Sequential Indirect Dual Immunohistochemistry with Primary Rabbit Antibodies on Cochlear Sections Using an Intermediate Heat-Denaturation Step
Maria Luque, Maria Luque, Rudolf Glueckert, Rudolf Glueckert
Abstract
Advanced immunohistochemical (IHC) protocols aim to visualize different molecules in situ simultaneously. These techniques are of utmost importance as a first step in studying possible interactions of proteins at the subcellular level. Colocalized stains in tissue sections indicate proximity of two proteins of interest. Frequently, double staining protocols are restricted by the lack of primary antibodies generated in different animal species for indirect IHC visualization. Here, we present a detailed protocol for mouse inner ear tissue using two different primary rabbit antibodies directed against transmembrane ion channel proteins of cochlear neurons. The two antibodies are combined for fluorescence (confocal) as well as dual multiplex colorimetric visualization in two sequential single IHC stainings. A heat-denaturation step is performed in between. Primary antibody specificity is tested by preadsorption with the immunogenic peptide, and positive and negative tissue controls are performed to confirm the reliability of the antibody detection. We describe the whole procedure in detail beginning with tissue extraction of the mouse inner ear and continuing with chemical fixation, cryoembedding, and preparation for manual and fully automated immunostaining, including steps for heat-induced antigen retrieval. The potential to use antibodies from the same host species for single and double IHC staining opens up multiple possibilities for detecting different targets in the same tissue section using resources and materials that are widely available. © 2021 The Authors. Current Protocols published by Wiley Periodicals LLC.
Basic Protocol 1 : Tissue preparation, cryoembedding, and sectioning
Basic Protocol 2 : Double colorimetric immunostaining with an automatic immunostainer
Basic Protocol 3 : Double manual fluorometric immunostaining with fluorescence
INTRODUCTION
Double and multiple immunostaining procedures are limited by the lack of available primary antibodies raised in different animal species. Previous studies have demonstrated various sequential staining protocols based on directly conjugated antibodies. Strategies such as blocking with serum from the species in which the antibodies were raised (Lewis Carl, Gillete-Ferguson, & Ferguson, 1993; Owen, Hakkinen, Wu, & Larjava, 2010; Shindler & Roth, 1996; Tornehave, Hougaard, & Larsson, 2000) or heat denaturation of the antibodies from the first immunostaining using a microwave oven have also been reported (Lan, Mu, Nikolic-Paterson, & Atkins, 1995; Tornehave et al., 2000). Here, we present a double staining protocol based on this intermediate step of heat-induced protein denaturation. After the first indirect immunostaining is performed, sections are covered with buffer and heated. The denaturation temperature depends on the antibody isotype. Our protocol is designed for use with the IgG isotype because it is frequently used for immunohistochemistry, and uses a temperature of 65°C that is suitable for this isotype (Akazawa-Ogawa, Nagai, & Hagihara, 2018). We perform protein denaturation for 8 min at 81°C to ensure the complete denaturation of all IgG domains. The main advantages of the protocols presented here, as compared to previous ones, are the lower expenditures of time, costs, and laboratory equipment that are needed. No reagents for direct antibody conjugation or additional blocking sera are required; moreover, the heat-denaturation step is applied for only 8 min between the two sequential stainings, saving time compared with primary antibody blocking procedures, which can be very time consuming. This study presents both an automated double staining protocol using multiplex colorimetric chromophores with an automated slide stainer (Roche®, Ventana® Discovery) and a manual protocol using fluorescent antibodies.
Inner ear tissue was used for both protocols, which sets high demands for tissue adherence to the slide. We describe how to extract, fix, decalcify, and embed the inner ear from mouse—which houses the vestibular system and the cochlea, the organ responsible for sound detection in mammals that includes the sensory epithelia and the spiral ganglion neurons (SGNs)—and include a detailed description of all the necessary steps. Finally, we present an example of the immunohistochemical procedure using antibodies localizing to two hyperpolarization-activated cyclic nucleotide–gated (HCN) channels in the neuronal membrane, HCN2 and HCN4 (Luque et al., 2021).
Basic Protocol 1: TISSUE PREPARATION, CRYOEMBEDDING, AND SECTIONING
This protocol describes the extraction and postmortem perfusing fixation of the inner ear in mice. Before working with animals, appropriate ethical approvals may be required. After whole inner ears are extracted, natural openings as well as additional holes in the otic capsule allow perfusion of fixative through the fluid compartments to enable proper fixation. A subsequent ethylenediaminetetraacetic acid (EDTA)-based decalcification of the bony tissue enables proper sectioning of the samples with a cryostat. Bone removal is accelerated with a rapid microwave histoprocessor. Once the bone has been decalcified, samples are cryoembedded and cut at 5- to 10-µm thickness on a cryostat.
The mouse strain we selected to use in this protocol is CBA/J, which is commonly used in auditory research because of its relatively good hearing performance with age (Liu et al., 2019). The inner ear extraction and postmortem fixation are critical steps for tissue preservation. Conservation of the 3D protein structure and especially the antibody epitopes in the cochlear tissue is important to obtain better results in immunostaining. We used young adult mice aged 21 days here because older mice presented lower staining intensities with these antibodies (Luque et al., 2021).
Materials
-
CBA/J strain mice, 21 days old (JAX no. 000656; Charles River)
-
4% (w/v) paraformaldehyde (formalin, PFA; see recipe)
-
0.1 M phosphate-buffered saline (PBS; see recipe)
-
1 g/L Na+ azide in 0.1 M PBS (see recipe)
-
20% (w/v) EDTA in 0.1 M PBS
-
10% and 15% (w/v) sucrose
-
Tissue-Tek O.C.T.™ compound (Scigen, ThermoFisher Scientific)
-
96% (v/v) ethanol
-
Thick (Dumont forceps no. 4) and fine forceps (Dumont forceps no. 5 and no. 55)
-
Scissors
-
Stereomicroscope (Zeiss OPMI 11)
-
5-ml glass vials with plastic lids
-
Horizontal shaker (Heidolph, Titramax 101)
-
Circular rotator (e.g., Stuart Tube Rotator SB2)
-
Histological cassettes
-
250-ml graduated beaker without spout and with lid
-
3- and 1-ml disposable Pasteur pipets
-
Histos 5 rapid microwave histoprocessor (Milestone®, Sorisole, Italy)
-
Cryomold (15 × 15 mm conical Peel-A-Way® mold, Ted Pella, Inc.)
-
Dry ice
-
Cryostat (Leica® CM 3050)
-
Superfrost™ slides (R. Langenbrick GmbH)
Tissue collection and fixation
1.Sacrifice each mouse by quick cervical dislocation.
2.Cut off the head and remove the skin after making an incision along the sagittal suture from dorsal to lateral.
3.Cut the skull with sharp scissors in the medial plane sagittally, and carefully remove the two halves of the brain with a small spoon.
4.Extract the right and left inner ears, located on each side of the skull.
5.Penetrate the round window with fine forceps and remove the stapes (oval window).
6.Make a small hole in the apical turn with fine forceps, close to the helicotrema.
7.Using a disposable 1-ml Pasteur pipet, gently perfuse ice-cold 4% PFA through the openings created in steps 5 and 6 to ensure that all perilymphatic compartments are flushed with fixative solution (Fig. 1).
