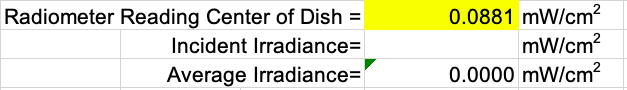Polychromatic UV Fluence (Dose) Response Determination
Daniel Ma, NATALIE HULL
Disclaimer
DISCLAIMER – FOR INFORMATIONAL PURPOSES ONLY; USE AT YOUR OWN RISK
The protocol content here is for informational purposes only and does not constitute legal, medical, clinical, or safety advice, or otherwise; content added to protocols.io is not peer reviewed and may not have undergone a formal approval of any kind. Information presented in this protocol should not substitute for independent professional judgment, advice, diagnosis, or treatment. Any action you take or refrain from taking using or relying upon the information presented here is strictly at your own risk. You agree that neither the Company nor any of the authors, contributors, administrators, or anyone else associated with protocols.io, can be held responsible for your use of the information contained in or linked to this protocol or any of our Sites/Apps and Services.
Abstract
The purpose of this protocol is to document the steps used for determination of UV doses for polychromatic UV sources such as UV LEDs, excimer lamps, medium pressure mercury lamps. The method is not limited to polychromatic light sources, but can also be used for monochromatic sources, such as low pressure mercury lamps. The method described in this protocol has been modified to account for polychromatic UV radiation sources (Linden and Darby, 1997) and is based on method is based on "Standardization of Methods for Fluence (UV Dose) Determination in Bench-Scale UV Experiments" (Bolton and Linden, 2003). If you cite your use of this protocol, you should also cite the relevant original literature. For further information on using UV LEDs and measuring UV intensity, refer to the following publications: Kheyrandish et al., (2018), Kheyrandish et al., (2017), Kheyrandish et al., (2018), Sholtes et al., (2019).
Before start
For microbial samples, sanitize bench top surfaces with 70% ethanol.
Steps
UV Dose Spreadsheet
UV Dose Spreadsheet: UV_Dose_Blank.xlsx
Here is an example spreadsheet filled out with radiometer factors, absorbance scan of sample, UV emission spectra of a low pressure mercury lamp, and sample geometry: UV_Dose_LP254.xlsx
This protocol will guide users through important parts of the UV Dose Spreadsheet.
Preparation of UV Light Source
Obtain UV Source Emission Spectra . Before your experiment, ensure that you have obtained the UV lamp emission spectra with a UV-VIS spectroradiometer (NIST-traceable is required ). Warm up the UV source. Typically, 15 minutes is sufficient for light emission to stabilize. UV source stabilization can be confirmed using a radiometer.
Set up the UV-VIS spectroradiometer and the acquisition software. Load calibration data provided with the calibrated UV-VIS spectroradiometer.
Measure the UV spectra by positioning the spectroradiometer below the UV source. Set scan parameters such as boxcar width, scans to average, and integration time. Obtain background scan (e.g., dark scan with no light exposure) before taking UV measurements.
Export the spectroradiometer measurements. If the spectroradiometer output does not provide irradiance measurements at integer value wavelengths, interpolation of the wavelengths and irradiance measurements is required to use the data in the UV Dose Spreadsheet.
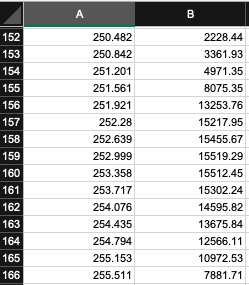
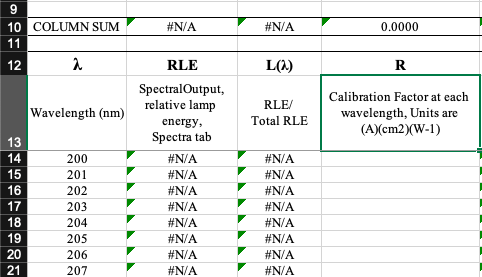
Interpolation can be performed on the emission spectra values to obtain integer wavelengths using the tab in the UV Dose Determination spreadsheet titled " Interpolation ". Interpolation can be also performed in Microsoft Excel or other computational software (e.g., in R Studio with the 'approx' function).
