Solvent Extraction of Polydimethylsiloxane (PDMS) Tubing to capture Volatile Organic Compounds from the Headspace and the Rhizosphere
Gareth Thomas, Anusha Mohan-Kumar, Alexander N. Borg, John Caulfield, Michael A. Birkett, József Vuts
Disclaimer
DISCLAIMER – FOR INFORMATIONAL PURPOSES ONLY; USE AT YOUR OWN RISK
The protocol content here is for informational purposes only and does not constitute legal, medical, clinical, or safety advice, or otherwise; content added to protocols.io is not peer reviewed and may not have undergone a formal approval of any kind. Information presented in this protocol should not substitute for independent professional judgment, advice, diagnosis, or treatment. Any action you take or refrain from taking using or relying upon the information presented here is strictly at your own risk. You agree that neither the Company nor any of the authors, contributors, administrators, or anyone else associated with protocols.io, can be held responsible for your use of the information contained in or linked to this protocol or any of our Sites/Apps and Services.
Abstract
This protocol describes a step-by-step guide for sampling Volatile Organic Compounds (VOCs) from the headspace/rhizosphere for synthetic blends and biological samples using polydimethylsiloxane (PDMS) silicon tubing, based on methods reported in Thomas et al. (2024) and Vuts et al. (2020). The advantages of using solvent elution when sampling headspace with PDMS tubing are that a liquid sample is generated, which can be used for multiple chemical (e.g. Gas Chromatography/coupled GC-Mass Spectrometry/Nuclear Mass Resonance Spectroscopy), behavioural (e.g. olfactometry) and electrophysiological (e.g. GC-electroantennography) tests, enabling a potential link to be established between VOC content and biological activity.
Steps
Conditioning PDMS tubes prior to headspace collection
Cut PDMS tubing (1 mm i.d. × 0.4 mm wall thickness) to desired length. The length of tubing can be variable depending on the headspace or rhizosphere set-up. In our previous work, the following lengths of PDMS tubing were used:
- For headspace Volatile Organic Compound (VOC) collections: 2.5 cm-7.5 cm
- For synthetic VOC blend collection from the rhizosphere: 30 cm
- For root VOC collection from the rhizosphere: 130 cm

Soak cut pieces of PDMS tubing in 100% methanol for a minimum of 24 hours.
Attach glass vessel to supply a constant flow of purified nitrogen, and turn on nitrogen supply. The PDMS tubes should be facing in the direction of the flow, enabling nitrogen to flow through the tubing.