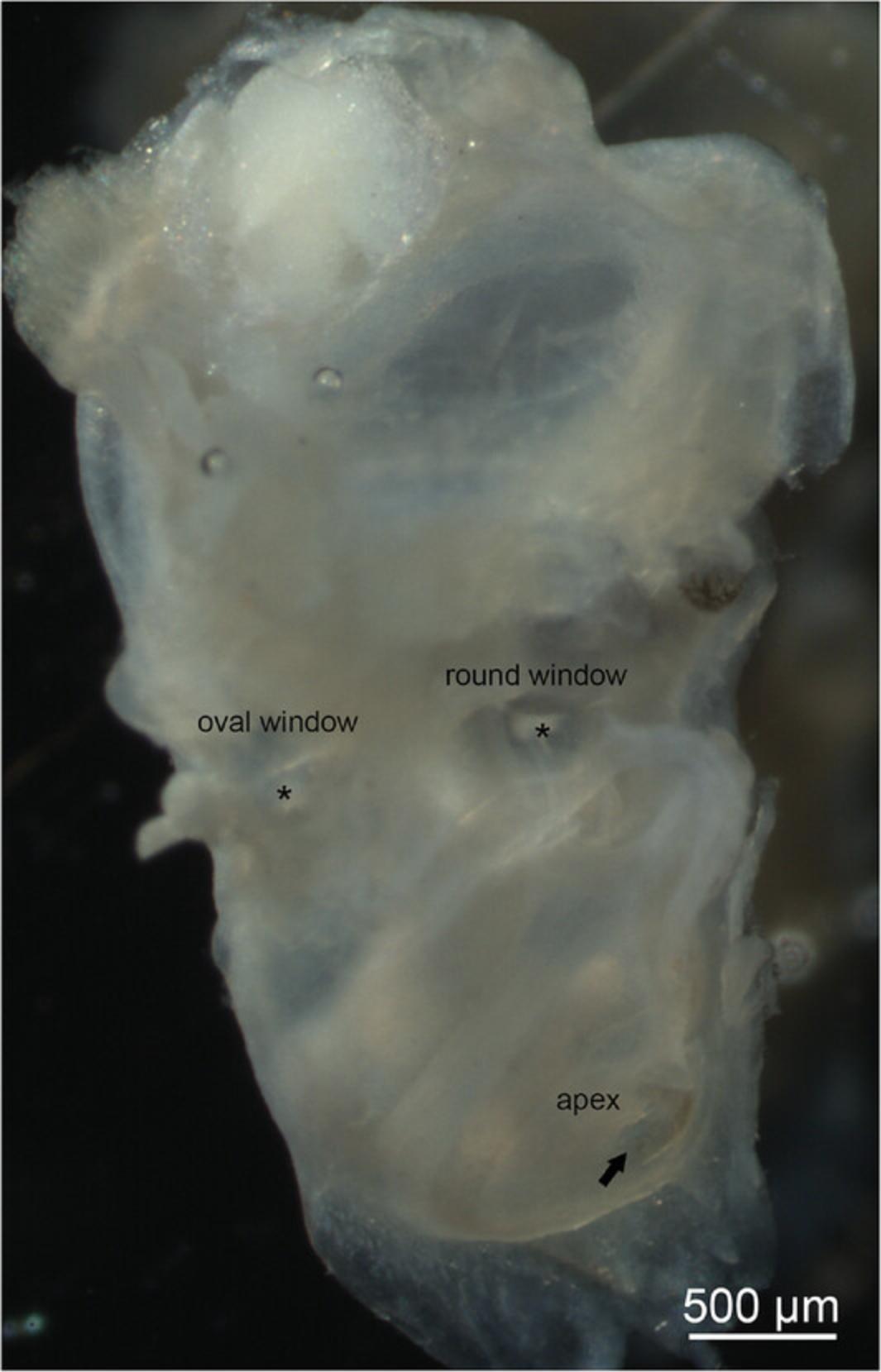
8.Transfer the specimens in 4% PFA to 5-ml glass vials with plastic lids and keep them overnight (16-18 hr) at 4°C with constant movement on a horizontal shaker set at 300 rpm.
9.Rinse the specimens five times for 5 min each time with 0.1 M PBS while agitating them gently on a circular rotator (Stuart Tube Rotator SB2, 20 rpm).
Decalcification and embedding
10.Transfer specimens to standard histological cassettes (Fig. 2A).
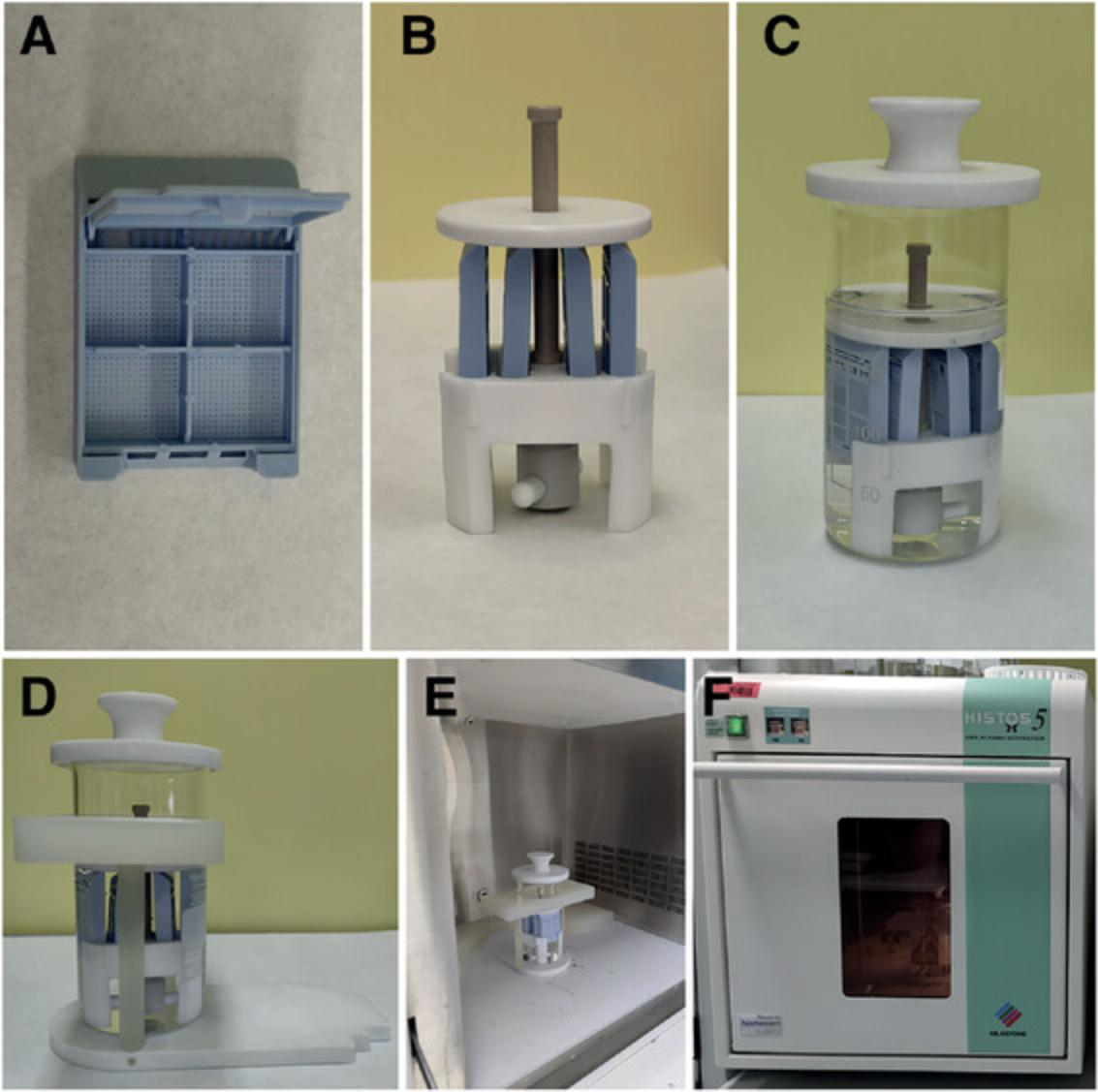
11.Place the cassettes into the cassette holder. The cassette holder attaches to a magnetic stirrer to allow agitation of the EDTA solution added in the next step (Fig. 2B).