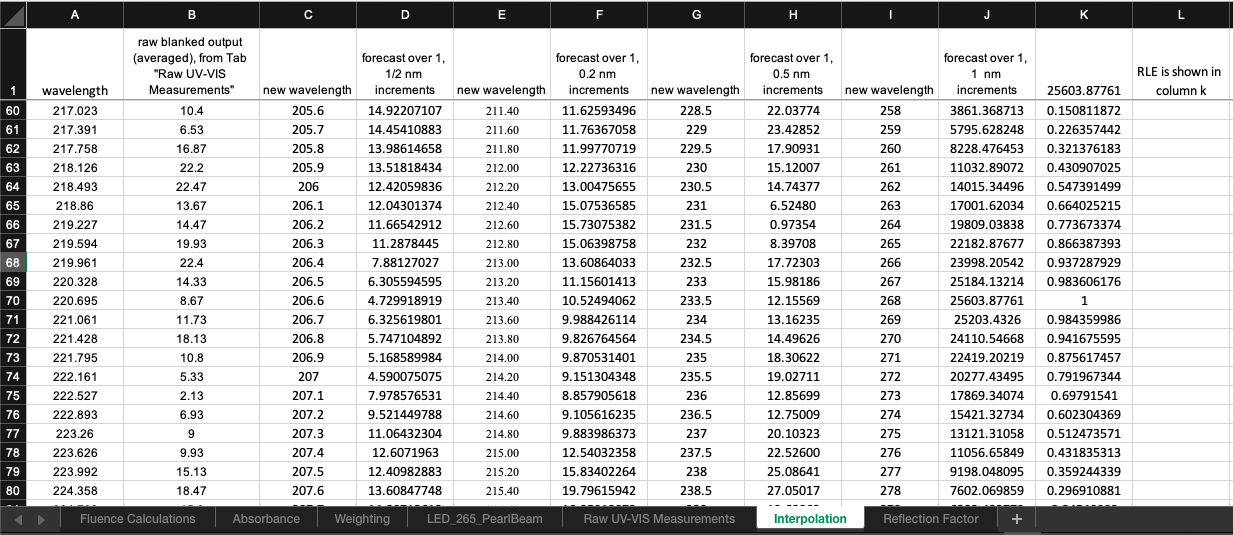
Check UV Spectra Characteristics . The peak and average wavelength can be obtained from the tab "Spectra Characteristics". The fp radiometer factor should be between 1–1.05. Values higher than this will adversely affect UV exposure times.
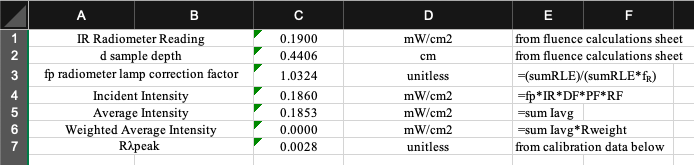
Sample Preparation
Select Sample Matrix. The UV dose responses of different sample matrices may be characterized using this protocol.
Sample types may include, but are not limited to, the following:
- Bio-molecular samples (e.g. bacteria, viruses, fungal spores, etc.)
- Environmental samples (e.g. wastewater, surface water, groundwater, etc.)
- Chemicals
Determine Volume of Working Solution Needed. To determine the minimum total volume of working solution (
= Sample volume needed per UV dose
Choose working stock concentration . The concentration should be chosen to quantify observable reductions for the highest UV dose. Consider personnel safety when choosing a stock concentration. Avoid risk of exposure to high concentrations of hazardous chemicals.
Prepare working solution for the chosen sample matrix. After calculating the volume needed and the target concentration, prepare the working solution of your sample by using the appropriate diluent, if necessary. Common diluents of samples include: DI water and phosphate buffered saline (PBS).
Preparing bio-molecular samples. If using bio-molecular samples, prepare microorganisms according to your laboratory protocols. Use best practices and aseptic technique. Biological triplicates are recommended for bio-molecular studies.
