Platelet Functional Testing Via High-Throughput Microtiter Plate-Based Assays
Hem Kumar Tamang, Hem Kumar Tamang, Emily N. Stringham, Emily N. Stringham, Benjamin E. Tourdot, Benjamin E. Tourdot
Abstract
Platelets play a critical role in hemostasis and thrombosis; therefore, in vitro assays that measure platelet reactivity are fundamental tools to gain insight into these physiologic processes, to diagnose platelet disorders, and to develop antithrombotic therapies. However, conventional platelet assays such as aggregometry, the clinical gold standard for assessing platelet function, are low throughput and require specialized equipment. Since platelets have a finite life span ex vivo , processes to miniaturize and multiplex assays allow a much broader overview of platelet function in significantly less time than conventional assays. Several groups have developed simplified, high-throughput approaches to quantify platelet activation with standard laboratory equipment to lower the barrier of entry to study platelet biology. This article describes a panel of optimized and validated high-throughput microplate assays to comprehensively assess platelet functionality, independently or in combination, to increase throughput and reduce costs. Specifically, following stimulation of platelets, a plate reader can be used to measure light transmission aggregation via absorbance; dense-granule secretion based on ATP-dependent luminescence generation; and cytosolic calcium levels with a cell-permeant, fluorescent Ca2+-sensitive dye. Additionally, platelets are an easily accessible component of the blood that share signaling pathways with other cells, making them ideal for high-throughput drug screens. The highly adaptable and complementary assays presented in this article can be used to decipher the molecular mechanism underlying platelet activation or to identify novel inhibitors. © 2023 The Authors. Current Protocols published by Wiley Periodicals LLC.
Basic Protocol 1 : Microtiter plate–based light transmission aggregometry
Basic Protocol 2 : Measuring dense-granule secretion in high-throughput microplate assays
Basic Protocol 3 : Microtiter plate–based calcium mobilization
Support Protocol : Platelet isolation and enumeration
INTRODUCTION
Platelets are small, anucleated cellular fragments derived from the cytoplasm of megakaryocytes that play a pivotal role in hemostasis and thrombosis (Noetzli, French, & Machlus, 2019). The formation of a platelet-rich hemostatic plug or pathogenic thrombus is a complex process that requires coordinated platelet activation in response to multiple stimuli. In order for the hemostatic plug or thrombus to grow and stabilize following vascular injury, platelets must adhere to the subendothelial extracellular matrix, secrete their granules, and form platelet–platelet interactions. Although specialized assays have been developed to selectively study each of these processes, aggregometry, which measures platelet–platelet interactions, remains the gold standard for diagnosing patients with platelet disorders and is the most commonly used assay to study platelet reactivity. The development of aggregometry revolutionized platelet biology by helping researchers gain insight into thrombus formation and develop antiplatelet therapy. However, many limitations are associated with platelet functional assays including conventional aggregometry. The main challenge is the need for expensive, specialized, and dedicated equipment like an aggregometer, which requires an experienced operator. Additionally, running a full agonist panel on a single donor with conventional assays requires a large sample volume and can be time consuming and labor intensive due to the limited number of samples that can be run simultaneously. This is especially consequential because platelets have a finite life span ex vivo , depending on the protocol used to prepare the platelets, due to the continuous decrease in platelet activity over time. Therefore, current techniques allow only a limited number of samples to be assayed reproducibly.
To address the limitations of conventional techniques, several platelet assays have been adapted to analyze platelet function in a microtiter plate, which allows a more in-depth high-throughput analysis of platelet function with a lower volume of platelets in significantly less time than conventional methods (Armstrong et al., 2009; Bye, Unsworth, & Gibbins, 2018; Chan, Armstrong, Papalia, Kirkby, & Warner, 2011). This article discusses detailed protocols for measuring platelet aggregation, dense-granule secretion, and Ca2+ mobilization in microtiter plates independently or combined. The simplest and most cost effective of these assays is microtiter plate light transmission aggregometry, as described in Basic Protocol 1, in which an agonist is added to platelets in a 96-well plate. As platelets activate in response to the agonist, they form clumps known as aggregates, which allow more light transmission. This can be quantified by measuring absorbance with a plate reader. Microplate readers and shakers are common pieces of laboratory equipment and are therefore accessible to most laboratories. The elimination of the need for an aggregometer greatly reduces the initial setup costs for this assay.
Additionally, a multimode plate reader capable of measuring luminescence and fluorescence can be used to perform microplate-based platelet-dense granule secretion and Ca2+ mobilization in real time or as dedicated endpoint assays (Chan, Leadbeater, Watson, & Warner, 2018). Similar to conventional lumiaggregometry, Basic Protocol 2 describes methodologies to measure dense-granule secretion in high-throughput microplate assays. Aggregation and luminescence can be read in parallel from the same sample in a 96-well plate with the simple addition of commercially available luciferase/luciferin mixtures either before or immediately after agonist stimulation. Furthermore, the rise in cytosolic Ca2+, which is essential to most platelet functions such as aggregation and granule secretion, can be easily monitored in the presence of Ca2+-sensitive dyes. Basic Protocol 3 provides a detailed description of high-throughput microplate assays to measure Ca2+ mobilization in platelets. Lastly, the Support Protocol offers practical advice on handling platelets, highly sensitive cells that are susceptible to preanalytical processes and incidental activation. Together the miniaturized platelet functional assays retain their sensitivity and provide a more accessible, highly adaptable platform to acquire large amounts of data or perform multiplexed drug screening.
