Standardised Methods for Feed Tabs Preparation to Assess Feeding Rate and Exposure to Insoluble Substances in Parhyale hawaiensis
Ibrahim Lawan, Dominique MJ Anderson, Theodore, B. Henry
Aquatic ecotoxicology
Insoluble substances
Dietary exposure
Toxicity testing
Parhyale hawaiensis
Feeding rate
DECOTABs
Chronic toxicity evaluation
Benzo(a)pyrene
Tropical marine organisms
Soluble substances
Disclaimer
The protocol provided here is intended for educational purposes only and should be conducted in appropriate laboratory settings. The developers of this protocol are not liable for any damages or consequences arising from its use. Users should ensure compliance with relevant safety regulations and ethical guidelines when conducting experiments involving live organisms.
Abstract
This protocol outlines standardised procedures for preparing feed tabs to assess feeding rates and conduct dietary toxicity testing of insoluble substances in Parhyale hawaiensis. By addressing the need for reliable testing methodologies, particularly those for evaluating the effects of insoluble pollutants on aquatic organisms, this protocol offers a robust framework for researchers. It facilitates comprehensive ecotoxicological studies, contributing to a better understanding of the environmental risks of toxicants (soluble or insoluble) using feeding metrics in marine ecosystems. This protocol uses benzo(a)pyrene (BaP), a polycyclic aromatic hydrocarbon (PAH), as a model toxicant because of its ecotoxicological concerns and physicochemical properties (e.g. low water solubility [0.00162 mg/L at 25°C], high lipid–water partition coefficient [log Klipw 7.35], and high octanol-water partition coefficient [log Kow 6.13]). Because BaP has low solubility, toxicity investigations must include assessment via diet as a more realistic and reliable method to assess its effects on aquatic organisms rather than just aqueous exposure.
Steps
Introduction
Dietary exposure and toxicological assessments of insoluble substances represent emerging frontiers in aquatic ecotoxicology, necessitating the development of standardised bioassays that accurately reflect real-world exposure scenarios (Connon et al., 2012). Current toxicity testing methodologies primarily rely on soluble substances introduced into the aqueous phase, which inherently limits their applicability for evaluating the effects of insoluble compounds (Gotz et al., 2021). Unlike their soluble counterparts, insoluble substances exhibit distinct chemical properties that lead to heterogeneous distribution and non-uniform uptake routes within aquatic environments (Hartmann et al., 2015). Therefore, there is a critical need to adapt test scenarios to address these challenges effectively. Dietary uptake has emerged as the predominant route of exposure to insoluble substances in aquatic organisms; however, this pathway is often overlooked in conventional testing protocols (Bundschuh et al., 2019). To address these limitations, we propose a standardised protocol utilising decomposition and consumption tablets (DECOTABs) (Gotz et al., 2021; Kampfraath et al., 2012) as a reliable food source for Parhyale hawaiensis , thereby facilitating systematic and rigorous toxicity testing via the oral pathway.
Parhyale hawaiensis , a tropical marine amphipod, has significant ecological importance and is a valuable model organism in various scientific disciplines (Paris et al., 2022). Recognised for its sensitivity to environmental stressors, P. hawaiensis has emerged as a promising candidate for tropical marine studies and laboratory investigations. Previous studies have established protocols for laboratory culturing, acute toxicity testing in water and sediment, internal dose determination, gene expression analysis, and assessment of DNA damage and chromosomal alterations in P. hawaiensis (Dos Santos et al., 2022). Leveraging these advancements, our objectives are twofold: 1) to develop a protocol for chronic toxicity evaluation with a particular focus on feeding rate by modifying the decomposition and consumption tablets (DECOTABs), and 2) to standardise a protocol for preparing feed tabs tailored for the toxicity testing of insoluble substances, with benzo(a)pyrene (BaP) serving as a model toxicant. The physicochemical properties of BaP, including its low water solubility (0.00162 mg/L at 25°C), high lipid–water partition coefficient (log Klipw lipw 7.35), and octanol-water partition coefficient (log Kow ow 6.13), underscore its profound insolubility and facilitate its bioaccumulation and toxicity in aquatic organisms (Hylland, 2006; Latimer & Zheng, 2003; Pampanin & Sydnes, 2013). In addition to addressing the necessity for reliable testing methodologies to evaluate the effects of insoluble pollutants in aquatic organisms, our protocol is poised to make significant contributions to ecotoxicology, especially when assessing the effects of both soluble and insoluble substances using feeding metrics.
Step I – Preparing Standard Feed Tabs
Prepare feed tabs with a total weight of five (5 g). All measurements below can be scaled up or down based on the target feed weight.
b. Heat the mixture in a microwave to about 100°C to dissolve the agar for approximately0h 0m 45s to 0h 1m 0s until it foams.
d. Before cooling, pour the mixture into 36 cylindrical moulds of 1.1 cm diameter and 0.6 cm height (custom-built aluminium device).

( NB: Aquatic animals, such as amphipods and fish, do not utilise cellulose because it is indigestible in their gut (Ighwela et al., 2012). Therefore, cellulose is nutritionally unavailable to these aquatic organisms and is used as a dietary binder, non-nutritive filler, anti-caking agent, bulking agent, and stabiliser. It is soluble in ether but insoluble in water).
Step II – Supplementing Insoluble Substance (BaP) in Feed Tabs
As described below, the selected insoluble substance, such as BaP , can be added to the feed supplement before adding agar .
a. Sum up the weights of the agar, cellulose, and feed supplement to know the total weight and determine the concentration of the insoluble substance that can be added per gram of diet.
b. Prepare the BaP-supplemented diet in a 300-ml glass beaker by combining the 2.72g of granulated feed supplements and 10mL acetone with or without BaP ( NB: high volume of carrier solvent was used to facilitate even distribution of toxicant).
c. Prepare diets with 0 (control), acetone-treated (control), and 50, 250, or 1250 μg BaP (nominal)/g diet and mix for approximately 0h 2m 0s ( NB: concentrations can be changed based on the insoluble substance used and the determined effective concentration).
e. Add 1.08g cellulose and complete the feed preparation as in Step I above .