Prepare environmental samples . If using environmental samples, obtain environmental samples and store in appropriate containers and temperatures. Environmental samples may be used to reflect environmental water quality (e.g., wastewater, river water, groundwater). Microorganisms may be spiked into environmental samples. Store chemicals in appropriate storage conditions (e.g., container, temperature, light) before UV exposures.
Prepare chemical samples . If using chemical samples, prepare stock solutions and working stock solutions using best practices for handling and preparing chemicals. Perform dilutions using an appropriate diluent. Store chemicals in appropriate storage conditions (e.g., container, temperature, light) before UV exposures.
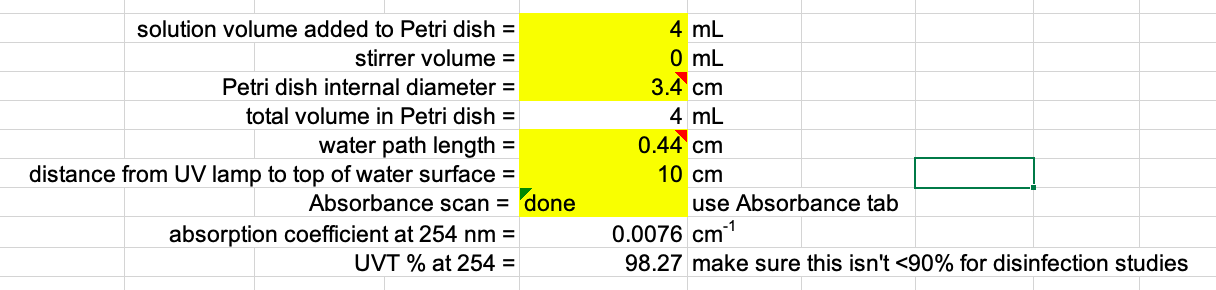
Measure sample absorbance. Use a UV-VIS spectrophotometer to obtain an absorbance spectra of working stock solution. Sample absorbance is required for determining the UV dose by accounting for the UV light absorbance at different wavelengths and for determining the Water Factor, one of the UV irradiance adjustment factors.
Prepare sample and dilutions for absorbance scan. Samples of high concentration may need to be diluted for UV-VIS measurements. Dilute samples using the appropriate diluent. Record the dilution factor, which will be used to correct absorbance scan data for the original solution.
Turn on UV-VIS spectrophotometer . Warm up at least 10 minutes. Longer warm up time for the lamps will increase performance. Adjust the default instrument settings if desired (e.g., scan speed, slit height, wavelength range, etc.).
Obtain absorbance measurements :
- Load a blank sample (e.g. cuvette with DI water).
- Zero the instrument. This zeroes the reading at the highest wavelength.
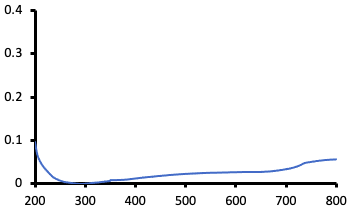
- Baseline the instrument. This zeroes the readings at all wavelengths. A baseline scan is shown in Figure A below.
- Optional : Obtain absorbance scan of distilled water immediately after the baseline (the absorbance scan values should be near 0 for all wavelengths). If the instrument was properly zeroed and a baseline acquired.
- Obtain sample absorbance scan(s).
- Empty cuvette. Dispose of sample in proper containers.
- Rinse cuvette at least once with working stock solution.
- Obtain at least duplicate absorbance scan per sample.
- Export tabulated absorbance scan data. Correct for any dilution factor, if necessary.
- Calculate the average absorbance from the replicate measurements.
- Paste the average absorbance at each wavelength in the Absorbance tab in the UV Dose Spreadsheet.
Measuring UV irradiance
Set up UV source and radiometer (must be NIST-calibrated). Select the distance between UV source and sample surface. Record this value in the UV Dose Spreadsheet.
