris)
-
Weighing papers (Whatman, cat. no. 10347672)
-
50-, 100-, and 250-ml round-bottom flasks
-
Stir bar
-
Rubber septum (Aldrich, cat. no. Z553921)
-
Ground glass stopper (Aldrich)
-
Eisco plastic joint clips (Fisher)
-
Rubber balloons for dry gas reactions (Aldrich)
-
Hei-PLATE stirring hotplates (Radleys)
-
Aluminum-backed Silica TLC plates F254 (Merck, cat. no. 1.05554.0001)
-
Heat gun for TLC development
-
254-nm UV lamp
-
Rotary evaporator (Büchi - R-200)
-
RV8 High Vacuum pump (Edwards) for evaporation of high boiling point solvent (water, pyridine, toluene; ∼1.0 × 10–3 bar)
-
MaximaDry™ diaphragm vacuum pump (Fisher Scientific) for rotary evaporation vacuum (∼1 mbar)
-
5-, 10-, and 20-ml Luer-lock all plastic (Polypropylene) disposable syringes (Fisher Scientific)
-
Synthware™ Büchner filter funnels (Fisher Scientific)
-
100-, 250-ml Separating funnel (Fisher Scientific)
-
Water pump for Büchner filtration
-
200-mm Liebig condenser
-
Biotage Isolera one
-
Biotage® Sfär Silica D: 60-μm flash chromatography columns
Synthesis of 2-acetamido-2-deoxy-6-O-triphenylmethyl α, β-D-mannopyranose (2)
1.Place 5 g (22.6 mmol, 1 eq.) of N -Acetyl-D-Mannosamine 1 into an oven-dried, 250-ml, 2-neck round-bottom flask fitted with a stir bar.
2.Seal the central neck with a rubber septum and the side neck with a ground glass stopper and plastic joint clip.
3.Purge the flask with dry nitrogen or argon (via a gas balloon), through the septum. Leave the gas balloon in place when the outlet needle is removed.
4.Add, using a polypropylene syringe and via the septum, 75 ml of anhydrous pyridine then heat, with stirring, to 60°C.
5.Add, quickly and via the side neck, 6.9 g (24.75 mmol, 1.1 eq.) of trityl chloride in one portion, then continue heating and stirring for 2 hr.
6.Monitor the reaction via thin-layer chromatography (70:30 n -hexane: ethyl acetate).
7.Transfer the solution into a 250-ml round-bottom flask and concentrate via rotary evaporation.
8.Extract the residue with 75 ml ethyl acetate and wash the extract with a saturated, aqueous, sodium chloride solution. Repeat the extraction three times and pool the organic layers and dry with anhydrous sodium sulfate.
9.Filter the solution using a paper filter and remove the solvent via rotary evaporation to attain the crude product as an off-white/tan solid 8.4 g.
Synthesis of 2-acetamido-1,3,4-tri-O-acetyl-2-deoxy-6-otriphenylmethyl-α,β-D-mannopyranose (3)
10.Add to a 150-ml round-bottom flask fitted with a stir bar, 2.2 g (4.75 mmol, 1 eq.) of crude 6-O-Trityl-N -Acetyl-Mannosamine 2 from step 10.
11.Seal the flask with a rubber septum and then purge the flask with dry nitrogen or argon via a gas balloon, through the septum.
12.Add, using a polypropylene syringe, 3 ml anhydrous pyridine and, with stirring, cool the suspension to 0°C in an ice/water bath.
13.Add 2.4 ml (25.39 mmol, 5.35 eq.) of acetic anhydride, via the septum, to the cooled solution. Following the addition, remove the ice bath and allow the solution to reach room temperature. Continue stirring at room temperature for 24 hr.
14.Evaporate the pyridine with a rotary evaporator, then co-evaporate the residue three times via rotary evaporator with 10-ml aliquots of anhydrous toluene.
15.Dissolve the residue in 100 ml dichloromethane and wash two times, each time with 50 ml deionized water.
16.Separate and collect the dichloromethane layer and dry it over sodium sulfate.
17.Remove the sodium sulfate by filtration via sintered glass Büchner funnel into a 250-ml round-bottom flask.
18.Remove solvent via rotary evaporation to yield 1.74 g of 3 as a pink/red solid.
Synthesis of 2-acetamido-1,3,4-tri-O-acetyl-2-deoxy-α, β-D-mannopyranose (4)
19.Add 1.5 g (2.54 mmol, 1 eq.) of crude 1,3,4-O-Triacetyl-6-O-Trityl-N -Acetyl Mannosamine 3 to a 100-ml round-bottom flask fitted with a stir bar.