( NB: Acetone was chosen as the carrier solvent because it can quickly evaporate under a stream of air and has been shown to cause minimal biological effects in previous studies (Hallare et al., 2006; Shatilina et al., 2020).
Step III – Drying Feed Tabs
a. Pour the mixture into 36 cylindrical moulds of 1.1 cm diameter and 0.6 cm height (custom‐built aluminium device).
b. The tablets may initially have a convex surface that quickly flattens during agar solidification at 6°C in refrigerator.
d. Remove the feed tabs from the moulds after approximately 0h 15m 0s in a refrigerator at 6°C.
g. Feed tabs can be stored at room temperature for use within months or at -20°C for long-term use.
Step IV – Evaluating Feed Tabs Water Stability and Adjustment Factor
In this step, the effects of drying and prewetting on the weight stability of the feed tabs at different handling steps were evaluated using a fine analytical balance.
Water Stability – The stability of feed tabs in water was evaluated by mimicking their replacement cycles during exposure to P. hawaiensis ( NB : this section evaluates the expected weight loss of the feed tabs without amphipods feeding on them).
Measure the initial dry weight ( dwI ) of at least four (4) feed tabs, transfer to seawater and prewet them for 24h 0m 0s before the experiment.
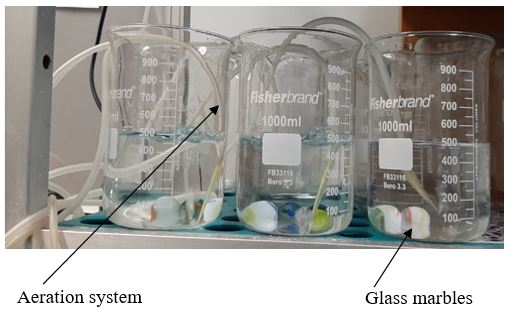
After 24h 0m 0s of prewetting, transfer them into a mock exposure chamber (a 1 L glass beaker filled with 500mL seawater with continuous aeration but no test organisms).
Add three (3) glass marbles in the beaker as hiding materials without amphipods and keep in a temperature-controlled room with a 12:12-h light:dark cycle at approximately 30°C for 48h 0m 0s ( NB: this is to mimic the amphipod exposure scenarios).
After the 48h 0m 0s, remove the feed tabs, gently blot on tissue papers, and dry them for 24h 0m 0s in the incubator at 45°C.
Place them in a desiccator or fume cupboard for 0h 30m 0s and weigh them again using an analytical balance to obtain the final dry weight ( dwF ).
The difference between dwI and dwF (mean weight loss [ MWL ]) per day provides stability of the feed tabs and allows for comparisons between them.
Use the dry weight difference in milligrams per day as an adjustment factor for the feeding rate calculation of the amphipod. This served as the expected weight loss of a particular feed tabs formulation without any organism feeding on them.
Step V – Feeding Experimental Organisms Feed Tabs and Feeding Rate Calculation
a. Experiment with a 1L glass beaker with an appropriate volume (500mL) of aerated artificial seawater.
Determination of the Feeding Rate
c. After 0h 30m 0s, weigh the feed tabs with an analytical balance to obtain the feed leftover final dry weight ( dwF ).
d. Calculate the feeding rate by subtracting leftover final dry weight ( dwF ) from the initial dry weight of feed tabs ( dwI )
e. Therefore, divide the estimated weight loss by the number of living P. hawaiensis at the end of 72h 0m 0s and calculate per day. Use the formula below:
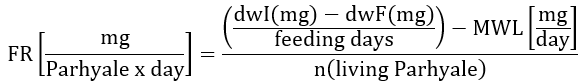
Where:
FR – Feeding rate.
dwI : initial dry weight of the feed tab before 24 h pre-wetting and 48 h feeding to the organisms.
dwF – final dry weight of the feed leftover after the organisms feed on it
n = number of living organisms at the end of a particular feeding cycle
MWL : adjustment factor as mean weight loss of the feed tab without organisms feeding on it.
d. Weight the feed tab before feeding the organisms to obtain initial dry weight ( dwI).
e. Prewet the feed tabs in seawater for 24h 0m 0s in a temperature-controlled room (exposure environment) before transferring them to the experimental beakers ( NB : prewetting facilitates organisms' easy feeding of the feed tabs).
f. Replace the feed tabs with new ones in an alternating cycle of three days (72h 0m 0s) throughout the exposure duration.
g. After every removal, dry the used feed tabs for 24h 0m 0s in an incubator at 45°C, weigh and record the final dry weight ( dwF )
h. The feeding rate (FR) per day of Parhyale with dwI and dwF of the specific feed tabs was adjusted by the number of feeding days and the mean weight loss ( MWL ) of the feed tabs per day calculated from the stability measurement.
a. As explained above, after feeding the organisms with weighed ( dwI ) and 24h-prewetted feed tabs, remove the leftover feed tabs with a spoon every 72h 0m 0s from all the experimental containers and transfer to a small pre-dried glass Petri dishes on a metal tray.
b. Dry the leftover feed tabs for 24h 0m 0s in an incubator at 45°C and then place in a desiccator or fume cupboard for 0h 30m 0s.








![(NB: trial days = 3 days (72 h] in this case) (NB: trial days = 3 days (72 h] in this case)](https://static.yanyin.tech/literature_test/protocol_io_true/protocols.io.81wgbx3qylpk/MWL.png)