12.Transfer the cassette holder into a beaker without a spout and fill the beaker up to 200 ml with 20% (w/v) EDTA in 0.1 M PBS, pH 7.2-7.4.
13.Cover the beaker with a lid and place it into a microwave support (Fig. 2C and D).
14.Transfer the microwave support with the samples into the microwave (Fig. 2E). Irradiate the samples at 800 W microwave power with stirring for 220 min at 37°C to decalcify the inner ears (Fig. 2F).
15.Wash the samples with 0.1 M PBS five times for 5 min each with stirring at room temperature.
16.Incubate the samples with 10% sucrose for 30 min at room temperature.
17.Incubate the samples with 15% sucrose for 30 min at room temperature on a shaker set at 300 rpm.
18.Incubate the samples with 15% sucrose overnight (20-22 hr) at 4°C on a shaker set at 300 rpm.
19.Incubate the samples with a 1:1 (v/v) mixture of 15% sucrose and O.C.T.™ compound (cryoembedding medium) overnight (20-22 hr) at 4°C with constant movement on a shaker set at 300 rpm.
20.Incubate the samples with O.C.T.™ compound overnight (20-22 hr) at 4°C with constant movement on a shaker at 300 rpm. Before introducing the samples into O.C.T.™, it is necessary to remove the air bubbles from the medium, so they do not enter inside the ear. For this purpose, a desiccator is used and vacuum is created for at least 1 hr to remove all air bubbles from the O.C.T.™ compound.
21.Prepare a mix of dry ice and 96% ethanol, and mix until it acquires a slurry consistency.
22.Prepare one cryomold per ear (the size needed will depend on the sample; for inner ears the 15 × 15-mm conical Peel-A-Way® molds from Ted Pella, Inc. are suitable). Fill the mold halfway with O.C.T.™ compound, and place under vacuum for at least 1 hr to remove air bubbles (see step 20).
23.Place the inner ear inside the mold. Use the stereomicroscope to orient the cochlea appropriately. The selected orientation depends on what the researcher wants to study; the two most common orientations for sectioning are parallel to the midmodiolar and horizontal plane (Fig. 3). In the “midmodiolar orientation,” the ear is placed with the oval window facing the base of the mold and the round window facing up (Fig. 3A and 3B). For the “horizontal orientation,” the vestibule is instead positioned towards the upper part of the mold and the cochlea on the base (Fig. 3C-E).
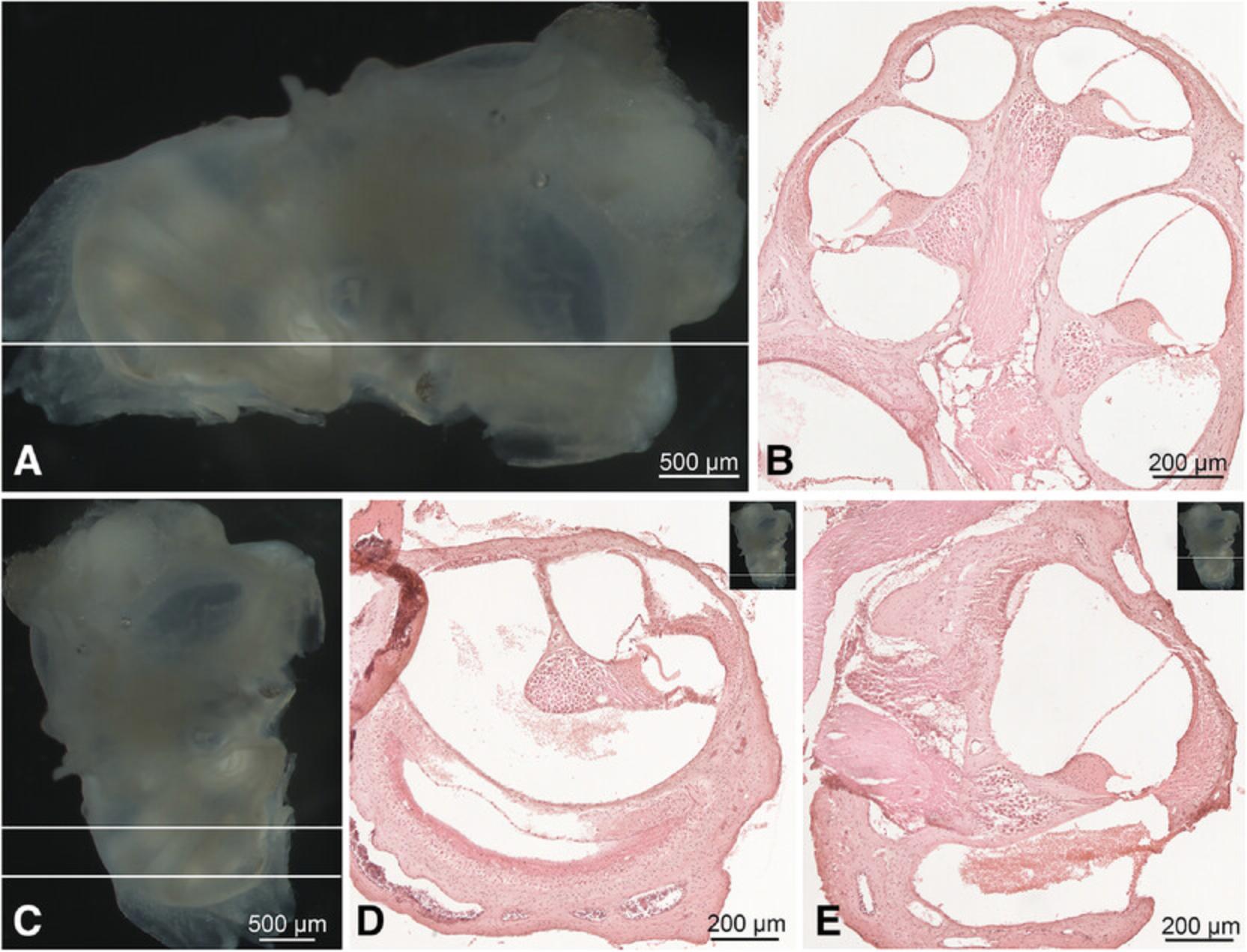
24.Fill up the mold containing the oriented cochlea completely with O.C.T.™. Pour the medium down slowly inside the mold to avoid changing the position of the cochlea and transfer it into the slurry mix prepared in step 21.The sample will be frozen within seconds.
