Purification of Methylglucose Lipopolysaccharides (MGLP)
Briana Marsico, Luisa M Nieto Ramirez, Karen Dobos
Abstract
Methylglucose lipopolysaccharides (mGLPs) are poly-methylated polysaccharides (PMPSs) that are acylated with short-chain fatty acids, uniquely synthesized by bacteria belonging to the Actinomycetales order. mGLPs are composed of ~20 glucose (Glc) units, most of them O-methylated (6- O -Me-Glc, Glc, and a 3- O -Me-Glc in a ratio of 11:8:1). Acyl functions in mGLPs are heterogeneous and contain acetyl, isobutyryl, succinyl, and octanoyl groups. mGLPs also known as 6-O-methylglucose-containing-lipopolysaccharides are believed to be essential in Mycobacterium tuberculosis and participate in the regulation of lipid metabolism, protecting fatty acids from degradation. Additionally, this cytosolic bacterial component is a potent stimulator of γ9δ2 T cells.
Before start
Steps
Total lipid extraction
Obtain approximately 100 g Sample Mycobacterium tuberculosis (Mtb) cells and freeze dry by lyophilization.
Determine the dry weight of the cells and transfer them to a 500 mL glass bottle.
Delipidate cells by adding 10mL of 10:10:3 [vol/vol/vol] chloroform: methanol: water per 1 gr of the Mtb cells. Add a stir bar and stir in a chemical fume hood. Make sure the bottle is tightly closed.
Transfer overnight extract into 35 mL Teflon Oak Ridge tube(s) and centrifuge at 27000x g,15°C
Note: The number of Teflon tubes would depend on the volume obtained in step 3.
Decant organic supernatant and preserve it in a labeled bottle.
Transfer the cells from tubes back to 500 mL bottle and repeat delipidation (steps 2 -5) two more times, reducing incubation time to 2 hours for each repeat. Be sure to vortex the cell pellet for one minute before repeating.
Collect all extracts into the same bottle (started on step 5).
Measure total volume and aliquot 0.5 mL into a previously weighed 2 mL glass vial. Dry under a Nitrogen bath and calculate mg/mL of total volume based on the net weight obtained.
Take 50 mg of your 10:10:3 total lipid and completely dry down via Nitrogen bath. Resuspend in MilliQ water at a concentration of 5 mg/mL (10 mL total). Use a water bath sonicator to thoroughly resuspend – may take several minutes. If necessary, can also add more water to further dilute and ensure complete resuspension.
Removal of high molecular weight molecules
Prepare your Amicon® Ultra-15 Centrifugal Filter Unit 10 kDa MWCO by adding 5 mL of MiliQ water and centrifuging at 3500x g,20°C. Dispose the flow-through.
Add your total lipid extract from step 9 to the Amicon® Ultra-15 Centrifugal Filter Unit 10 kDa MWCO and centrifuge at 3500x g,20°C, keeping the flow-through . Check volume level: if there is only a small amount of flow-through, increase speed to 3750 – 4000 x g.
Repeat centrifugation until most of the volume is passed and the retentate volume is ~ 1 mL.
Rinse 10 kDa filter membrane using Pasteur pipette by adding 2 – 3 mL of MiliQ water, pipette up and down several times. Centrifuge for 10 minutes and collect the flow-through .
Concentration of mGLP (~3.5-4 kDa)
Transfer pooled flow-through (from steps 11-13) to a Cytiva Microsep Advance Centrifugal Devices with Omega Membrane 3kDa (maximum capacity 4 mL).
Centrifuge at 3500x g,20°C,0h 0m 0s for 10 minutes and dispose of flow-through. Add more of the 10 kDa flow-through until max capacity (4 mL) is reached again in the Cytiva Microsep.
Continue this process until all the 10 kDa flow-through has been passed through the 3 kDa filter and the retentate volume is reduced to ~ 1 mL.
Transfer retentate from previous step to 13 x 100 tube and thoroughly rinse 3 kDa filter 2X with 1 mL of water, pipetting up and down to collect any residual product before transferring to 13 x 100 tube. Record the total volume obtained.