20.Add 35 ml (498.9 mmol, 196.11 eq.) of 80% (v/v) acetic acid in water and fit the flask with a water-cooled 200-mm Liebig condenser and plastic joint clip.
21.Heat the mixture, with stirring, to 60°C and continue for 4 hr.
22.Monitor the reaction progress by TLC (ethyl acetate: n -hexane, 90:10) R f product: 0.18.
23.Filter through a sintered glass Büchner funnel into a 100-ml round-bottom flask and concentrate the mixture via rotary evaporation to yield an off-white solid.
24.Purify the crude product by automated flash chromatography using a Biotage Isolera one chromatographic system fitted with a Biotage® Sfär Silica D (25g) column, using ethyl acetate and n -hexane as the eluents. Yield 600 mg (68%) as a white solid.
25.Characterize the compound by 1H NMR.
Basic Protocol 2: PREPARATION OF 3-ACETAMIDO-6-((((((S)-1-ETHOXY-4-METHYL-1-OXO-PENTAN-2-yl) AMINO) (PHENOXY)PHOSPHORYL) OXY) METHYL) TETRAHYDRO-2H-PYRAN-2,4,5-TRIYL TRIACETATE (5)
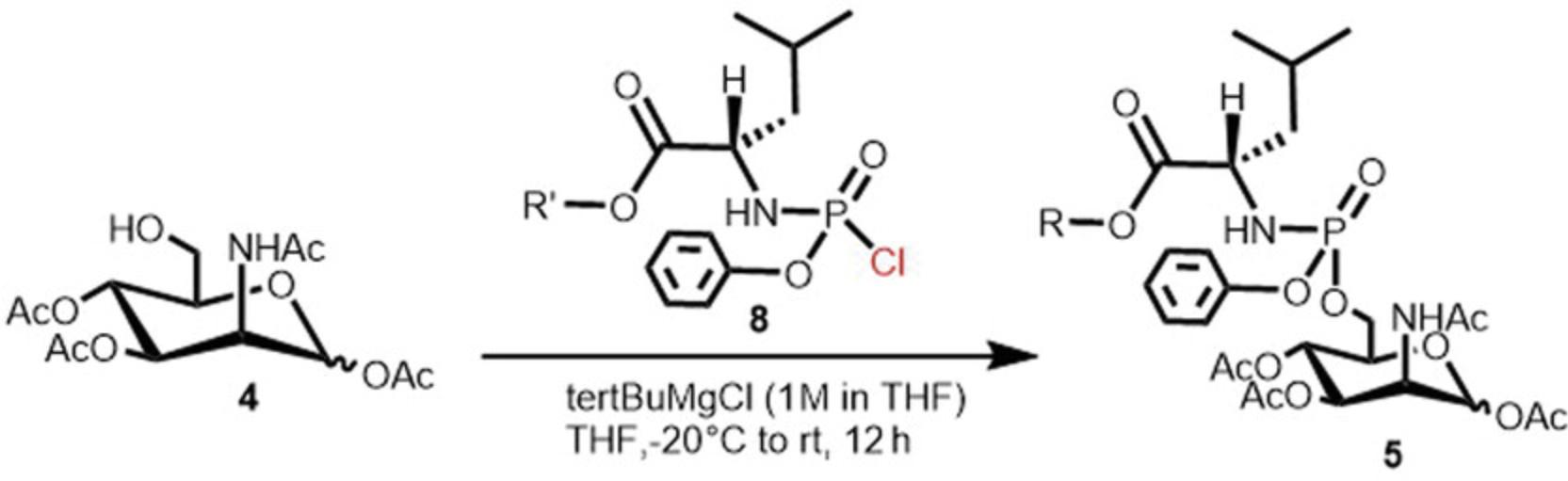
The preparation of ManNAc phosphoramidate prodrugs can be completed via either the Grignard route or the N -methyl imidazole route reported in the literature (Serpi et al., 2013). In our experience with carbohydrate phosphate prodrugs, the Grignard method (Fig. 2) has been found to be superior as the NMI route produces lower yields and causes difficulties in the purification of the products. To a solution of acetylated sugar 4 , (1 equiv) dissolved in anhydrous THF, under argon, a slight excess of tBuMgCl solution in THF, (1 M, 1.5 equiv) at −20°C under an argon atmosphere to allow the activation of 6-OH by the formation of magnesium salt. After 30 min, always at low temperature, a solution of an appropriate phosphorochloridate (1.5 equiv) in dry THF is added dropwise to the mixture. Then, the reaction is then allowed to reach room temperature and stirred for 12 hr until the starting material disappear as judged by TLC analysis. Prodrugs are isolated as a mixture of Sp and Rp diastereoisomers of α and β anomers by column chromatography in yields of 35%–60% depending on the specific phosphorochloridate used, representing an overall yield of 11%–20% of 5 from ManNAc 1. The most significant factor limiting the yield of this process is the purification of 4 and 5 as both compounds produce significant tailing in column and flash chromatography with later fractions of 5 coeluting with impurities and, therefore not being included in the final weights.