STRATEGIC PLANNING
Improper whole-blood collection or sample handling can significantly alter platelet responsiveness to agonists or lead to inadvertent agonist-independent spontaneous platelet activation. Although preanalytical procedures have been kept to a minimum in these protocols, several critical preanalytical variables should be considered and rigorously standardized before testing platelet function to ensure accurate and consistent results. Furthermore, verify blood donors have no history of bleeding disorders and have refrained from using substances known to modulate platelet activation, including abstaining from cyclooxygenase (COX) inhibitors, nonsteroidal anti-inflammatory drugs, or aspirin for 7 days before the blood draw.
Blood Collection
The act of drawing blood is a potential source of artifactual platelet activation. To reduce the most common sources of platelet activation, tissue factor contamination, and red blood cell lysis, we recommend the following: (1) Ensure a clean needle stick with a large-bore needle, 21 gauge or larger. (2) Discard the first vacutainer to capture any tissue factor generated as part of the initial needle stick, and remove the dead space in the line to ensure proper fill volume. (3) Confirm smooth blood flow during collection. Vacutainers should be inverted immediately after collection to ensure the blood is adequately mixed with the anticoagulant. Without the addition of an anticoagulant, whole blood would clot within minutes of collection (Østerud & Brox, 1983). However, the addition of anticoagulants that chelate Ca2+ or inhibit thrombin introduce a number of challenges, which have been reviewed in depth (Mannuß, 2020; May & Heptinstall, 2004). For experiments with platelets suspended in plasma typically known as platelet-rich plasma (PRP), we recommend collecting blood in sodium citrate, a weak Ca2+ chelator, whereas for isolating platelets acid citrate dextrose (ACD) or sodium citrate can be used.
Sample Handling
Proper handling of the sample following blood collection is critical to obtain robust platelet reactivity data. For instance, time between blood draw and sample preparation should be minimized to reduce spontaneous platelet activation. Prolonged storage of platelets at cold temperatures can activate platelets (Kattlove & Alexander, 1971); therefore, whole blood, PRP, and platelets should be kept at room temperature or 37°C. Furthermore, store samples in polypropylene tubes, and avoid unnecessary agitation before use. Platelets should be allowed to rest for 30 min following resuspension to recover. Below we discuss methods to validate your method of platelet isolation and handling to ensure spontaneous activation is kept to a minimum. All platelet functional tests should be performed within 4 to 6 hr of blood draw, depending on how the platelets were prepared and stored. Alternatively, a timecourse can be performed with submaximal concentrations of agonists to determine when platelets lose >10% of their initial activity based on your isolation protocol.
NOTE : All protocols involving humans and human samples must first be reviewed and approved by an institutional review board or independent ethics committee or must follow local guidelines for the use of human samples. All participants must provide informed consent.
Basic Protocol 1: MICROTITER PLATE–BASED LIGHT TRANSMISSION AGGREGOMETRY
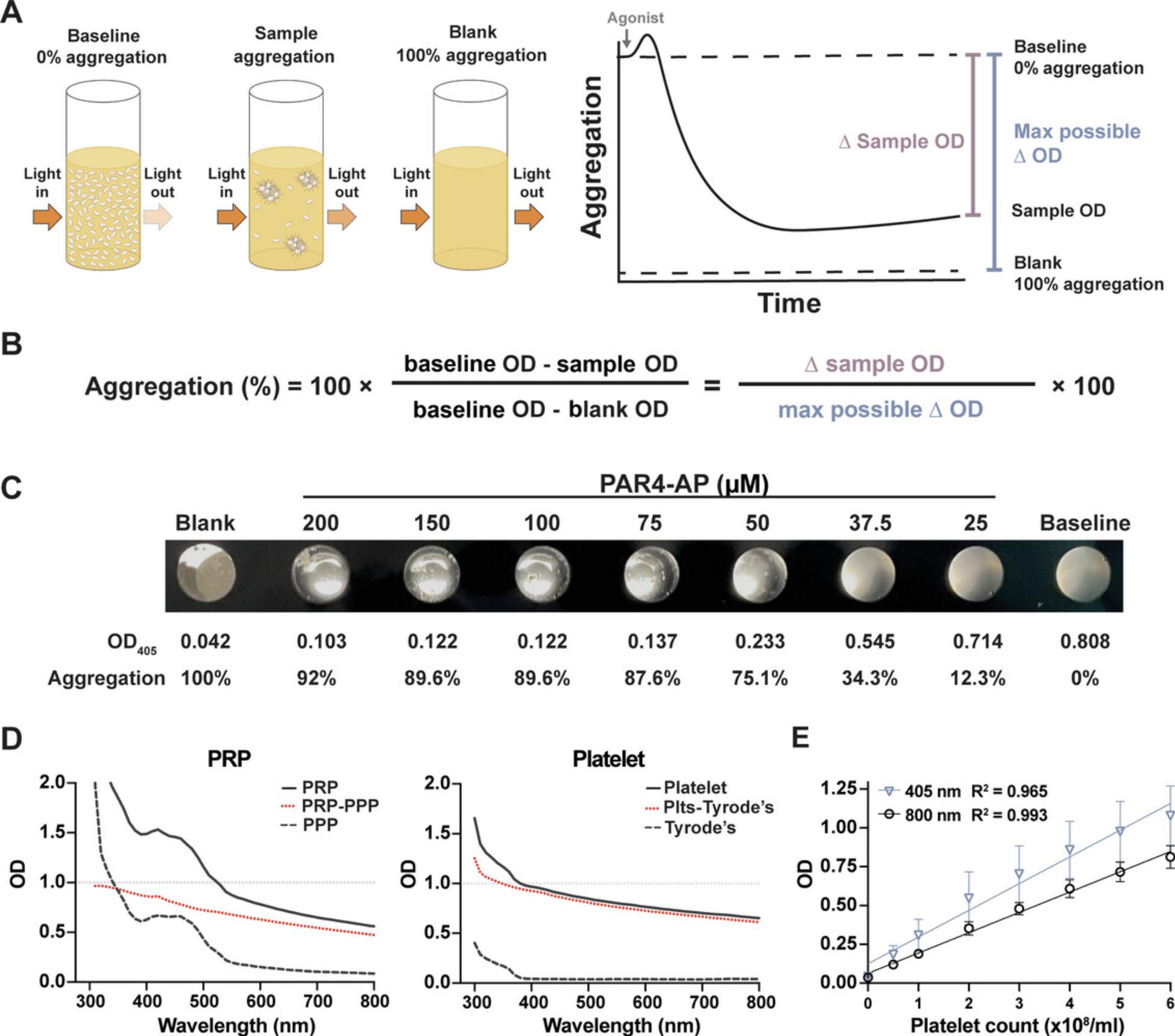