25.After 1 min, the samples will be completely frozen. Place the O.C.T.™ blocks containing the ears on dry ice for 20 min to allow the adhesive alcohol to dry.
Cryosectioning
26.Section the samples with a cryomicrotome. For inner ear sectioning, the temperature of the chamber is set at −20°C and object temperature at −22°C.
27.Collect sections sequentially on Superfrost™ slides until approximately a 25-slide distance from the apex (at 10-µm section thickness). Then, start a second column in each slide with sections from the central modiolar region with good presentation of the middle turn. Collect a third column with the basal turn and a fourth and fifth column with the basal hook region of the tonotopic axis. In this case, all the slides present a section from each cochlear turn, apart from the vestibule and the cerebellum.
Basic Protocol 2: DOUBLE COLORIMETRIC IMMUNOSTAINING WITH AN AUTOMATIC IMMUNOSTAINER
This protocol details double colorimetric immunostaining with 3,3′diaminobenzidine (DAB) and Fast Red salt chromophores. Many epitopes require an antigen retrieval before immunostaining is performed. This pretreatment of the sections is needed to make the epitopes accessible for the antibodies especially after aldehyde fixation. After a heat pretreatment in buffer solutions, immunostaining with colorimetric or fluorometric detection techniques are performed.
Materials
-
Slides containing O.C.T.™-fixed mouse inner ear sections
-
4% (w/v) PFA (see recipe)
-
0.1 M PBS (see recipe)
-
Ventana Discovery reagents IHC:
-
DAB MAP KIT (Streptavidin-biotin-peroxidase Kit with CuSO4 DAB visualization, cat. no. 760-124, Roche Medical Systems Inc.), including reaction reagents blocker-D, inhibitor D, SA-HRP D, DAB D, DAB H2O2 D, and Copper D
-
RED MAP KIT (streptavidin-biotin-phosphatase kit with Fast Red salt visualization, cat. no. 760-123, Roche Medical Systems Inc.), including reaction reagents blocker R1, SA-Alk phosphatase R, activator R, naphthol R, Fast Red R, and Fast Red R2
-
Hematoxylin (cat. no. 790-2208)
-
Bluing reagent (cat. no. 760-2037)
-
Ventana Discovery buffers IHC:
-
Discovery wash (cat. no. 950-100)
-
Liquid coverslip (LCS, cat. no. 650-010)
-
Reaction buffer (cat. no. 950-300)
-
CC1m-VENTANA® buffer (Tris-EDTA based buffer, pH 8, cat. no. 950-500, Roche Medical Systems Inc.)
-
CC2m-VENTANA® buffer (Citrate based buffer, pH 6, cat. no. 760-107, Roche Medical Systems Inc.)
-
Ventana antibody diluent (cat. no. ADB250)
-
Anti-HNC2 primary antibody (Alomone Labs, cat. no. APC-030, lot. AN1650, RRDI AB_2313726)
-
Anti-HCN4 primary antibody (Alomone Labs, cat. no. APC-052, lot. AN2025, RRDI AB_2039906)
-
Biotin-SP-conjugated donkey anti-rabbit secondary antibody (Jackson Immunoresearch Labs, cat. no. 711-065-152, RRDI: AB_2340593, Lots.136387, 133152, 140405)
-
Xylol
-
Entellan® mounting medium (Merck, cat. no. 107961)
-
Ventana Discovery automated immunostainer (Roche Medical Systems Inc.)
-
Zeiss microscope
1.Fix the sections to the slide with 150 ml 4% PFA at 50°C in the microwave for 19 min at 24-W microwave power to enhance section adhesion to the slide.
2.Rinse with 0.1 M PBS for 5 min.
3.Introduce the slides in the Ventana Discovery automated immunostainer (Fig. 4A and 4B).

4.Place the reagents from the DAB MAP and RED MAP KITS on the reagent tray of the Ventana Discovery instrument (Fig. 4C).
5.Set the instrument to start the immunostaining. Details of the immunostaining protocol are provided on the printed label attached to the slide. If appropriate, different retrieval buffers and heat pretreatments cycles can be applied depending on the localization of the antigen and antibody (see Critical Parameters).
6.Heat and EDTA buffer: Different heat steps are applied automatically by the immunostainer (1× room-temperature preincubation for 2 min, 1× 95°C for 8 min, 5× 100°C for 4 min, 1× room temperature for 8 min) while incubating the sections in EDTA buffer (CC1m).
7.To reduce endogenous peroxidase activity, treat the slides with an excess of H2O2 substrate (inhibitor D from DAB MAP kit) for 4 min.
8.Incubate the primary antibody from the first immunostaining for 1 hr. Use antibodies diluted in Ventana antibody diluent, which contains 0.3% protein in 0.1 M PBS (pH 7.3) and 0.05% ProClin 300 as preservative.
9.Rinse the slides twice with reaction buffer.
10.Incubate the secondary antibody for the first immunostaining for 30 min.
11.Perform DAB incubation. First, apply blocker D (high-protein salt blocking solution) for 2 min to reduce background staining, and then incubate with the enzyme streptavidin–horse peroxidase for 16 min. Rinse slides four times and then add diaminobenzidine (DAB) D and DAB H2O2 (substrate for the peroxidase) for 8 min. Finally, apply Copper D (CuSO4) for 4 min to intensify the staining (Fig. 4D).
12.Heat the slide to 85°C and incubate for 8 min. The primary and secondary antibody complexes will be denatured, but the DAB precipitate will survive this treatment.
13.Rinse the slide with reaction buffer and apply the second primary antibody for the second immunostaining raised in the same species. Incubate for 1 hr.
14.Incubate the sections for 30 min with the secondary antibody, biotinylated donkey anti-rabbit at a 1:400 dilution.
15.Apply the streptavidin alkaline phosphatase and incubate 30 min. Rinse the slide with reaction buffer and incubate 4 min with Activator R and Naphthol R (substrate for alkaline phosphatase). Apply Fast R and fast R 2 (Fast Red salts) sequentially to the slide, incubating for 8 min each (Fig. 4E).
16.Apply hematoxylin counterstain and incubate for 2 min.
17.Apply bluing reagent for 2 min to turn hematoxylin staining into blue color.
18.Rinse the slides with reaction buffer and remove them from the immunostainer.
19.Rinse slides with distilled water and soapy water to remove the oily coating applied by the immunostainer. Rinse three more times for at least 1 min each in distilled water.
20.Leave the slides to dry overnight at room temperature, as Fast Red salts are soluble in alcohols (or alternatively use an aqueous mounting medium).
21.Mount slides the next day as follows. First, incubate slides in xylol for 2 min. Let the slides dry for 10 min and mount with Entellan® mounting medium. Gently apply 4-5 drops of the mounting solution and cover them with coverslips (24 × 50 mm). Let them dry.