Additional Materials (also see Basic Protocol 1)
-
2-Acetamido-1,3,4-tri-O-acetyl-2-deoxy-α, β-D-mannopyranose 4 (see Basic Protocol 1)
-
Argon (or nitrogen) gas
-
Sure/Seal™ Tetrahydrofuran (Aldrich)
-
Sure/Seal™ Tertbutyl magnesium chloride 1M in THF (Aldrich)
-
Sodium chloride (Fisher Scientific)
-
Ethyl (Chloro(phenoxy)phosphoryl)-L-Leucinate (8 ; see the Support Protocol)
-
Cerium (IV) sulfate (Merck, cat. no. 359009)
-
Sulfuric acid (Merck, cat. no. 1.12080)
-
50-, 100-, and 250-ml round-bottom flasks
-
Disposable hypodermic needles (Fisher Scientific)
-
Ice/salt bath
Synthesis of (3S,4R,5S,6R)-3-acetamido-6-((((((S)-1-ethoxy-4-methyl-1-oxopentan-2-yl) amino)(phenoxy)phosphoryl)oxy)methyl)tetrahydro-02H-pyran-2,4,5-triyl Triacetate (6)
1.Place 1 g (2.88 mmol, 1 eq.) of 2-Acetamido-1,3,4-tri-O-acetyl-2-deoxy-α, β-D-mannopyranose 4 into a 50-ml, oven-dried, round-bottom flask fitted with a stir bar.
2.Seal the flask with a rubber septum.
3.Purge the flask with dry nitrogen or argon via a gas balloon, through the septum venting the system with another needle for about 30 s to 1 min.
4.Leave the gas balloon in place when the outlet needle is removed so the system is fully under argon.
5.Add, using a polypropylene syringe via the septum, 15 ml of anhydrous THF and then cool the solution to about –20ºC using an ice/salt bath.
6.Add, with stirring and using a polypropylene syringe, 4.3 ml (4.3 mmol, 1.5 eq.) of a 1.0 M solution of tertbutyl magnesium chloride in THF, dropwise to avoid local heating of the solution.
7.Continue stirring while the solution attains room temperature slowly.
8.Add dropwise using a polypropylene syringe, 1.44 g (4.3 mmol, 1.5 eq.) ethyl (Chloro(phenoxy)phosphoryl)-L-leucinate (8) dissolved in 5 ml anhydrous THF over the course of 10 min. Remove the ice bath and allow the reaction to warm to room temperature while stirring for 12 hr.
9.Monitor the reaction via TLC (10:90 diethyl ether: ethyl acetate) hourly, after 10 hr the R f of the product is typically ∼0.5.
10.Remove the solvent using a rotary evaporator connected to a diaphragm pump.
11.Purify the crude product by automated flash chromatography using a Biotage Isolera one chromatographic system fitted with a Biotage® Sfär Silica D (25 g) column, using ethyl acetate and n -hexane as the eluents. Yield 700 mg (37.7%).
12.Characterize the compound by 31P NMR, 1H NMR, 13C NMR and Mass Spectrometry (Electrospray ionization).
