Preparation of a reduced Cr2+ solution for sulfide distillation using a Jones Reductor
Sebastian Rubiano-Rincon, Patrick Larkin
Abstract
This protocol uses a Jones Reductor to prepare reduced Cr2+ for the distillation of H2S from marine sediments. It is an adaptation of a protocol described by Backlund et al (DOI:https://doi.org/10.2137/1459606054224147)
Steps
Jones Reductor Setup and Use
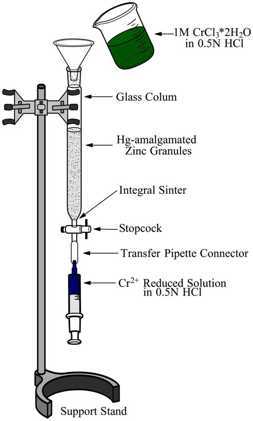
Set up in a support stand a glass-column (~40-cm long, 1.5 cm interior diameter) with an integral sinter at the bottom. Secure a stopcock at the bottom of the column and turn the handle to the closed position.
Measure 40-50 g of granular zinc in a 250 mL beaker and wash for 1 minute with ~50 mL 1N HCl by stirring with a glass stir rod. Carefully decant the HCl solution into a waste beaker. Repeat the washing with 1N HCl and decantation two more times.
Wash the granular zinc from the previous step with ~50 mL DI water for 1 minute by stirring with a glass stir rod. Carefully decant the water. Repeat the washing with DI water and decantation one more time.
Cover the washed zinc granules with ~50 mL of 2% HgCl2 solution. Stir the contents for 5 to 10 min with the aid of a stirring bar and/or glass rod or spatula.
Decant the HgCl2 solution from the zinc granules into a waste beaker, and wash them with ~50 mL of DI water as before. Decant and repeat the washing two more times. The resulting amalgamated zinc should have a bright silvery luster.
Transfer the amalgamated zinc granules to the glass column using a plastic funnel and fill the column without going over the shoulder of the column. This completes the set-up of the Jones Reductor (See Figure No. 1).
Run 50 mL of DI water down the column into the waste beaker to wash the amalgamated zinc granules. Once done, close the stopcock and make sure the zinc granules are covered with water. Note: if the reductor is not to be used immediately, it must be left full of water in order to prevent atmospheric oxidation of the Zn granules.
Activate the amalgamated zinc granules by running ~75 mL of 0.5N HCl down the column into the waste beaker. Once done, close the stopcock and leave the zinc column submerged in HCl solution.
Begin percolating the 1M CrCl3*6H2O solution through the Jones reductor by opening the stopcock and letting the solution run down the column. An efficient reduction of the chromium ions is verified by a color change from dark green (Cr3+) solution to a bright blue (Cr2+) solution (See Figure No. 1).
-
As soon as the drops exiting the column turn bright blue, close the stopcock.
-
Obtain an 8-cm long piece of narrow (5 mm OD) plastic tubing (the stems of tansfer pipets will work). Attach one end to the stopcock and the other end to the tip of a plastic (polypropylene) syringe.
-
Open the column stopcock and slowly start drawing the reduced Cr2+ solution from the column into the syringe. Prevent accumulation of air pockets inside the syringe as they may re-oxidize the solution.
-
Collect 50 mL of the reduced Cr2+ solution into one plastic syringe. Make sure to keep adding the CrCl<sub>3</sub>*6H2O (green) solution to the zinc column, leaving it always covered.
-
Once the collection is done, close the stopcock and quickly cap the syringe. If necessary, gently push out any air bubbles from the syringe before capping. Repeat 50 mL collections as necessary.
-
Store Cr<sup>2+</sup> solution at room temperature for subsequent sulfide distillation procedures.
Clean Up
Clean the zinc column by passing ~25 mL of 0.25N HCl, followed by ~25 mL of DI water. Leave the zinc column full of water for future use (2-3 weeks maximum).
Rinse each piece of glassware with DI water to dispose any solid or liquid into a waste bottle.
Wash glassware in warm, 1% Citranox solution. Rinse with Distilled H2O (3x), followed by DI H2O (1x). Dry in oven for 2 hours at 100oC or air dry.
Waste Management
All liquid waste must be disposed in properly labelled waste bottles. Make sure to list every chemical in the waste label in the bottle.
All solid waste must be disposed of in a solid waste bucket.
IMPORTANT: any residues of the Hg-amalgamated zinc granules, including the reducing column, MUST BE DISPOSED into a metallic mercury waste bag.