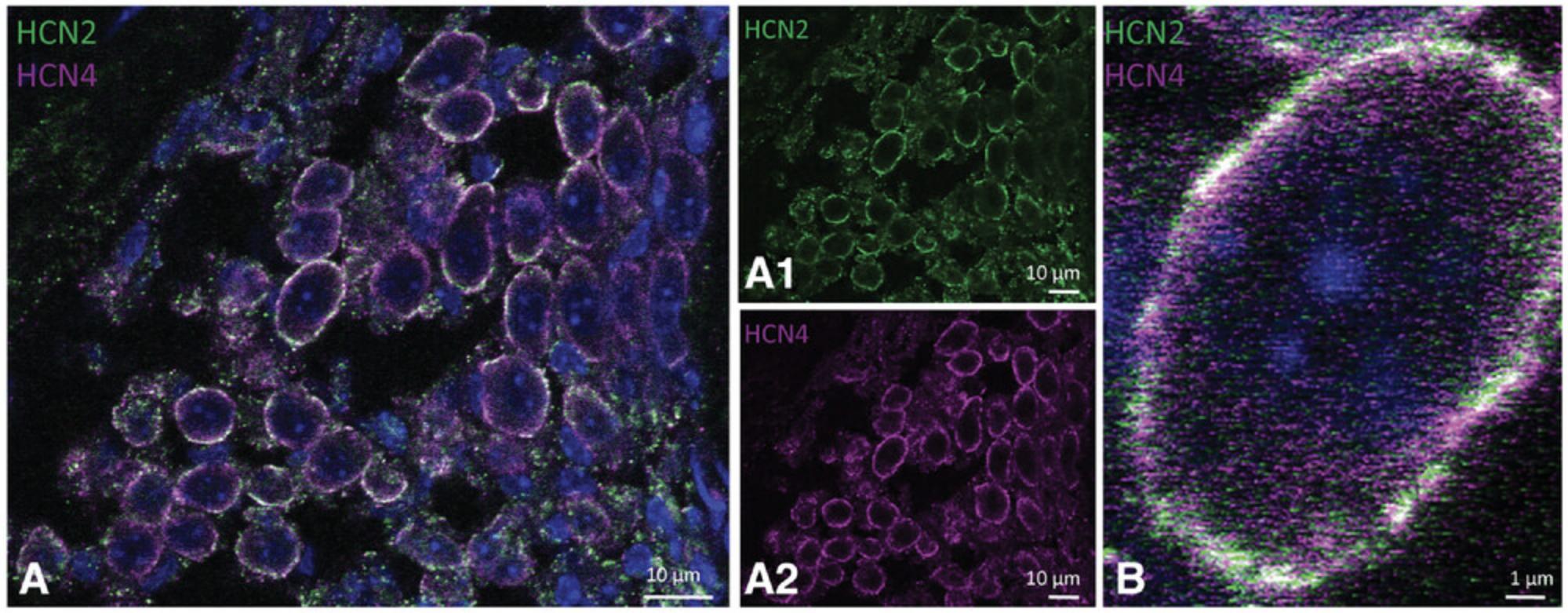
22.Image the slides. Sample results are shown in Figure 5, with SGNs in CBA/J mouse showing coexpression of HCN2 (DAB, brown) and HCN4 (Fast Red) (Fig. 5A). After color deconvolution of the image, single channels are visualized: HCN2 (Fig. 5A1, DAB) and HCN4 (Fig. 5A2, Fast Red). High magnification of the SGNs shows HCN2 and HCN4 staining at the soma membrane and cytoplasm (Fig. 5B). Color deconvolution shows HCN2 staining (Fig. 5B1, DAB) and HCN4 (Fig. 5B2, Fast Red).
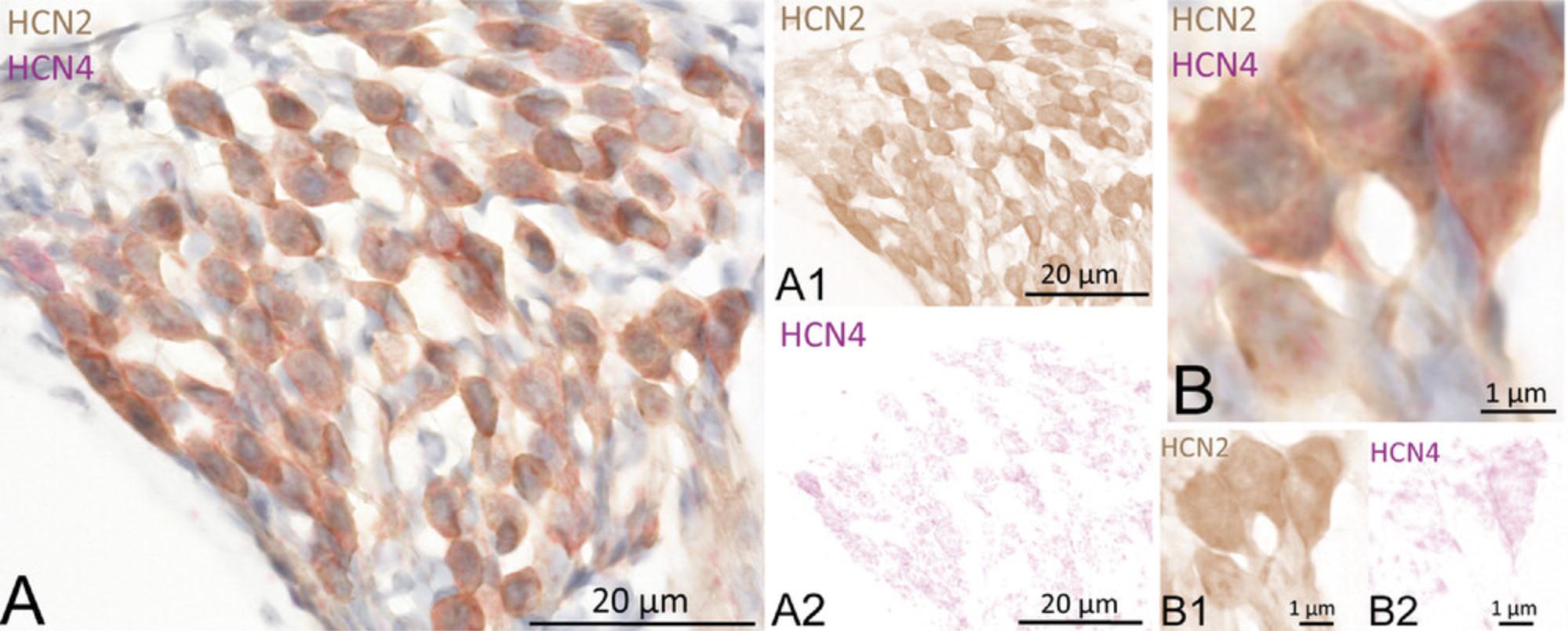
Basic Protocol 3: DOUBLE MANUAL FLUOROMETRIC IMMUNOSTAINING WITH FLUORESCENCE
This protocol details double fluorescence immunostaining with Alexa Fluor® 488 and Dylight 649 as fluorophores. Antigen retrieval may be also necessary for fluorescent staining. This pretreatment can be performed in a Ventana immunostainer. Some antibodies, such as HCN antibodies**,** do not require antigen retrieval; Triton X-100 permeabilization on the slide is sufficient for the antibody to access its epitope.