This protocol describes light transmission aggregometry performed in a microtiter plate to assess agonist-dependent platelet–platelet interactions. Briefly, platelets are aliquoted into the appropriate wells of a half-area, clear-bottom 96-well plate. After adding an agonist, the plate is shaken for 5 to 10 min at 37°C, and the absorbance is read on a plate reader. A simple previously published equation can be used to calculate the final percentage of aggregation for each sample (French et al., 2018). A diagram depicting how the aggregation formula was derived is shown in Figure 1A,B. For most assays, platelet activation should be measured in a dynamic, submaximal range of agonist concentrations. See Table 1 for suggested concentrations of common agonists; however, the investigator should optimize agonist concentrations (Fig. 1D). PRP may be substituted for platelets in the protocol, as long as platelet-poor plasma (PPP) is used as the blank rather than Tyrode's buffer and absorbance is measured at 595 nm instead of 405 nm (Fig. 1D). Platelets should be resuspended at 3.0 × 108 platelets/ml. If a hematologic analyzer is unavailable, platelet count can be adjusted based on optical density (OD) at 405 or 800 nm (Fig. 1E). For aggregation assays with PRP, it is unnecessary to adjust the platelet count (Chan et al., 2011; Ho & Chan, 1995; Munnix et al., 2021).
| Agonist | Supplier, cat. no. | Suggested dose response | Receptor | Notes |
|---|---|---|---|---|
| PAR1-AP | GL Biochem, custom order | 0.156, 0.3125, 0.625, 1.25, 2.5, 5, 10, 20 μM | PAR1 | One-letter amino acid peptide sequence: SFLLRN-NH2 |
| PAR4-AP | GL Biochem, custom order | 18.75, 25, 37.5, 50, 75, 100, 150, 200 μM | PAR4 | One-letter amino acid peptide sequence: AYPGKF-NH2 |
| Thrombin, α-thrombin (Factor IIa) | Enzyme Research Laboratories, HT 1002a | 0.156, 0.3125, 0.625, 1.25, 2.5, 5, 10, 20 nM | PAR1, PAR4 | Cleaves fibrinogen to fibrin; use tetrapeptide GPRP to block fibrin cross-linking when using high concentrations of thrombin |
| Collagen, native collagen fibrils (type I) from equine tendons | CHRONO-LOG, 385 | 0.156, 0.3125, 0.625, 1.25, 2.5, 5, 10, 20 μg/ml | GPVI, α2β1 | labile in physiologic solution |
| ADP | Sigma-Aldrich, A2754 | 0.156, 0.3125, 0.625, 1.25, 2.5, 5, 10, 20 μM | P2Y12, P2Y1 | Isolated platelets may be refractory to ADP |
| Epinephrine | Sigma-Aldrich, E4250 | 0.78, 1.56, 3.125, 6.25, 12.5, 25, 50, 100 μM | α2a | Isolated platelets respond poorly to epinephrine |
| AA | Cayman Chemical, 90010 | 7.8, 15.6, 31.25, 62.5, 125, 250, 500, 1000 μM | TPα/β | Use to assess whether donor was on a COX inhibitor |
| Anti-CD9 antibody (clone ALB6) | Beckman Coulter, IM0117 | 0.156, 0.3125, 0.625, 1.25, 2.5, 5, 10, 20 μg/ml | FcγRIIA | Slow kinetics compared with other agonists |
| Fibrinogen | Sigma-Aldrich, F3879 | 100-250 μg/ml | αIIbβ3 | Stimulation of washed platelets with a weak agonist may require the addition of exogenous fibrinogen to see aggregation |
-
AA, arachidonic acid; COX, cyclooxygenase; GPRP, glycine-proline-arginine-proline; GPVI, glycoprotein VI; PAR(1/4)-AP, protease-activated receptor-(1/4)-activating peptide.
Materials
-
Platelet agonist(s) (see Table 1)
-
Platelets or PRP (see Support Protocol)
-
Pharmacologic inhibitors:
- Iloprost (CAS no. 78919-13-8; e.g., Cayman Chemical, cat. no. 18215)
- Cangrelor (CAS no. 163706-36-3; e.g., Cayman Chemical, cat. no. 22086)
-
Tyrode's buffer (see recipe)
-
96-well, conical (V-bottom) polypropylene plate (e.g., USA Scientific, cat. no. 1833-9600)
-
96-well, half-area, clear, flat-bottom plates, not treated/nonsterile (e.g., Corning, cat. no. 3695)
-
10- and 200-μl multichannel pipettes
-
Orbital plate shaker (e.g., Eppendorf ThermoMixer)
-
Plate reader (e.g., BioTek Synergy H1 Hybrid Multi-Mode Reader)
-
Computer running data analysis software (e.g., GraphPad Prism)
1.Prepare working stocks of each agonist in a separate V-bottom 96-well plate such that 10 μl of each working stock can be used to achieve the desired final concentration in a volume of 100 μl.