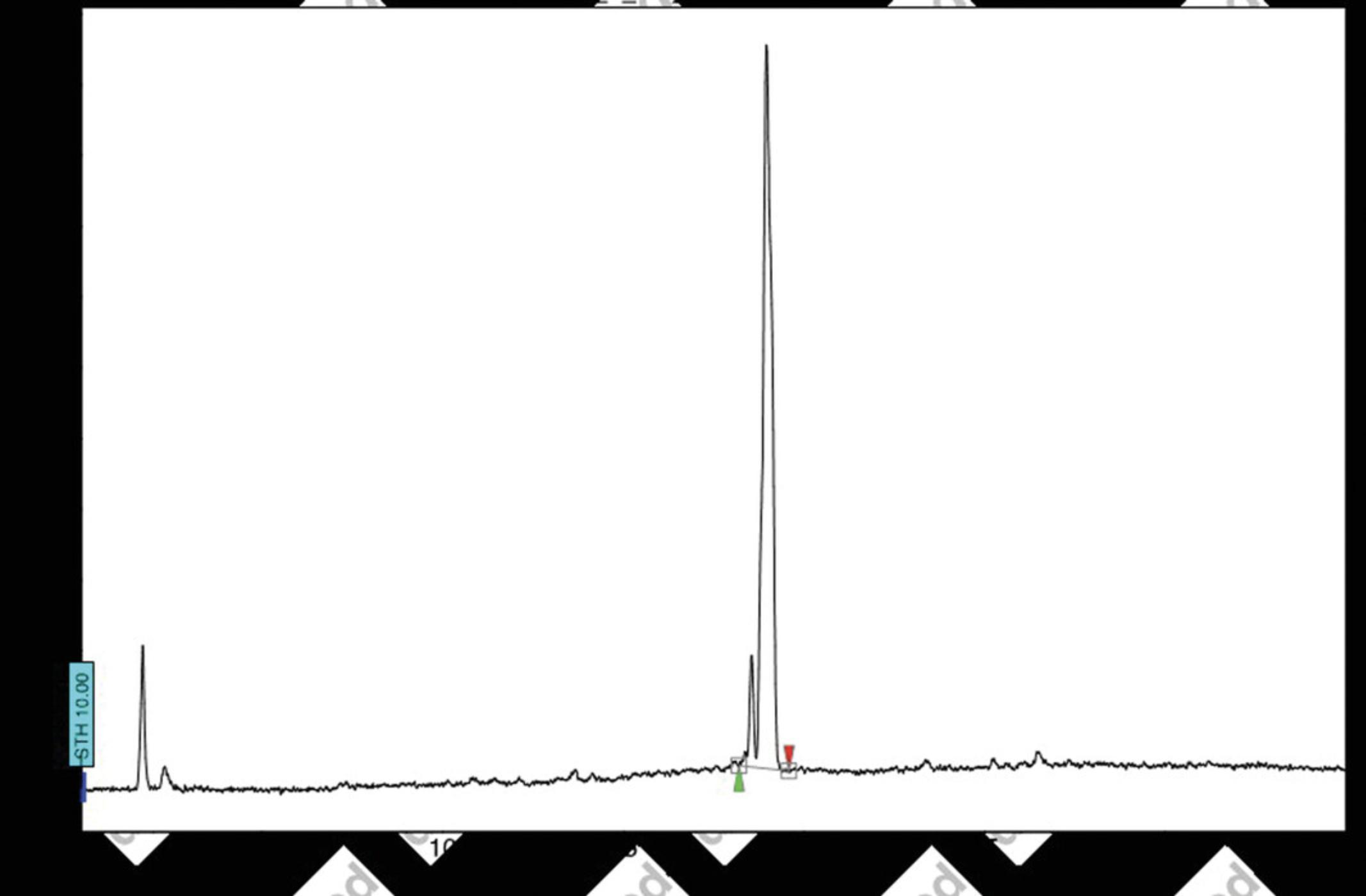
13.Analyze the purity of the prodrug via reverse-phase analytical HPLC. The following HPLC conditions are recommended:
- Column: 150 × 4.6 mm; particle size: 5 μm, C18 (Varian Pursuit XRs 5 C18)
- Phase A: water; phase B: MeCN
- Gradient: 90% mobile phase A to 100% mobile phase B over 30 min
- Flow rate: 1 ml/min; detection wavelength: 210 and 254 nm; elution time: 32 (2 min for column re-equilibration)
- Retention time for the diastereoisomers is tR 18.55 min, 18.96 min
Support Protocol: PREPARATION OF ETHYL (CHLORO(PHENOXY)PHOSPHORYL)-L-LEUCINATE
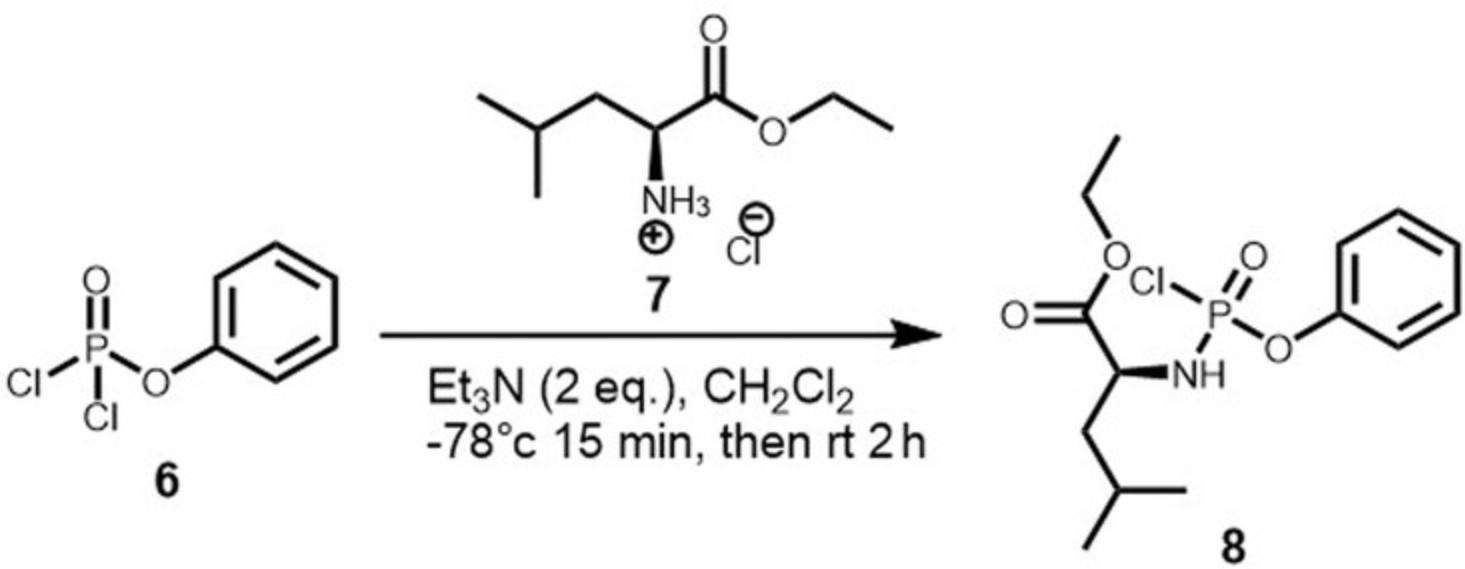
This supporting protocol describes the general synthesis of Ethyl (Chloro(phenoxy)phosphoryl)-L-leucinate, the key intermediates for the synthesis of ManNAc Prodrugs 5 (Fig. 4). The same protocol can be applied for the synthesis of all other phosphorochloridate by just changing the amino acid esters and the dichlorophosphate. Phenyldichlorophosphate used in this supporting protocol is commercially available. Other aryloxy-dichloridates (naphthyl etc.) can be prepared with the detailed procedure described in our previous protocol (Serpi et al., 2013). For the synthesis of 5 , phenyl dichlorophosphate and the L-Leucine ethyl ester hydrochloride, are dissolved in anhydrous dichloromethane (DCM) under argon atmosphere and cooled down to −78°C. Triethylamine (2 equiv) is added dropwise and the resulting mixture is stirred for about 15–20 min at low temperature (−78°C). Then, the reaction mixture is allowed to reach room temperature and was left stirring for 2 hr. The solvent is removed under reduced pressure, the solid residue is suspended in diethyl ether, and stirred under argon atmosphere for 20 min, to ensure complete extraction of the product from the ammonium salt. The salt is then filtered off, and the ether is evaporated giving the chloridate 5 as a colorless oil, which is used in the next step without further purification.