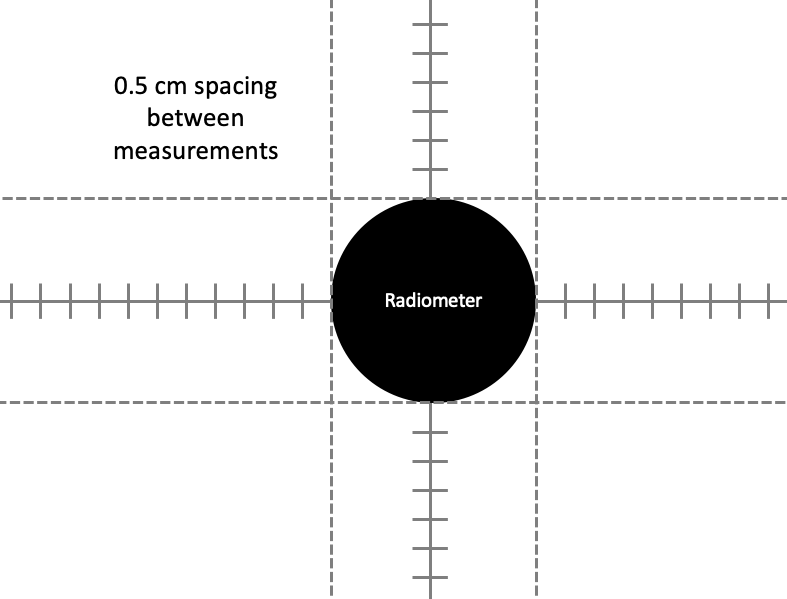
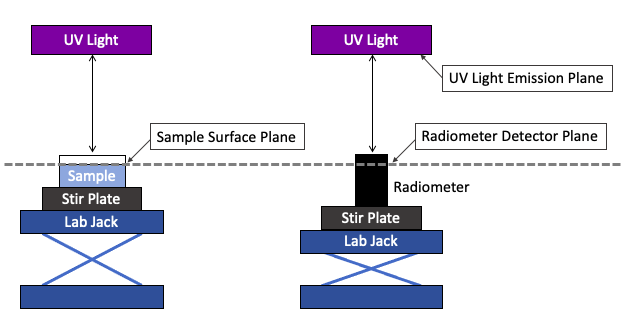
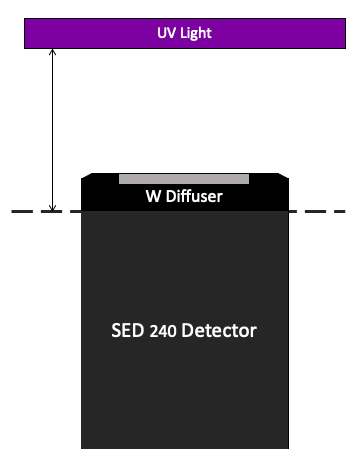
To obtain UV irradiance measurements, set up the radiometer so that the radiometer detector plane is at the same level as the sample surface during UV exposures (Figure A). Use an adjustable lab jack to achieve the same sample surface plane during sample exposure and radiometer measurements. Check the location of the reference plane of the radiometer, as shown in Figure B.
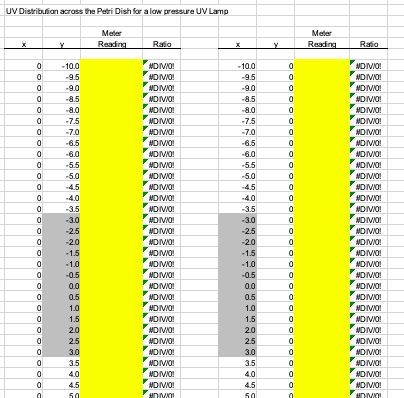
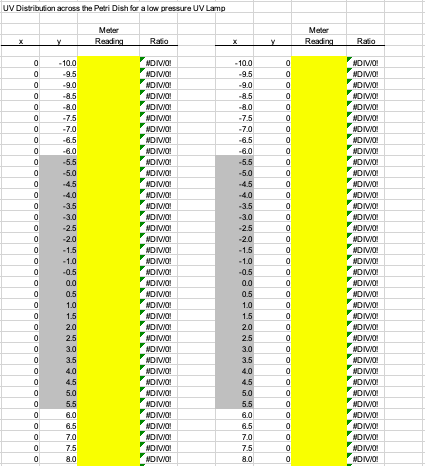
Obtain Petri Factor . The UV dose calculation requires the Petri Factor to account for the spatial non-uniformity of the UV source emission over the surface of the water sample in the container (e.g., Petri dish). The Petri Factor is obtained by measuring the UV irradiance at the sample surface plane at 0.5 cm intervals in the x- and y-direction starting from the center of the sample dish.