2.Aliquot 90 μl platelets into the wells of a 96-well, half-area, clear, flat-bottom plate. If applicable, treat platelets with pharmacologic inhibitor or vehicle control before aliquoting them into individual wells.
3.Transfer 10 μl agonist(s) from the 96-well V-bottom plate to wells containing platelets using a multichannel pipette.
4.Shake plate 5 min at 1000 rpm, 37°C.
5.Aliquot 90 μl platelets and 10 μl Tyrode's buffer into an unused well to use as unstimulated resting platelet suspension representing 0% aggregation. Aliquot 100 μl Tyrode's buffer into another well to use as a buffer-only control representing 100% aggregation.
6.Read absorbance of the plate at 405 nm.
7.Calculate percent aggregation:
![Details are in the caption following the image Microtiter plate light-transmission aggregometry with pharmacologic inhibitors. (A) Platelets were stimulated with 0.5 or 5 μg/ml collagen in the presence of increasing concentrations of ibrutinib, a Bruton's tyrosine kinase inhibitor. Data represent mean ± SD. (B) Platelets were incubated with increasing concentrations of a single-chain variable fragment (scFv, clone IV.3) that is known to block the function of FcγRIIA and then stimulated with anti-CD9 antibodies, an FcγRIIA-dependent agonist. Data represent mean ± SD. (C) ADP- and epinephrine-mediated aggregation of platelets treated with cangrelor, a P2Y<sub>12</sub> receptor inhibitor. Concentration–response curves of platelets stimulated with (D) thrombin, (E) collagen, and (F) anti-CD9 antibodies in the presence of 20 nM iloprost, 1 μM cangrelor, or vehicle control (dimethyl sulfoxide [DMSO]); n = 4. Data represent mean ± SD.](https://static.yanyin.tech/literature_test/cpz1668-fig-0003-m.jpg)
Basic Protocol 2: MEASURING DENSE-GRANULE SECRETION IN HIGH-THROUGHPUT MICROPLATE ASSAYS
Following stimulation, platelets initiate several positive feedback mechanisms, including the secretion of dense granules. Dense granules contain small molecules including ADP, ATP, and serotonin that elicit platelet activation. This protocol uses a microtiter luciferin–luciferase bioluminescence assay to measure ATP release from agonist-stimulated platelets as a marker for dense-granule secretion. The method is based on the consumption of extracellular ATP by the enzyme luciferase, which oxidizes its substrate luciferin to oxyluciferin. The decay of oxyluciferin emits visible light that can be quantified by a plate reader (Fig. 4A). The luciferin and luciferase are provided at excess, so the rate-limiting step in luminescence generation is the amount of ATP released. Thus, the luminescence generated is proportional to the amount of ATP secreted by the platelets. Importantly, luminescence is linear when measuring the exogenous addition of ATP to platelets at concentrations tested up to 40 μM (Fig. 4B).
![Details are in the caption following the image Measuring dense-granule secretion in high-throughput microplate assays. (A) Graphical illustration depicting dense-granule secretion from stimulated platelets. The presence of a luciferin/luciferase mixture can convert the extracellular ATP released from dense granules into light via a chemical reaction. (B) The luminescence (relative luminescence units [RLU]) generated by the addition of exogenous ATP (0.078 to 40 μM) to platelets incubated with CHRONO-LUME is linear (n = 4). Data represent mean ± SD. (C) ATP secretion from platelets stimulated with increasing concentrations of collagen (0.078 to 20 μg/ml) was measured at various gains on a multimode plate reader. The RLU increases as the gain increases, but the percent ATP secretion measured relative to the maximum RLU for each gain remains constant. (D) Platelets were stimulated with a range of thrombin or collagen concentrations, and dense-granule secretion was measured as a single-endpoint read. (E) Lumiaggregation, in which parallel aggregation and luminescence readings are taken on the same well, was performed with protease-activated receptor-4-activating peptide (PAR4-AP)-stimulated platelets treated with 4 mM arginine-glycine-aspartate-tryptophan (RGDW), 20 nM iloprost, 100 μM aspirin, or vehicle control (Ctrl; 0.1% ethanol). (F) Six technical replicates were performed with 50 μM PAR4-AP to determine the reproducibility of real-time ATP secretion measurements. (G) Real-time measurement of ATP secretion following PAR4-AP stimulation of platelets. Data can be presented as representative tracings plus maximum secretion or composite tracings (n = 3). Data represent mean ± SD. (H) Real-time ATP secretion monitored in whole blood stimulated with anti-CD9 antibodies in the presence of 20 nM iloprost (ilo; n = 3), 1 μM cangrelor (can; n = 6), or vehicle control (Ctrl; n = 6). Data represent mean ± SD.](https://static.yanyin.tech/literature_test/cpz1668-fig-0004-m.jpg)
Most modern plate readers allow users to adjust the gain of luminescence. The relative luminescence units (RLU) of a sample increases as the gain increases, but the percent change compared with maximum release is constant (Fig. 4C). This assay can be performed as either a real-time or static-endpoint assay with aggregation (lumiaggregometry; Fig. 4D-G). Platelets incubated with CHRONO-LUME can be stimulated and shaken for 5 min, and a single luminescence read can be performed (Fig. 4D). As noted above, lumiaggregometry can be performed in a 96-well plate simply by adding 1 vol. CHRONO-LUME to 24 vol. platelets before aliquoting platelets into individual wells (i.e., 40 μl CHRONO-LUME to 960 μl platelets, then 90 μl mixture to each well; Fig. 4E). Then follow the same protocol for 96-well plate aggregation in Basic Protocol 1, but measure both absorbance and luminescence at the end of plate shaking. The high sensitivity of luminescence detection can also be used to measure dense-granule secretion in whole blood or PRP, which limits preanalytical procedures and allows testing dense-granule secretion under more physiologic conditions (Sun, Tandon, Yamamoto, Yoshitake, & Kambayashi, 2001; Fig. 4H).
