Post-Surgical Dissection of Ovaries
Stephen Fisher, Marielena Grijalva, Rong Guo, Sarah A Johnston, Hieu Nguyen, John Renz, Jean G Rosario, Steven Rudich, Brian Gregory, Junhyong Kim, Kate O'Neill
Abstract
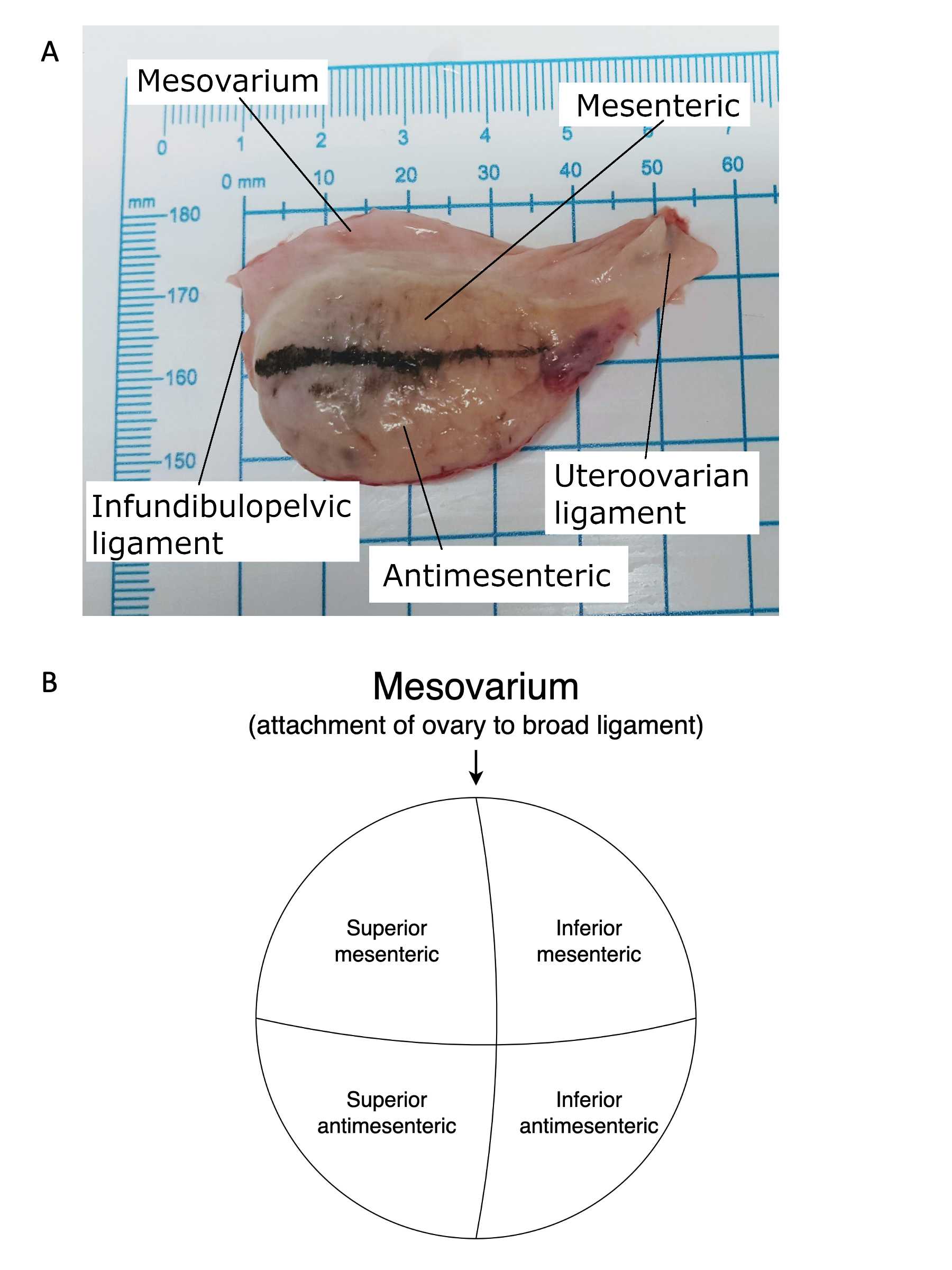
This protocol details dissection of the ovaries in preparation for 10X Visium, 10X Multiomics, pathology review, and biobanking. Care must be taken to systematically divide the ovary and to maintain orientation of the specimens as they are removed using the three points of fixation for the ovary (the uteroovarian ligament, the infundibulopelvic ligament and the mesosalpinx). Once the ovary is isolated it is difficult to discern which aspect of the ovary is superior to the ovary's attachment to the mesosalpinx and which is inferior. Colored marking dyes can be used to help maintain orientation provided they do not interfere with downstream analyses.
Before start
Dilute phosphate buffered saline (Gibco; 14200-075) to 1X with nuclease-free water.
Steps
Receive excised female reproductive system on ice en bloc. Orient the specimen with the anterior aspect of the uterus facing up based on presence of vesicouterine reflection.
Using a disposable scalpel, incise the mesovarium. Orient ovary with the uteroovarian ligament on the right, infundibulopelvic ligament on the left, and mesovarium on top for the right ovary (Figures 6).
Dry the ovary thoroughly with a Kimwipe.
Use marking dye to maintain orientation of the ovary with the uteroovarian ligament on the right, infundibulopelvic ligament on the left, and mesovarium on top.
Take the weight and, using a disposable ruler, take spatial measurement of each ovary.
Return ovaries to ice.
Place the first ovary in the pre-chilled mold (Figure 2).
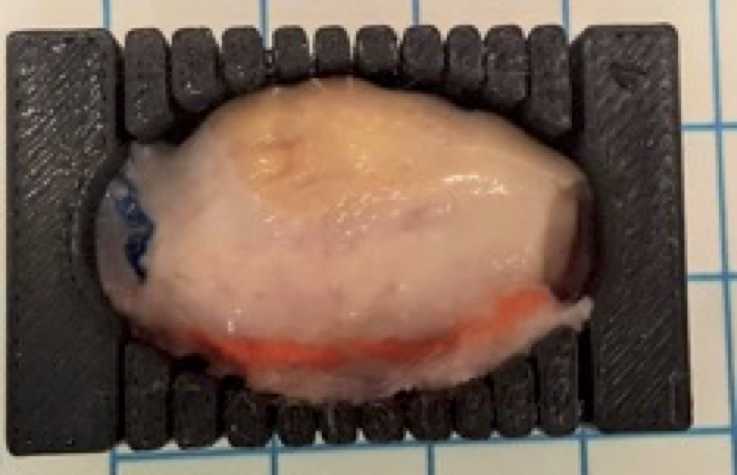
Divide the ovary along the long axis of the ovary from the uteroovarian to the infundibulopelvic ligament using the guidelines of the mold (Figure 1a).
Take each slice and orient mesovarium ridge at 12 o’clock.
Divide each slice along the line of the mesovarium ridge to divide the ovary into portions superior to and inferior to the mesovarium.
Take each half and divide it again in half to separate the ovary into the mesenteric and antimesenteric portions (Figure 1b).
For later 3D anatomical positioning, the quadrant of the tissue piece and the slice number along the long axis should be tracked for each tissue specimen.
Tissue can be processed with protocols OCT-Embedded Tissue Preparation, Tissue Fixation Preparation, or Snap-Frozen Tissue Preparation depending on the desired downstream processing.
Repeat steps with the contralateral ovary, using a fresh, ice-cold ovary mold.