Ensure the radiometer factor is set to the calibration value for the peak wavelength. The peak wavelength and its corresponding radiometer factor can be found in the Fluence Calculation tab.
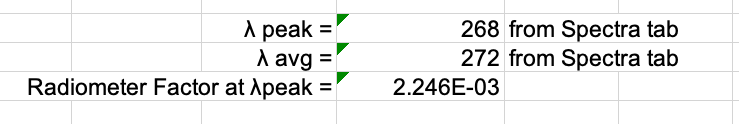
Depending on the size of the dish, the UV Dose Spreadsheet will indicate the minimum number of measurements to take in each direction.
Check that the UV irradiance adjustment factors in the Fluence Calculations tab are within the recommended range of values:
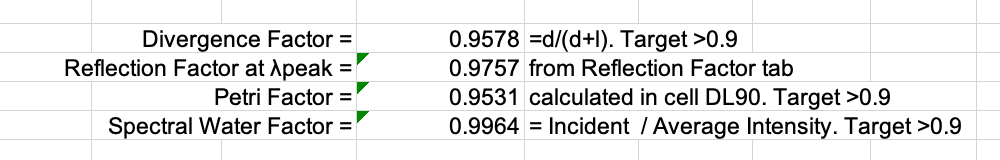
The center of dish irradiance (
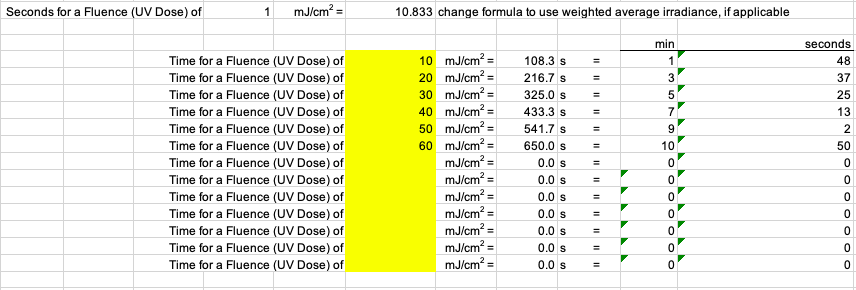
Calculate exposure times for pre-determined UV doses. The zero (0; unexposed) dose sample is very important must be taken. Consider taking the zero dose sample multiple times during the experiment (e.g., beginning, middle, end). Dose responses should have at least 3-5 points in addition to the zero dose. Check the literature for expected dose responses for the specific microorganism.
Performing UV exposures
Perform UV exposures by transferring working solution of sample to sample container (sterile container for microbial samples), adding a stir bar (sterile for microbial samples), and placing on the magnetic stir plate. Expose the sample to UV irradiation for the calculated exposure times. Expose samples in random order (e.g., not ascending or descending order of UV dose values).
After the exposure time is complete, replace the shutter to block off UV irradiation. Transfer irradiated samples to appropriate containers. For microbial samples, use sterile technique and equipment (e.g., pipetting) to transfer sample to sterile containers (e.g., test tubes). Save samples for downstream analysis and quantification.
Acknowledgements
We acknowledge the past and present Hull Lab members who contributed to the development and review of this protocol. The UV dose spreadsheet provided in this protocol has undergone iterative changes. It has been modified from UV dose spreadsheets from the Linden Lab and changes have been made based on personal communications from Dr. Jim Bolton.