Materials
-
Platelet agonists (see Table 1)
-
CHRONO-LUME reagent (e.g., CHRONO-LOG, cat. no. 395)
-
Platelets (see Support Protocol)
-
96-well, conical (V-bottom) polypropylene plate (e.g., USA Scientific, cat. no. 1833-9600)
-
96-well, half-area, white- or black-walled, clear, flat-bottom plate (e.g., Corning, cat. nos. 3883 or 3880, respectively)
-
Plate reader (e.g., BioTek Synergy H1 Hybrid Multi-Mode Reader)
-
10- and 200-μl multichannel pipettes
-
Orbital plate shaker (e.g., Eppendorf ThermoMixer)
1.Prepare working stocks of each agonist in a separate V-bottom 96-well plate such that 5 μl of each working stock can be used to achieve the desired final concentration in a final volume of 50 μl.
2.Add 2 μl CHRONO-LUME (a luciferin/luciferase mixture) to 43 μl platelets, and incubate 5 min in the wells of a 96-well, half-area, white- or black-walled, clear, flat-bottom plate.
3.Program a kinetic read on a multimode microplate reader to perform steps 4 to 7.
4.Read baseline luminescence before agonist stimulation.
5.Eject plate and transfer 5 μl agonist to platelets from V-bottom 96-well plate using a multichannel pipette.
6.Read plate luminescence after agonist stimulation.
7.Shake plate continuously 5 min at 800 rpm while reading luminescence every 30 s.
8.Report data as RLU.
Basic Protocol 3: MICROTITER PLATE–BASED CALCIUM MOBILIZATION
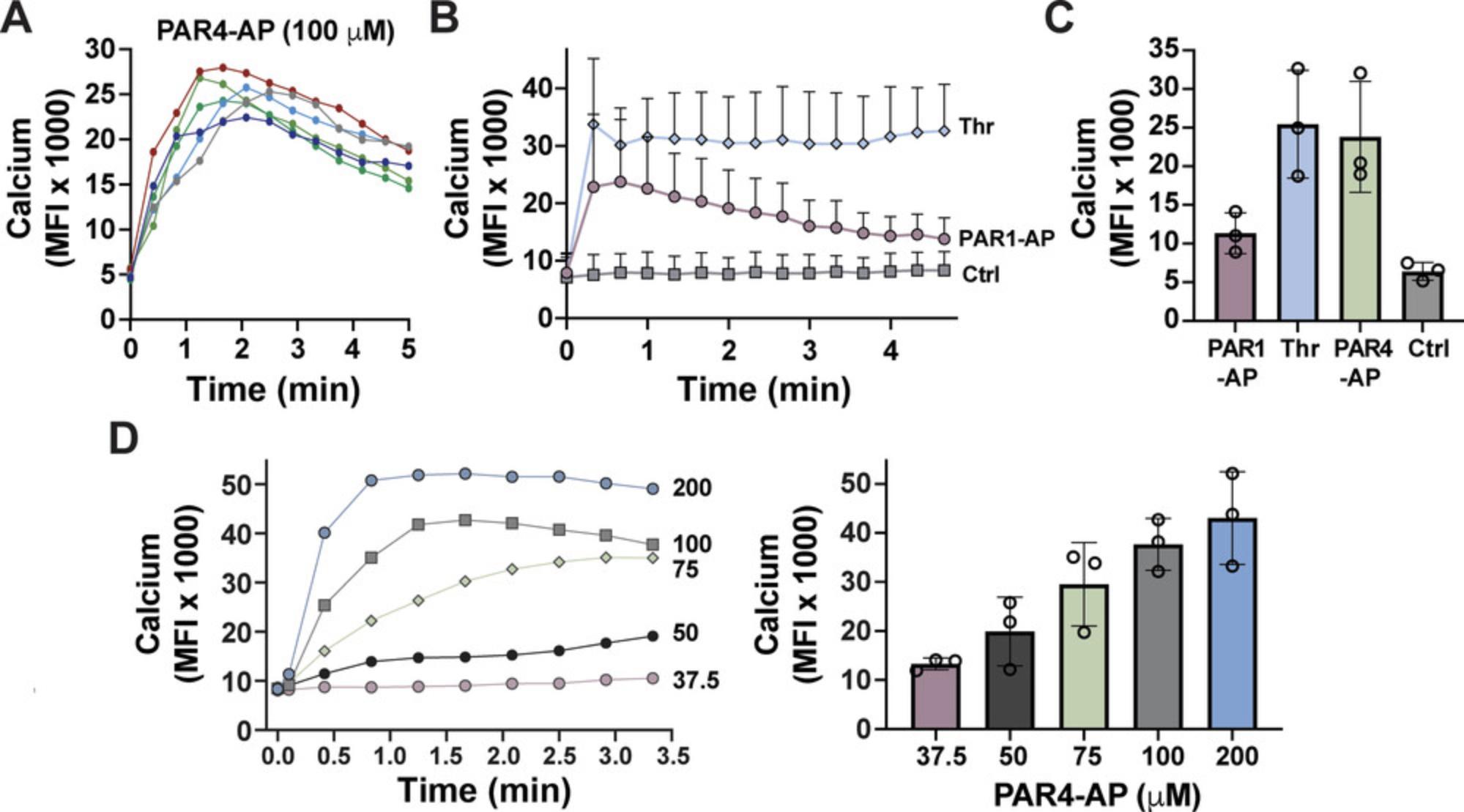
The rise in intracellular Ca2+ is an early second messenger that is common and essential to most platelet functions, including platelet aggregation and granule secretion. Upon activation of platelets, basal cytosolic Ca2+ levels go from ∼100 nM to low micromolar levels depending on the type and amount of agonist used. This protocol describes the use of a nonratiometric, fluorescent Ca2+-sensitive dye, Fluo-4 AM, to measure rises in intracellular Ca2+ following agonist stimulation. Ca2+ mobilization is transient following stimulation with many agonists; therefore, it is best to measure changes in Ca2+ mobilization in real time (Fig. 5A-C). However, for drug screens, Ca2+ mobilization can be measured as a single endpoint by simply adding 1 µl Fluo-4 AM per 499 µl platelets and performing Basic Protocol 1.