Determine the total amount of crude material in 3 kDa retentate by aliquoting 0.5 mL into a pre-weighted 2 mL vial. Calculate mg/mL of total 3KDa crude material.
Aliquot 0.5 mg of 3 kDa retentate into a 13 x 100 tube and completely dry down on a N2 bath. Resuspend in 500 uL of water and dilute 1:10 by adding 4.5 mL of additional water.
Prepare your Sep-Pak C18 cartridge, adding in order the following solutions, using a 10 mL glass syringe:
a. 4 mL 95% acetonitrile
b. 5 mL 100% methanol
c. 2 mL 80% methanol in chloroform
Equilibrate your cartridge by adding 3 mL of water; leave enough water in the column so that it is saturated and a droplet is visible at the bottom. Let sit for 5 minutes.
Load your diluted crude sample into the column and slowly elute.
Wash with 4 mL of water (no need to collect the flow-through).
Perform a series of elutions with the following, with a wash step in between each elution, using 4 mL of water :
a. 4 mL 30% methanol in water
b. 4 mL 60% methanol in water
c. 4 mL 90% methanol in water
Collect each elution and washes steps in a labeled and pre-weighted 13 x 100 tube.
Repeat Sep-Pak chromatography for as much material is needed. Around 6 to 8 cycles of Sep-Pak preparations would be needed for 50 mg of the 10:10:3 extract (obtained in step 9).
In the N2 bath, dry the 60%, and 90% fractions obtained in the Sep-Pak runs, separately (mGLP is enriched mostly in the 60% fraction and in a less extent in the 90% fraction).
Determine the dried weight of the 60% and 90% fraction and resuspend in 0.5 mL of water. Record the concentration obtained for each fraction.
After combining the 60% and 90% fractions, the yield of purified mGLP is about 4% of the 10:10:3 obtained material from step 9 (starting with 50 mg of the 10:10:3 extract, the yield of is approximately 4 mg).
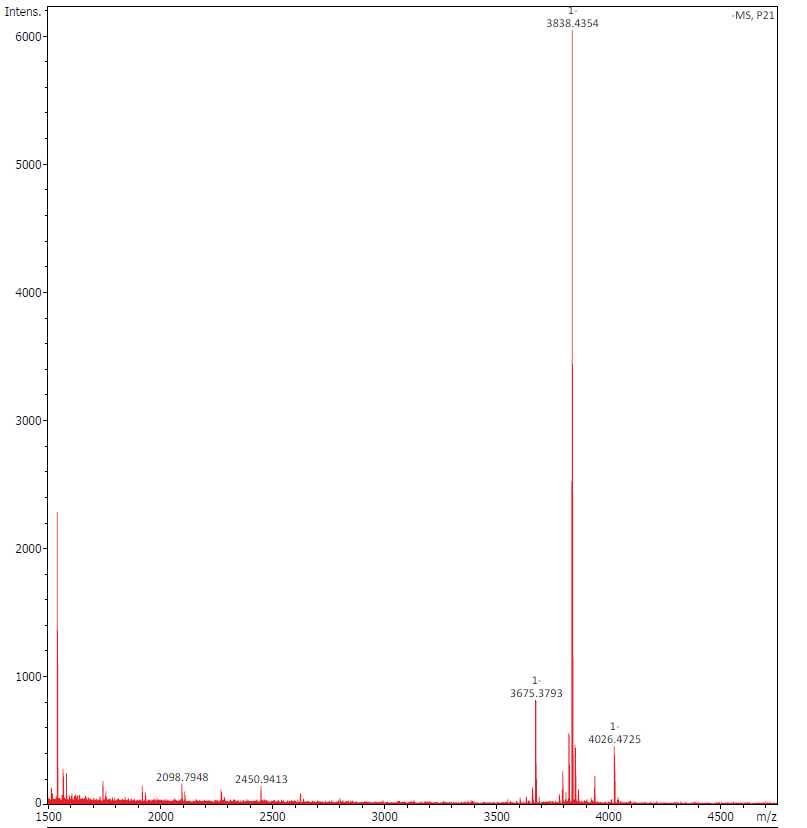
Perform the MALDI-TOF for QC.