Additional Materials (also see Basic Protocol 1)
-
Argon (or nitrogen) gas
-
Phenyl dichlorophosphate (Aldrich, cat. no. P22389)
-
L-Leucine ethyl ester hydrochloride (Aldrich, cat. no. P22389)
-
Sure/Seal™ Anhydrous Triethylamine (TEA; 99.5% pure; Sigma-Aldrich)
-
Sure/Seal™ Anhydrous diethyl ether (Aldrich Et2O)
-
Drierite or anhydrous calcium chloride (ACS reagent)
-
Sure/Seal™ Anhydrous dichloromethane (CH2Cl2) (Aldrich)
-
Dry-ice/acetone bath
-
Magnetic stirring and heating plate
-
Rotary evaporator equipped with a vacuum pump and calcium chloride (or Drierite) valve
-
Glass-sintered filter funnel (max pore size 16–40 μm)
-
Water aspirator
-
500-ml round-bottom, two-necked flasks
-
Vacuum pump
Prepare ethyl (chloro(phenoxy)phosphoryl)-L-leucinate phosphorochloridate (8)
1.Under a N2 or Ar atmosphere, first place 4.08 g (20.86 mmol) phenyl dichlorophosphate 6 , then 4 g (18.96 mmol) of L-leucine ethyl ester hydrochloride 7 or other amino acid ester and 150 ml anhydrous dichloromethane in a 250-ml round-bottom flask.
2.Seal the flask with a rubber septum. Purge the flask with dry nitrogen or argon via a gas balloon, through the septum venting the system with another needle for about 30 s to 1 min.
3.Leave the gas balloon in place when the outlet needle is removed so the system is fully under argon.
4.Cool the above mixture at −78°C by means of a dry ice-acetone bath.
5.Add using a polypropylene syringe, 5.29 ml (37.92 mmol) anhydrous triethylamine, dropwise over 15 min and stir the resulting mixture on a magnetic plate for 15 min at −78°C.