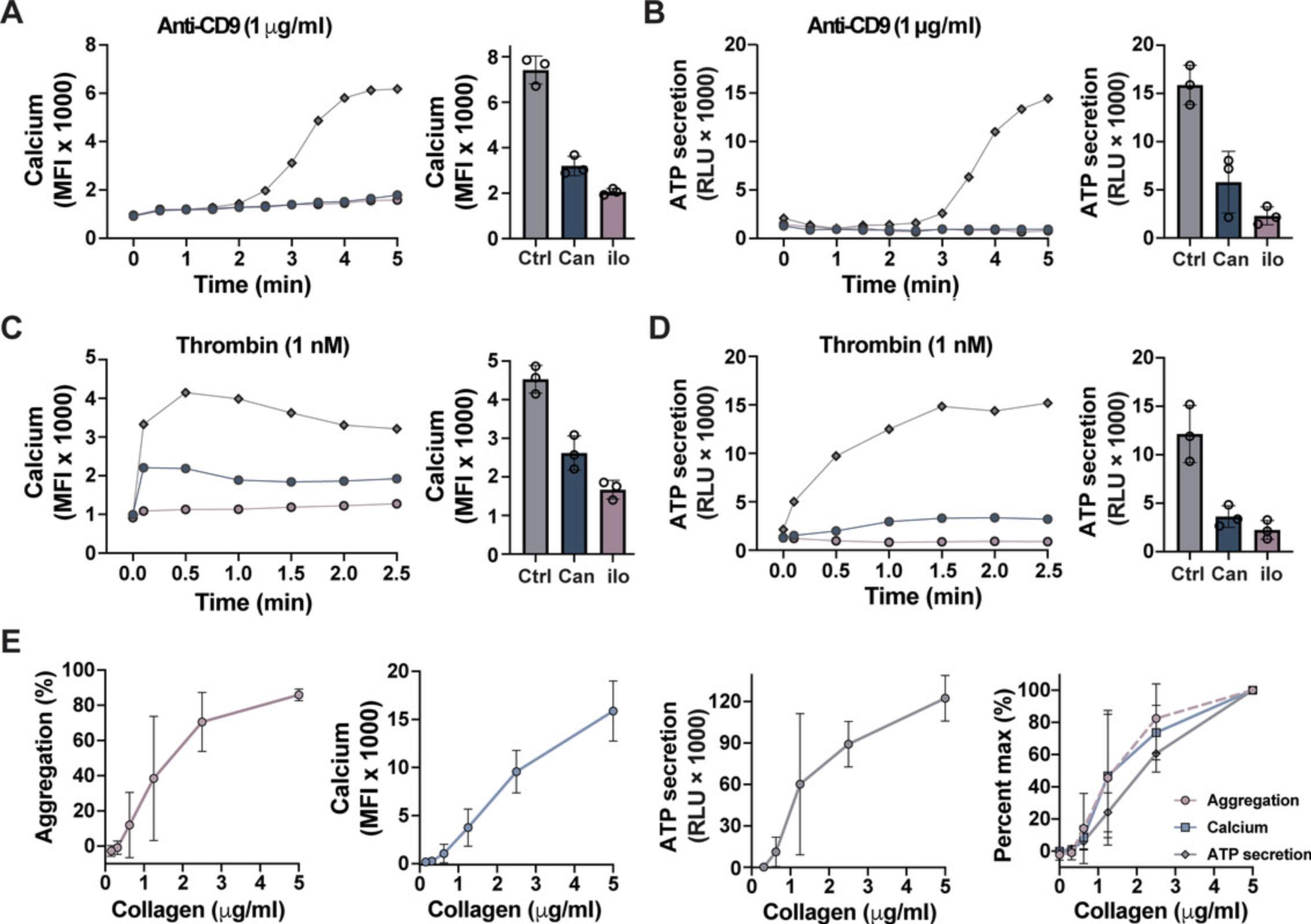
Additionally, Ca2+ can be multiplexed with dense-granule secretion for real-time measurements (Fig. 6A-D) or as an endpoint assay with aggregation and dense-granule secretion (Fig. 6E).
Materials
-
Platelet agonists (Table 1)
-
1 μg/ml Fluo-4 AM (e.g., Invitrogen, cat. no. F14201) in dimethyl sulfoxide (DMSO)
-
Platelets (see Support Protocol)
-
1 M CaCl2 (e.g., Invitrogen, cat. no. F14201)
-
96-well, conical (V-bottom) polypropylene plate (e.g., USA Scientific, cat. no. 1833-9600)
-
37°C incubator
-
96-well, half-area, black-walled, clear, flat-bottom plate (e.g., Corning, cat. no. 3880)
-
Plate reader (e.g., BioTek Synergy H1 Hybrid Multi-Mode Reader )
-
10- and 200-μl multichannel pipettes
-
Orbital plate shaker (e.g., Eppendorf ThermoMixer)
1.Prepare working stocks of each agonist in a separate V-bottom 96-well plate such that 5 μl of each working stock can be used to achieve the desired final concentration in a final volume of 50 μl.