Materials
-
Slides containing O.C.T.™-fixed mouse inner ear sections
-
Distilled water
-
Blocking solution (see recipe)
-
0.1 M PBS (see recipe)
-
Primary antibody solution (see recipe)
-
Anti-HCN2 primary antibody (Alomone Labs, cat. no. APC-030, Lot. AN1650, RRDI: AB_2313726)
-
Anti-HCN4 primary antibody (Alomone Labs, cat. no. APC-052, Lot. AN2025, RRDI: AB_2039906)
-
DY 649 secondary antibody (Vector Laboratories, cat. no. DI-1649, Lot. Z0820, RRDI: AB_2336420)
-
Alexa Fluor® 488 AffiniPure secondary antibody (Molecular Probes; cat. no. A-21206, Lot. 1674651, RRID: AB_2535792)
-
Secondary antibody solution (see recipe)
-
4% PFA (see recipe)
-
Vectashield™ with DAPI (Vector Laboratories Inc., cat. no. H-1200, RRDI:AB_2336790)
-
Clear nail polish
-
Hydrophobic pen marker: Super pap pen liquid blocker mini neu (Science services, cat. no. N71312-N)
-
Staining jar or chamber
-
Heating plate (Präzitherm, Harry Gestigkeit GMBH)
-
24 × 50-mm coverslips
1.Circle the sections on the slides with a hydrophobic pen so that the different solutions used in the protocol will be retained on the slide in small volumes.
2.Place slides in a humid chamber and introduce wet paper towels into the chamber to generate moisture (Fig. 6A).

3.Place the slides in distilled water inside a staining jar to rehydrate them and remove the cryoembedding medium from the sections.
4.Carefully apply 300-400 µl of blocking solution to the areas of the slides marked by the hydrophobic pen, and incubate the slides inside the humid chamber for 1 hr.
5.Prepare dilutions of primary antibody in a suitable solution that also contains some protein to avoid antibody aggregation and a detergent for permeabilization (see recipe for primary antibody solution).
6.Discard the blocking solution. Pipet 300 µl of primary antibody dilution onto the slide. Keep overnight inside the humid chamber at 4°C to allow the antibody solution to slowly penetrate the section with a low binding affinity due to the cold temperature. This reduces nonspecific binding of the primary antibody.
7.Incubate the primary antibody for 1 hr at room temperature.
8.Working in the dark, prepare dilutions of secondary antibody in secondary antibody solution. The fluorochromes attached to the secondary antibodies quench when exposed to light so that the staining signal decreases. Use specific microcentrifuge tubes for fluorescence that protect the antibodies against light. When this is not possible, aluminum foil can be used to wrap standard microcentrifuge tubes to prevent the antibodies from being exposed to light.
9.Discard primary antibody solution and rinse the slides in 0.1 M PBS three times for 5 min each.
10.Apply secondary antibody dilution and incubate the sections for 2 hr at room temperature in the dark.
11.Thoroughly rinse the slides five times with 0.1 M PBS for 10 min each time.
12.Postfix the antibody-fluorochrome complex with 4% PFA for 30 min.
13.Rinse the slides twice with 0.1 M PBS for 5 min each.
14.Heat the slides in a heating plate for 8 min at 81°C. To avoid allowing the sections to dry out, apply 0.1 M PBS to the slide surface after 5 min (Fig. 6B and 6C).
15.Let the slide equilibrate to room temperature. Discard the remaining 0.1 M PBS from the slide.
16.Perform a second immunostaining with a second primary antibody raised in the same species as the first primary antibody applied in step 6.
17.Repeat steps 6-11 using the antibodies selected for the second immunostaining.
18.Rinse the slides in 0.1 M PBS five times for 10 min each.
19.Mount the slides with an aqueous mounting medium such as Vectashield™ with DAPI. Apply two to three drops to each slide, and cover them with 24 × 50-mm coverslips.
20.Keep the slides at 4°C for 10 min. Seal the coverslips to the slide with nail polish. Store the slides at 4°C until ready to check the slides on the microscope.
21.Check the slides under a microscope to assess the staining quality, and proceed to imaging the slides.
REAGENTS AND SOLUTIONS
Blocking solution
Dissolve 0.3% Triton X-100 and 30% normal donkey serum (NDS) in 0.1 M of PBS. To prepare 10 ml, pipet 3 ml NDS and 30 µl Triton X-100 in 7 ml of 0.1 M PBS. Cut off the tip of the pipet before adding Triton X-100, which is a highly viscous detergent, so a bigger opening of the pipet tip facilitate its addition to the sample. Prepare solution fresh every time as needed.
Phosphate-buffered saline (PBS)
1 M PBS : To prepare 1 l, dissolve 53.4 g Na2HPO4·2H2O and 15.39 g NaH2PO4·2H2O in 1000 ml distilled water. Store at room temperature for longer periods.
0.1 M PBS : To prepare 1 l, dilute 100 ml of 1 M PBS in 900 ml distilled water. Store at 4°C for 2-3 weeks. For longer periods, check the pH and check that no fungi have grown inside.
0.1 PBS + Na+ azide
Dissolve 1 g/L Na+ azide in 1 L distilled water. This solution should be stored at 4°C for longer periods.
Paraformaldehyde (PFA), 4%
To prepare 1 l of solution, dissolve 40 g paraformaldehyde in 600 ml distilled water. Add 4-6 pearls of solid NaOH and stir while heating until the solution reaches 60°C. Stop the heating and wait until it reaches room temperature. Filter the solution with filter paper and a funnel. Add 24.37 g/L NaH2PO4·2H2O. Adjust the pH to between 7.2 and 7.4 with NaOH and fill up to 1 l with distilled water.
Primary antibody solution
1% normal donkey serum (NDS) + 0.3% Triton X-100 in 0.1 M PBS (see recipe). To prepare 50 ml of solution, pipet 0.5 ml NDS and 150 µl Triton X-100 into 49.5 ml of 0.1 M PBS. Prepare fresh every time as needed.
Secondary antibody solution
1% of NDS in 0.1 PBS. To prepare 50 ml of solution, pipet 0.5 ml NDS into 49.5 ml of 0.1 M PBS. Prepare fresh every time as needed.
COMMENTARY
Background Information
Different approaches have been reported for performing double stainings with two antibodies raised in the same species, specially using fluorescence antibodies. Strategies such as the use of direct conjugated primary antibodies are frequently employed (Mao & Mullins, 2010). In this case, the first immunostaining can be performed along with the incubation with the primary antibody and detection with a fluorochrome-labeled secondary antibody. After blocking the sections with serum derived from the species in which the secondary antibody is raised, the directly conjugated primary fluorochrome complex is applied (Owen et al., 2010). Indirect methods including separate primary and secondary antibody incubations are preferred, however, because the direct methods have been demonstrated to have lower antibody sensitivities (Im, Mareninov, Diaz, & Yong, 2019). Another approach is to block the sections after the first indirect immunostaining for 3 hr with normal serum derived from the same host species as the primary antibodies (Ansorg, Bornkessel, Witte, & Urbach, 2015; Lewis Orbach Carl et al., 1993). A final option is to apply denaturation treatments using a microwave, as we have done here (Tornehave et al., 2000). The main advantages of our technique compared to previous ones are the shorter timeframe and lack of special reagents or materials.