6.After this period, remove the cooling bath and allow the mixture to reach room temperature.
7.Stir the mixture at this temperature for 60–120 min.
8.Monitor the reaction with 31P NMR.
9.Evaporate the solvent under reduced pressure on a rotary evaporator.
10.Under argon, suspend the solid in anhydrous diethyl ether to obtain a white suspension.
11.Quickly filter the suspension of triethylammonium hydrochloride using a sintered Büchner funnel connected to a water aspirator into a 250-ml round-bottom flask.
12.Evaporate the ether solution of chloridate 5 using a rotary evaporator equipped with a calcium chloride valve and a membrane vacuum pump.
13.Analyze the oily residue by 31P NMR and 1H NMR.
REAGENTS AND SOLUTIONS
Pancaldi's reagent
For 1 L of visualizing solution, you will need:
- 42 g ((NH4)6MoO4 (Ammonium Molybdate; Merck, cat. no. M1019)
- 2 g Ce (SO4)2 (Cerium sulfate; Merck, cat. no. 359009)
- 625 ml H2SO4 (Sulfuric acid; Merck, cat. no. 112080)
- H2O to 1 L
All reagents should be added slowly in a fume hood.
Dissolve the inorganic salts in a minimum amount of water, add the sulfuric acid slowly to this solution (CAREFUL here because the solution will become hot). Once the mixture is at room temperature add water to volume. Storage is possible in a 250-ml wide-mouth jar. This stain has a long shelf-life so long as solvent evaporation is limited. Surround the jar with aluminum foil as the stain may be photo-sensitive and exposure to direct light may shorten the shelf-life of this reagent. Μark the observed spots with a dull pencil following heating as this stain will fade on the TLC plate after a using a couple of days.
COMMENTARY
Background Information
Carbohydrates are a class of vital compounds for human beings. It is not surprising that many functionalized, synthetic carbohydrates can work as medicines as well. Particularly in glycosylation disorders, those that are generated by a deficiency in the production of carbohydrates. Congenital disorder of glycosylation is a term that applies to a large group of over 130 rare genetic, metabolic disorders due to defects in a complex chemical process known as glycosylation (Hennet & Cabalzar, 2015). One of these diseases, called GNE myopathy is caused by a defect in an enzyme Glucosamine (UDP-N-Acetyl)-2-Epimerase/N -Acetylmannosamine Kinase responsible for the synthesis of N -acetyl Mannosamine-6-phosphate a key precursor in the biosynthesis of sialic acid (Carrillo, Malicdan, & Huizing, 2018; Celeste et al., 2014). This enzyme has the double function of epimerization of N -acetyl glucosamine into its epimer mannosamine, and the subsequent immediate attachment of a phosphate group to the 6-end of the sugar to form the phosphate sugar ManNac-6P. ManNac-6P is then converted into Neu5Ac-9-P (sialic acid) by Neu5Ac-9-P synthase. Lack of sialic acid on the surface of muscle cells seems responsible for the presence of this disease that causes progressive muscle weakness (Penner et al., 2006). In this protocol, we report the preparation of ManNAc -6-phosphate prodrugs as a potential therapeutic agent for GNE myopathy. The rationale is that when the prodrug is activated by enzymatic removal of phosphate masking groups and acetyl groups from the sugar hydroxyl groups, it will release the product of the defective GNE enzyme (providing a source of ManNac-6P) thereby restoring the level of sialic acid and rescuing the GNE phenotype (Morozzi et al., 2019). This strategy is advantageous over the administration of simple ManNac as a nutritional supplement (clinical trial NCT04231266) because of the following: due to their high polarity, carbohydrate molecules do not passively cross the gut membrane and require specific transporter proteins to reach the bloodstream. Their poor absorption by the gut necessitates the administration of high dosage (up to 10–12 g/day) to reach a suitable concentration. This causes severe digestion problems in the patient, which has led in several cases to their abandoning of the trial or the treatment. By delivering a more lipophilic prodrug (the acetyl groups contribute to this lipophilicity) the passive diffusion will allow the administration of a significantly lower dosage of the compound, with a drastic reduction of the side effects (Morozzi et al., 2019). Secondly, the direct delivery of the ManNac-6P will bypass, completely, the defective enzyme. Indeed ManNac, even when reaching the cellular location needs to be phosphorylated and because GNE mutations also affect the kinase moiety, ManNac can still be poorly converted into its phosphorylated precursor and, therefore, poorly transformed into sialic acid.