2.Add 1 μl of 1 μg/ml Fluo-4 AM (a fluorescent Ca2+-sensitive dye) to 498 μl platelets, and incubate 10 min at 37°C. Immediately before aliquoting platelets, add 1 μl of 1 M CaCl2 (2 mM final).
3.Aliquot 45 μl Fluo-4 AM–treated platelets into the wells of a 96-well, half-area, black-walled, clear, flat-bottom plate.
4.Program a kinetic read on a multimode plate reader to perform steps 5 to 7.
5.Read baseline fluorescence (494 nm excitation/506 nm emission) before agonist stimulation.
6.Eject plate and transfer 5 μl agonist to Fluo-4 AM–treated platelets from V-bottom 96-well plate using a multichannel pipette.
7.Read plate fluorescence after agonist stimulation.
8.Shake plate continuously 4 min at 800 rpm while reading fluorescence every 20 s.
9.Report data as mean fluorescence intensity.
Support Protocol: PLATELET ISOLATION AND ENUMERATION
This protocol provides a detailed procedure for the isolation of PRP or platelets from human whole blood. PRP and platelets can be separated from other cellular components of blood by centrifugation.
Materials
-
10× ACD (see recipe)
-
50 U/ml apyrase (e.g., Sigma-Aldrich, cat. no. A6410)
-
50 μg/ml prostaglandin E1 (PGE1; CAS 745-65-3; e.g., Cayman Chemical, cat. no. 13010)
-
Tyrode's buffer (see recipe)
-
21-G needle
-
5-ml sodium citrate or ACD vacutainer (e.g., BD, cat. nos. 363083 or 364606, respectively)
-
Benchtop centrifuge (e.g., Eppendorf 5810)
-
15- and 50-ml polypropylene conical tubes
-
Transfer pipette (e.g., Thermo Fisher Scientific, cat. no. 13-711-7M)
-
Automated hematology analyzer (e.g., Drew Scientific, Hemavet 950FS)
-
Microtiter plate
-
Plate reader (e.g., BioTek Synergy H1 Hybrid Multi-Mode Reader )
-
Orbital plate shaker (e.g., Eppendorf ThermoMixer)
Prepare platelet-rich plasma
1.Draw up to 50 ml blood from the antecubital vein of a consenting donor with a 21-G needle into 5-ml sodium citrate or ACD vacutainers.
2.Centrifuge citrated whole blood in vacutainers 10 min at 200 × g , room temperature, with low acceleration set to “2” and brake set to “0” (off) if using an Eppendorf 5810.
3.Transfer PRP to a clean 15- or 50-ml polypropylene conical tube with a plastic transfer pipette, being careful not to disturb the buffy coat or red blood cell layers.
4.Centrifuge remaining whole blood in vacutainers 10 min at 2000 × g , room temperature, with acceleration set to “9” and brake on to obtain PPP. Transfer PPP to a clean polypropylene conical tube, being careful not to disturb the buffy coat and red blood cell layers.
Isolate platelets from platelet-rich plasma
5.Add 1 vol. of 10× ACD to 9 vol. PRP, and then add apyrase to a final concentration of 0.02 U/ml and/or PGE1 to 50 ng/ml. Mix gently by inverting three times.
6.Centrifuge PRP 10 min at 2000 × g , room temperature, with acceleration set to “2” and brake set to “2.”
7.Remove PPP supernatant, and gently resuspend platelet pellet(s) in Tyrode's buffer.
8.Count platelets using a hematology analyzer.
9.Test platelets for amount of spontaneous, agonist-independent aggregation to examine quality of platelet preparation: Aliquot 90 μl platelets and 10 μl Tyrode's buffer into the wells of microtiter plate; perform in duplicate. Aliquot 100 μl Tyrode's buffer into a well as a blank to measure maximal light transmittance.
10.Read OD of wells at 405 nm on a plate reader.
11.Shake plate 10 min at 1000 rpm, 37°C.
12.Reread OD of wells at 405 nm on a plate reader.
13.Calculate percent spontaneous aggregation.
REAGENTS AND SOLUTIONS
ACD, 10×
- 2.5% (w/v) sodium citrate tribasic
- 1.5% (w/v) citric acid
- 2.0% (w/v) D-glucose
- Store at room temperature for up to 6 months
- Before use, sterilize using a 0.22-μm filter (e.g., Sigma-Aldrich, cat. no. S2GPU10RE)
Use deionized distilled water to prepare solution.
Tyrode's buffer
- 10 mM HEPES
- 11.9 mM NaHCO3
- 127.2 mM NaCl
- 5.0 mM KCl
- 0.4 mM NaH2PO4
- 1.0 mM MgCl2·6H2O
- 5.0 mM D-glucose
- 0.35% (w/v) BSA (e.g., Thermo Fisher Scientific, cat. no. BP9706)
- Adjust pH to 7.4 using 1 M NaOH
- Store at 4°C for up to 3 months or at room temperature for up to 1 week
- Before use, sterilize using a 0.22-μm filter (e.g., Sigma-Aldrich, cat. no. S2GPU10RE)
Use deionized distilled water to prepare solution.
Bring to room temperature before use.