Critical Parameters
Fixation time and temperature
The staining intensity obtained depends on the fixative and the duration and temperature of fixation. Improper fixation of the specimen results can decrease the quality of the tissue considerably. Longer fixation times reduce staining intensity because more epitopes are covered by aldehyde-mediated cross-links. We found the best conditions to be overnight fixation (16-18 hr) at 4°C, and 4% PFA proved to provide the best compromise between slowing down autolysis and allowing tissue penetration of the fixative. Shorter fixation times (such as 1-2 hr) at room temperature could also be used with similar intensities of staining, but different antibodies need to be tested to find the best conditions for each.
Movement and perfusion
While samples are being fixed with 4% PFA or incubated with sucrose and O.C.T.™, it is important to agitate the tissue to facilitate penetration, independently of the temperature. Higher temperatures will always accelerate the process, but agitation of the samples is very beneficial.
Other pretreatment options
Depending on the antibody used and the antigen involved, different protocols for slide pretreatment can be used, and these are critical for the immunostaining results. Other options besides those detailed in the protocols here include the following.
- Citrate buffer. Some antibodies performed better after the slide was heated (steps 5 and 6, Basic Protocol 2) while being incubated with citrate buffer (CC2m). pH 6 is slightly acidic.
- Heat pretreatment with Tris-EDTA buffer, pH 9, using an autoclave. After the sections are fixed onto the slide (step 1, Basic Protocol 2), slides are placed in a slide-staining jar (75 × 25 mm) with 70 ml EDTA buffer and covered with a glass lid. The jar is then placed inside the autoclave and the slides are incubated for 50 min at 1 bar (the maximum temperature applied is 121°C for 20 min). The sections should be allowed to cool down inside the autoclave for 90 min before immunostaining is begun.
- Longer pretreatment. The pretreatment may be lengthened by increasing the number of heating cycles. Increasing the antigen retrieval time elicits the detachment of tissue from the slide and a decrease in tissue preservation. It is important to find a good compromise between high staining intensity and conservation of tissue sections.
Positive and negative controls
In both immunostaining protocols, negative and positive controls need to be carried out in parallel with the double staining. These controls are summarized in Tables 1 (controls performed for Basic Protocol 2) and 2 (controls performed for Basic Protocol 3).
| Primary antibody, first immunostaining | Secondary antibody, first immunostaining | Heat 95°C, 8 min | Primary antibody, second immunostaining | Secondary antibody, second immunostaining |
|---|---|---|---|---|
| HCN2 | DAB | - | - | - |
| HCN4 | Fast Red | - | - | - |
| HCN2 | DAB | + | HCN4 | Fast Red |
| HCN4 | DAB | + | HCN2 | Fast Red |
| Primary antibody, first immunostaining | Secondary antibody, first immunostaining | Fixation: 4% PFA, heat 81°C, 8 min | Primary antibody, second immunostaining | Secondary antibody, second immunostaining |
|---|---|---|---|---|
| HCN2 | Alexa Fluor® 488 (A488) | - | - | - |
| HCN4 | Dylight (DY) 649 | - | - | - |
| HCN2 | A488 | + | Primary antibody diluent | Secondary antibody diluent |
| HCN2 | DY 649 | - | - | - |
| HCN2 | A488 | - | - | DY 649 |
| HCN2 | Secondary antibody diluent | + | Primary antibody diluent | A488 |
| HCN2 | Secondary antibody diluent | + | Primary antibody diluent | DY 649 |
| HCN2 | Secondary antibody diluent |
Fix for only 30 min with 4% PFA |
Primary antibody diluent | DY 649 |
| Primary antibody diluent | Secondary antibody diluent | + | HCN4 | DY 649 |
| Primary antibody diluent | A488 | + | - | - |
| Primary antibody diluent | DY 649 | - | - | - |
The antibodies used in these protocols were previously described, and positive and negative controls were performed to assure specificity of the staining (Luque et al., 2021).
Troubleshooting
Low signal even at high antibody concentrations
Try different antigen retrieval protocols described here (Critical Parameters, section on pretreatment). This usually uncovers the epitope and yields higher staining signal. Each protocol needs to be tested due to possible nonspecific binding of the antibody.
Longer incubations with the primary and secondary antibodies may also be performed. For fluorescence IHC, incubation with the primary antibody for 48-72 hr at 4°C and 2 hr at room temperature are suitable. In colorimetric staining, incubation times can also be prolonged, but need to be tested for each antibody.
Signal from the first DAB staining is lost or reduced due to the secondary immunostaining; post-fixative step 30 min
If the intensity of the staining from the first antibody used decreases with colorimetric staining, a fixation step can be performed between the two immunostaining steps. For HCN2-HCN4, this was not necessary because the staining precipitates adhered well within the delicate cytoskeleton meshwork of the neurons, but other combinations may require a “fixative meshwork” to retain DAB stain. A fixation step with 4% PFA for 30 min better preserves the staining with the DAB chromophore.
Nonspecific background in the sections
In colorimetric IHCs, a blocking step before the primary antibody incubation may be added to the protocol. Blocking with up to 2% (w/v) bovine serum albumin gave good results. Other commercially available blockers may be applied.
Understanding Results
It is important to validate the specificity of the staining after each run. Positive control staining should be carried out in parallel with the experimental staining to confirm the specificity of the antibodies and exclude the presence of cross-reactivity between different proteins on the sections (Burry, 2011).
To test the specificity of the primary antibody staining, a preabsorption of specific binding sites of the antibody with the peptide that was used to generate it (immunogenic peptide) can be performed (1 hr at 37°C with movement). The primary antibody without the control peptide should be diluted to the same volume and incubated in parallel. Finally, cochlear sections can be incubated with the primary antibody with and without previous immunogenic peptide incubation. If the staining is specific, no signal should be detected when the staining is performed with the antibody-control peptide complex because the variable sites of the antibodies should be saturated with the control peptide.
Secondary antibody specificity is checked in each experiment using a slide incubated without the primary antibody. No staining should be observed on this control slide.
Time Considerations
See Figure 8 for a summary of the timing for different steps of the three protocols. Preparing the tissue to perform the immunohistochemical protocols takes 6 days. After days 1, 2, and 3, the specimens may be stored in 0.1 M PBS with Na+ azide until needed.