Critical Parameters
There are not many critical parameters in this protocol. Use dry glassware, anhydrous chemical reagents, and dry argon flow is recommended. We have found that the quality of the trityl chloride used in the first step of Basic Protocol 1 is fundamental for the success of the reaction. Old bottles of reagent do not perform well in terms of the yield of the final product. Purification of trityl chloride by crystallization from toluene solves this problem quite neatly. Tritylated ManNac should be acetylated immediately to avoid detritylation therefore, handling of this protected carbohydrate under dry and acid-free conditions is recommended for the success of this protocol. Careful control of the temperature during Grignard addition is another key parameter to control. Its addition should be performed at −20°C and in a dropwise manner to avoid a local increase in temperature. Without this care migration of the acetyl group can occur with consequent formation of phosphate regioisomers. Monitor the formation of the different phosphorus species during this one-pot process by analyzing aliquots of the reaction mixture via 31P-NMR. All the reactions must be monitored by TLC visualizing these compounds with Pancaldi's reagent.
Troubleshooting
The synthetic procedures described in this unit are intended for use only by persons with prior training in experimental organic chemistry and thus with knowledge of the common chemical laboratory techniques. Generally, the procedures in this protocol are quite robust and the critical steps are very few. However, care must be taken in certain steps in order to obtain the best outcome. Table 1 below collects the main problem that may arise following the above procedures and the strategy to overcome them.
| Problem | Possible cause | Solution |
|---|---|---|
| Poor yield in tritylation reaction (Basic Protocol 1) |
Inferior quality of Trityl chloride Purification via acidic silica gel. Trityl group is acid sensitive, and the acidity of the silica gel can deprotect the material |
Use a new bottle or recrystallize the old one before use. Store it under dry conditions Purification is usually not needed for this step. If desired, column chromatography can be performed by neutralization of the silica acidity by treat the packed column with 1% Et3N in DCM before the elution or add triethylamine (1% in the eluent) |
| Trityl removal reaction (Basic Protocol 1) | If the reaction is conducted for more than 2 hr an impurity with lower Rf starts to form and its removal by column chromatography can be tedious | Monitor the reaction by TLC until the starting material disappear and stop the reacting at this point. Usually, 2 hr are sufficient for this purpose |
| Detritylated product heavily contaminated with trityl compounds (Basic Protocol 1) | The high MW of the trityl moiety combined with the aqueous nature of the deprotection reaction affords large amounts of trityl byproducts. If the solvent is removed at this stage, these are concentrated with the intended product and purification becomes very time-consuming and wasteful of solvents | Following completion of reaction cool the solution to room temp in a water bath and allow to stand at room temperature for 30 min. The trityl byproducts are deposited at the bottom of the flask. The solution is carefully filtered through a sintered glass funnel to remove a large proportion of the trityl contaminants, allowing for much easier purification |
| Phosphoramidation reaction (Basic Protocol 2) | Acetyl migration | Careful control of the reaction temperature during Grignard addition is fundamental for the success of this step. Higher temperatures cause acetyl migration with consequent formation of phosphorus regioisomers |
Time Considerations
The reaction time for the synthesis of these prodrugs and its analogs is overall short compared to other multistep synthesis. Careful storage of trityl chloride will save time that could be spent to recrystallize the regent of wait for its arrival. We have found that the synthesis of acetylated ManNac 4 can be accomplished in three full working days thanks to our optimization of some literature procedures. This time includes all the necessary purifications. The phosphoramidating agent (the Support Protocol) can be prepared just under 3 hr in multigram quantitates and can be safely storage for months in a sealed vial in a freezer without decomposition. Prodrug preparation require an additional 24 hr (including chromatographic purification). Several prodrugs can then be prepared quite easily once bulk of starting materials are accumulated.
Acknowledgments
Funding for this project has been provided by Demeter/Ichorion Therapeutics which is warmly acknowledged.
Author Contributions
Fabrizio Pertusati : Conceptualization, Funding acquisition, Writing — original draft, Writing — review & editing; James Morewood : Data curation, Visualization, Writing — review & editing.
Conflict of Interest
The authors do not have any conflict of interest.
Open Research
Data Availability Statement
Please include a data availability statement. You can choose from a list of standard templates found in the Author Guidelines on our website.