Place glass vessel containing PDMS tubes into a modified heating oven (170˚C) for a minimum of 1.5 hours.
After 1.5 hours, use heat-resistant gloves to remove the glass vessel from the oven and allow to cool. Keep nitrogen flow on whilst the vessel is cooling.
Conditioning glass chamber/metal plates prior to headspace collections
Rinse Teepol detergent off glass chamber using distilled water.
Rinse glass chamber with 100% acetone.
Repeat steps 7-9 for the metal plates.
Place the glass chamber and metal plates in a modified heating oven (150˚C) for a minimum of 1.5 hours.
Remove the glass chamber and metal plates from the oven using heat-resistant gloves and allow to cool.
Application of synthetic VOC blend for sampling from aerial headspace
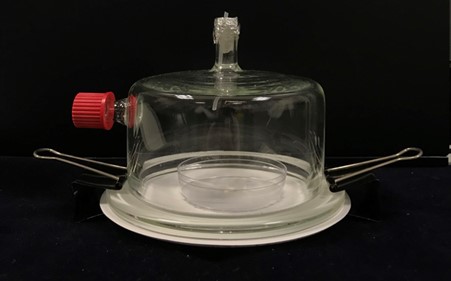
Place a filter paper disc (12.5 cm) onto a metal plate (12 cm diam.).
Place a Petri dish (5 cm diam.) on top of the filter paper.
Place a small filter paper disc (0.6 cm diam.) onto the Petri dish.
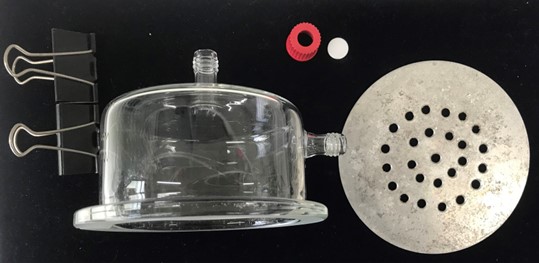
Using a small square of tin foil, cover the outlet at the top of the glass chamber.
Attach the septum and red screw cap to the inlet at the side of the glass chamber.
Using forceps, insert a small hole through the top of the tin foil to place the PDMS tubing through.
Using cotton gloves, carefully place the PDMS tubing through the hole in the foil, so it is suspended above the headspace. Where possible, avoid skin contact with conditioned PDMS tubing, using cotton gloves/forceps, to reduce the chance of contamination of PDMS tubing with skin VOCs.
Prepare a synthetic VOC blend of compounds in redistilled solvent (diethyl ether/hexane), such that 10 µL of the blend will yield a 100 µg dose (10 µg/µL blend).
N.B. this dose is based on the methods from Thomas et al. (2024).
Apply 10 µL of a synthetic VOC blend of compounds onto the filter paper disc, and immediately place the glass chamber over the Petri dish.
After one hour, remove the PDMS tubing from the headspace using cotton gloves, and refer to 'Eluting sorbed compounds from the PDMS tubing'.
N.B. certain parameters (e.g. dose of synthetic VOC blend/length of tubing/length of sampling time) can significantly influence compound recovery (Thomas et al., 2024; Vuts et al., 2020).
To determine background VOCs, the carrier solvent alone (diethyl ether/hexane) should be applied to the filter paper as a blank control, which should be performed each time the experiment is set up.
Sampling VOCs from headspace of biological sample, e.g. orange (Citrus sinensis)
Place filter paper (12.5 cm) on top of the metal plate.
Place orange fruit onto filter paper.
Using a small square of tin foil, cover the outlet of the glass chamber.
Attach the septum and red screw cap to the inlet at the side of the glass chamber.
Using forceps, insert a small hole through the top of the tin foil to place the PDMS tubing through.
Place the glass chamber (10 cm height × 12 cm diam.) on top of the metal plate, confining the orange within the headspace.
Using cotton gloves, carefully place the PDMS tubing through the hole in the foil, so it is suspended above the headspace.
Attach the glass chamber to the metal plate using bulldog clips.
After one hour, remove the PDMS tubing from the headspace using cotton gloves, and refer to 'Eluting sorbed compounds from the PDMS tubing'.
To determine background VOCs, an empty glass chamber should be used as a blank control, which should be set up exactly the same way as the treatment containing the orange fruit, and should be performed each time the experiment is set up.
Eluting sorbed VOCs from the PDMS tubing
Remove the PDMS tubing from the headspace, and attach the tubing to the end of a glass Pasteur pipette.
Place a 1.1 mL pointed GC vial underneath the PDMS tubing.
Measure 1 mL of redistilled solvent (diethyl ether/hexane) into an LC-MS vial.
Take up 1 mL of redistilled solvent (diethyl ether/hexane) and pass through the clamped glass Pasteur pipette, which will pass through the PDMS tube and into the vial.
To remove the remaining solvent in the PDMS tube, attach pipette bulb teat to the Pasteur pipette and pass a bolus of air.
Solvent will pass through the pipette and PDMS tubing into the pointed glass vial, eluting the VOCs sorbed to the PDMS tubing, to generate a liquid extract. This can be analysed multiple times by gas chromatography (GC), coupled GC-mass spectrometry (GC-MS), GC-electroantennography (GC-EAG) and behavioural assays.
In the case of GC analysis via thermal desorption, directly place the PDMS tubing after step 33 into an empty Tenax tube with a glass wool plug. Seal Tenax tubes with PDMS into glass ampoules under a stream of nitrogen and store at -20˚C until ready for GC/GC-MS analysis.
Application of synthetic VOC blend for sampling from the rhizosphere
Heat-sterilise sand at 80˚C for 48 h.
Wash 250 mL glass beakers with Teepol detergent and then rinse with distilled water and acetone; oven-dry at 150˚C for a minimum of 2 hours.
Mix sand with distilled water to achieve a 15% moisture content (v/v).
Add sand to 250 mL beaker to achieve a ca. 1 cm depth of sand at the bottom of the beaker.
Fill up beaker with sand medium and compact with a metal spoon.
N.B. soil can also be used as a medium in a similar way.
Prepare synthetic VOC blend in redistilled solvent (diethyl ether/hexane), such that 100 µL of the blend will yield a 1 mg dose (100 µg/µL blend).
N.B. this dose is based on the methods from Vuts et al. (2020).
Make a 0.5 cm diam. hole in the medium using a hollow glass rod.
Insert a 100 µL glass micropipette through the glass rod down the hole to mid-depth of medium, and inject 100 µL of the synthetic VOC blend. Cover the hole with sand.
After 1 h, place a 1.1 mL screw-cap GC vial on one side of the tubing, insert a Pasteur pipette into the other end and pass 1 mL of redistilled solvent (diethyl ether/hexane) through the tubing. Solvent will pass through the pipette and PDMS tubing into the pointed glass vial, eluting the VOCs sorbed to the PDMS tubing, to generate a liquid extract. This can be analysed multiple times by gas chromatography (GC), coupled GC-mass spectrometry (GC-MS), GC-electroantennography (GC-EAG) and behavioural assays.

To remove the remaining solvent in the PDMS tube, attach pipette bulb teat to the Pasteur pipette and pass a bolus of air.
Sampling VOCs from the plant rhizosphere, e.g. maize (Zea mays)
Grow maize (cv. Delprim) in soil for 16 days.
Wash 250 mL glass beakers with Teepol detergent and then rinse with distilled water and acetone; oven-dry at 150˚C for a minimum of 2 hours.
Sterilize sand (0.25–0.71 mm grain size) in the oven for 48 hours at 80˚C.
Mix the sand with distilled water (v/v) to obtain a substrate with 15% moisture content.
Bend a 19 cm long glass tube (0.5 cm diam.) into a U shape (height: 8 cm and base: 3 cm), using a Bunsen burner.
Coil PDMS tube (130 cm) around the glass tube ensuring that the hollow inside of the PDMS tube is not obstructed anywhere, to allow for a flow of solvent during elution. Fasten the PDMS tubing at the top of the U shape with PTFE tape. This is a PDMS probe.

Transfer one 16-day-old plant to the beaker containing the sand.
Fill the remainder of the beaker with sand.
Release five second instar larvae of western corn rootworm ( Diabrotica v. virgifera ) onto the surface of the sand, and allow them to feed on the maize roots for 5 days.
After 5 days, place a 1.1 mL screw-cap GC vial on one side of the tubing, insert a Pasteur pipette into the other end and pass 2 mL of redistilled solvent (diethyl ether/hexane) through the tubing. Solvent will pass through the pipette and PDMS tubing into the pointed glass vial, eluting the VOCs sorbed to the PDMS tubing, to generate a liquid extract. This can be analysed multiple times by gas chromatography (GC), coupled GC-mass spectrometry (GC-MS), GC-electroantennography (GC-EAG) and behavioural assays.

To remove the remaining solvent in the PDMS tube, attach pipette bulb teat to the Pasteur pipette and pass a bolus of air.