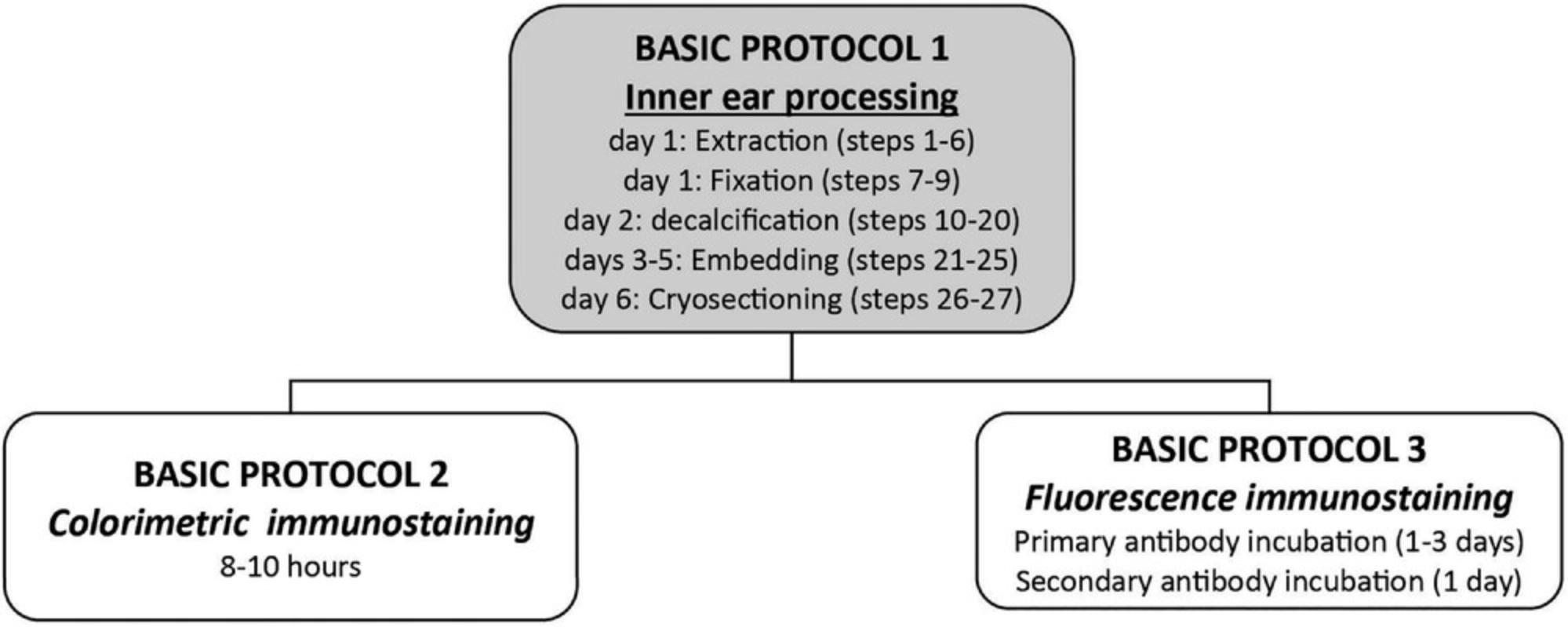
The first protocol with colorimetric staining is performed in 1 day, and results may be checked the same day. The staining protocol with fluorescence secondary antibodies takes longer as it depends on the primary antibody incubation time that is different for each antibody.
In total, for the colorimetric staining the protocol will take 7 days, whereas at least 8-9 days are needed for the manual fluorescence immunostaining.
Times may be shortened at some steps: e.g., the day of cryosectioning could be the first day of the manual immunofluorescence, or cryosectioning could be performed after the blocks are dried from the embedding.
Acknowledgments
The authors gratefully acknowledge MedEl® (Ion Channel Project) and the Austrian Science Fund (FWF project 3154-B27-Gapless Man: Machine Interface) for funding this study, and especially Carolyn Garnham and Heval Benav from MedEl® Innsbruck.
Author Contributions
Maria Luque : Data curation, formal analysis, investigation, methodology, writing–original draft; Rudolf Glueckert : funding acquisition, investigation, methodology, project administration, supervision, writing–review and editing
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Data available on request from the authors
Literature Cited
- Akazawa-Ogawa, Y., Nagai, H., & Hagihara, Y. (2018). Heat denaturation of the antibody, a multi-domain protein. Biophysical Reviews , 10(2), 255–258. doi: 10.1007/s12551-017-0361-8
- Ansorg, A., Bornkessel, K., Witte, O. W., & Urbach, A. (2015). Immunohistochemistry and multiple labeling with antibodies from the same host species to study adult hippocampal neurogenesis. Journal of Visualized Experiments: JoVE , (98). doi: 10.3791/52551
- Burry, R. W. (2011). Controls for immunocytochemistry: An update. Journal of Histochemistry and Cytochemistry , 59(1), 6–12. doi: 10.1369/jhc.2010.956920
- Coleman, B., Rickard, N. A., de Silva, M. G., & Shepherd, R. K. (2009). A protocol for cryoembedding the adult guinea pig cochlea for fluorescence immunohistology. Journal of Neuroscience Methods , 176(2), 144–151. doi: 10.1016/j.jneumeth.2008.09.007
- Im, K., Mareninov, S., Diaz, M. F. P., & Yong, W. H. (2019). An Introduction to Performing Immunofluorescence Staining. Methods in Molecular Biology , 1897, 299–311. doi: 10.1007/978-1-4939-8935-5_26
- Lan, H. Y., Mu, W., Nikolic-Paterson, D. J., & Atkins, R. C. (1995). A novel, simple, reliable, and sensitive method for multiple immunoenzyme staining: Use of microwave oven heating to block antibody crossreactivity and retrieve antigens. Journal of Histochemistry and Cytochemistry , 43(1), 97–102. doi: 10.1177/43.1.7822770
- Lewis Carl, S. A., Gillete-Ferguson, I., & Ferguson, D. G. (1993). An indirect immunofluorescence procedure for staining the same cryosection with two mouse monoclonal primary antibodies. Journal of Histochemistry and Cytochemistry , 41(8), 1273–1278. doi: 10.1177/41.8.7687266
- Liu, H., Li, G., Lu, J., Gao, Y. G., Song, L., Li, G. L., & Wu, H. (2019). Cellular differences in the cochlea of CBA and B6 mice may underlie their difference in susceptibility to hearing loss. Frontiers in Cellular Neuroscience , 13, 60. doi: 10.3389/fncel.2019.00060
- Luque, M., Schrott-Fischer, A., Dudas, J., Pechriggl, E., Brenner, E., Rask-Andersen, H., … Glueckert, R. (2021). HCN channels in the mammalian cochlea: Expression pattern, subcellular location, and age-dependent changes. Journal of Neuroscience Research , 99(2), 699–728. doi: 10.1002/jnr.24754
- Mao, S. Y., & Mullins, J. M. (2010). Conjugation of fluorochromes to antibodies. Methods in Molecular Biology , 588, 43–48. doi: 10.1007/978-1-59745-324-0_6
- Owen, G. R., Hakkinen, L., Wu, C., & Larjava, H. (2010). A reproducible technique for specific labeling of antigens using preformed fluorescent molecular IgG-F(ab')2 complexes from primary antibodies of the same species. Microscopy Research and Technique , 73(6), 623–630. doi: 10.1002/jemt.20803
- Shindler, K. S., & Roth, K. A. (1996). Double immunofluorescent staining using two unconjugated primary antisera raised in the same species. Journal of Histochemistry and Cytochemistry , 44(11), 1331–1335. doi: 10.1177/44.11.8918908
- Tornehave, D., Hougaard, D. M., & Larsson, L. (2000). Microwaving for double indirect immunofluorescence with primary antibodies from the same species and for staining of mouse tissues with mouse monoclonal antibodies. Histochemistry and Cell Biology , 113(1), 19–23. doi: 10.1007/s004180050002