Literature Cited
- Aich, U., Campbell, C. T., Elmouelhi, N., Weier, C. A., Sampathkumar, S. G., Choi, S. S., & Yarema, K. J. (2008). Regioisomeric SCFA attachment to hexosamines separates metabolic flux from cytotoxicity and MUC1 suppression. ACS Chemical Biology , 3(4), 230–240. doi: 10.1021/cb7002708
- Ashmus, R. A., & Lowary, T. L. (2014). Synthesis of carbohydrate methyl phosphoramidates. Organic Letters , 16(9), 2518–2521. doi: 10.1021/ol500894k
- Carrillo, N., Malicdan, M. C., & Huizing, M. (2018). GNE myopathy: Etiology, diagnosis, and therapeutic challenges. Neurotherapeutics , 15(4), 900–914. doi: 10.1007/s13311-018-0671-y
- Celeste, F. V., Vilboux, T., Ciccone, C., de Dios, J. K., Malicdan, M. C., Leoyklang, P., … Huizing, M. (2014). Mutation update for GNE gene variants associated with GNE myopathy. Human Mutation , 35(8), 915–926. doi: 10.1002/humu.22583
- Hennet, T., & Cabalzar, J. (2015). Congenital disorders of glycosylation: A concise chart of glycocalyx dysfunction. Trends in Biochemical Sciences , 40(7), 377–384. doi: 10.1016/j.tibs.2015.03.002
- Kannan, T., Vinodhkumar, S., Varghese, B., & Loganathan, D. (2001). Synthesis of glycosyl phosphoramidates: Novel isosteric analogues of glycosyl phosphates. Bioorganic & Medicinal Chemistry Letters, 11(18), 2433–2435. doi: 10.1016/s0960-894/(01)00469-3
- McGuigan, C., Serpi, M., Bibbo, R., Roberts, H., Hughes, C., Caterson, B., … Verson, C. R. (2008). Phosphate prodrugs derived from N-acetylglucosamine have enhanced chondroprotective activity in explant cultures and represent a new lead in antiosteoarthritis drug discovery. Journal of Medicinal Chemistry , 51(18), 5807–5812. doi: 10.1021/jm800594c
- Morozzi, C., Sedlakova, J., Serpi, M., Avigliano, M., Carbajo, R., Sandoval, L., … Pertusati, F. (2019). Targeting GNE myopathy: A dual prodrug approach for the delivery of N-acetylmannosamine 6-phosphate. Journal of Medicinal Chemistry , 62(17), 8178–8193. doi: 10.1021/acs.jmedchem.9b00833
- Muus, U., Kranz, C., Marquardt, T., & Meier, C. (2004). cycloSaligenyl-mannose-1-monophosphates as a new strategy in CDG-Ia therapy: Hydrolysis, mechanistic insights and biological activity. European Journal of Organic Chemistry , 2004(6), 1228–1235. doi: 10.1002/ejoc.200300681
- Penner, J., Mantey, L. R., Elgavish, S., Ghaderi, D., Cirak, S., Berger, M., … Hinderlich, S. (2006). Influence of UDP-GlcNAc 2-epimerase/ManNAc kinase mutant proteins on hereditary inclusion body myopathy. Biochemistry , 45(9), 2968–2977. doi: 10.1021/bi0522504
- Serpi, M., Bibbo, R., Rat, S., Roberts, H., Hughes, C., Caterson, B., … McGuigan, C. (2012). Novel phosphoramidate prodrugs of N-acetyl-(D)-glucosamine with antidegenerative activity on bovine and human cartilage explants. Journal of Medicinal Chemistry , 55(10), 4629–4639. doi: 10.1021/jm300074y
- Serpi, M., Madela, K., Pertusati, F., & Slusarczyk, M. (2013). Synthesis of phosphoramidate prodrugs: ProTide approach. Current Protocols in Nucleic Acid Chemistry , 53(1), 15.15.11–15.15.15. doi: 10.1002/0471142700.nc1505s53
- Subratti, A., Ramkissoon, A., Lalgee, L. J., & Jalsa, N. K. (2021). Synthesis and evaluation of the antibiotic-adjuvant activity of carbohydrate-based phosphoramidate derivatives. Carbohydrate Research , 500, 108216. doi: 10.1016/j.carres.2020.108216
Key References
- Serpi, M., Madela, K., Pertusati, F., & Slusarczyk, M. (2013). Synthesis of phosphoramidate prodrugs: ProTide approach. Current Protocols in Nucleic Acid Chemistry , 53(1), 15.15.11–15.15.15. doi: 10.1002/0471142700.nc1505s53
This reference is key for the preparation of the phosphoramidating agent used in this protocol. It contains the detailed procedure for the preparation of the aryl dichlorophosphate, the amino acids ester p-toluenesulfonate and hydrochloride salts and the procedure for their coupling.
- Morozzi, C., Sedlakova, J., Serpi, M., Avigliano, M., Carbajo, R., Sandoval, L., … Pertusati, F. (2019). Targeting GNE myopathy: A dual prodrug approach for the delivery of N-acetylmannosamine 6-phosphate. Journal of Medicinal Chemistry , 62(17), 8178–8193. doi: 10.1021/acs.jmedchem.9b00833
This reference report a structure activity relationship of a small prodrug family one of which is described in this protocol with the proof -of-concept of their activity against GNE myopathy diseases.