COMMENTARY
Background Information
The formation of platelet-rich clots at the site of vascular damage in humans has been recognized since the late 1800s (Mannuß, 2020). However, without the ability to methodically study platelet activation in vitro , the molecular and physiologic mechanism underlying the accumulation of platelets at the site of vascular injury remained unknown. In the 1960s, Gustav Born developed a method to quantify platelet reactivity in vitro after determining that light transmittance was proportional to the number of platelets in suspension, now commonly referred to as aggregometry (Born, 1962; Born & Cross, 1963). The aggregometer was a significant breakthrough clinically for diagnosing individuals with platelet disorders and was instrumental in developing antiplatelet therapies.
Furthermore, the advent of aggregometry, coupled with later advances in flow cytometry and microfluidic devices, revolutionized the study of platelet function leading to the discovery of crucial platelet agonists, their cognate surface receptors, and the signaling pathways they elicit. Although hemostasis and thrombosis are not identical processes, a similar sequence of events occurs in both. The formation of a platelet-rich hemostatic plug or pathogenic thrombus can be divided into two separate but overlapping stages: (1) platelet–matrix adhesion, including tethering, activating, and spreading platelets on subendothelial extracellular matrix components predominantly mediated by adhesion receptors, and (2) platelet–platelet interaction, including cohesion, typically referred to as platelet aggregation and stabilization. Both platelet aggregation and stabilization are driven by the interaction of integrin αIIbβ3 with fibrinogen or another multivalent ligand and reinforced by platelet-derived soluble agonists, including dense-granule secretion (reviewed in depth: Tourdot & Holinstat, 2017; van der Meijden & Heemskerk, 2019; Versteeg, Heemskerk, Levi, & Reitsma, 2013).
Platelet-rich plugs grow in a well-defined hierarchal structure owing to the distribution and concentration of agonist (Stalker et al., 2013). Although platelet adhesion receptors interact with numerous subendothelial extracellular matrix components following vascular injury, collagen (a glycoprotein VI and integrin α2β1 agonist) is often used for in vitro platelet activation due to its potency compared with other matrix components. The most potent soluble platelet agonist is thrombin, a serine protease activated as part of the coagulation cascade, which is restricted to the site of vascular injury and results in a core of highly activated, densely packed platelets. Around the core is an outer shell of loosely packed platelets, which protrudes into the intravascular space and is chiefly driven by the soluble platelet-derived mediators including dense-granule secretion of small molecules like ADP and formation of thromboxane A2 (Stalker et al., 2013; Tourdot & Holinstat, 2017; van der Meijden & Heemskerk, 2019; Versteeg et al., 2013). The selection of agonists for in vitro assays is usually done based on their physiologic role in the formation of hemostatic plug or to test specific intracellular signaling pathways. Usually, both immunoreceptor tyrosine-based activation motif (ITAM)-containing receptor and G protein–coupled receptor (GPCR) agonists are selected. When studying a novel pharmacologic inhibitor or the role of a protein in platelets, a panel of agonists responsible for each of these processes can be used to characterize which signal transduction pathways are altered.
The primary limitation to conventional platelet functional assays is the need for specialized equipment, such as an aggregometer. Additionally, these assays are often time consuming and labor intensive due to their low throughput. To address these limitations, microtiter plate functional assays were developed (Fratantoni & Poindexter, 1990). Microtiter plate assays retain their ability to detect changes in platelet reactivity in the presence of antiplatelet therapies and are being validated as clinical diagnostic tools (Chan et al., 2011; Chan et al., 2018; Hsu et al., 2022). Further platelet functional tests have been adapted to microtiter plate assays, including dense-granule secretion and Ca2+ mobilization. For library drug screens, ultrahigh-throughput versions of these protocols that use 384- or 1536-well plates have been developed (Fernández et al., 2022; Martins Lima et al., 2019). The assays presented in this article have numerous applications in platelet research and drug discovery. These protocols are highly adaptable, and either real-time or endpoint data measurements can be collected, allowing a cross-comparison of a large number of agonists with multiple functional readings.
Critical Parameters
The preanalytical variables, including collection, processing, and storage of samples before running the functional assay, are sensitive to user manipulation and are the most critical factor to optimize in order to achieve robust results (Tiffany & Henry, 1983). Different isolation protocols can significantly change the responsiveness of platelets to an agonist. In an extreme example, the release of ADP from platelet dense granules during isolation can cause the desensitization of ADP receptors resulting in platelets that are refractory to ADP stimulation, whereas other preparations have robust responses to ADP (Cazenave et al., 2004). Based on an international survey of experts, there is a general, but not universal, consensus on the best practices for preanalytical processing of samples before platelet functional tests (Cattaneo et al., 2009; Cattaneo et al., 2013). The lack of standardization in laboratory practices can in part be attributed to the inability to decipher what is an artifactual platelet response and what is true platelet reactivity. For example, one study reported that platelets stored at ambient temperature have an increase in reactivity compared with platelets stored at 37°C (Mindukshev et al., 2022), whereas another study attributed the increased reactivity of platelets stored at ambient temperature to being an artifact caused by preactivation during storage (Maurer-Spurej et al., 2001). Standardization has been further hampered by conflicting studies on key variables that have been discussed in depth by others (Cattaneo et al., 2009, 2013; Cazenave et al., 2004; Hechler, Dupuis, Mangin, & Gachet, 2019; Jarvis, 2004). Briefly, the following parameters are often contested including: (1) choice of the anticoagulant, (2) blood collection method (vacutainers, syringe, drip), (3) storage temperature (ambient or 37°C; Mindukshev et al., 2022), (4) components of Tyrode's buffer (BSA, divalent cations, apyrase), and (5) platelet concentration (Tiffany & Henry, 1983).
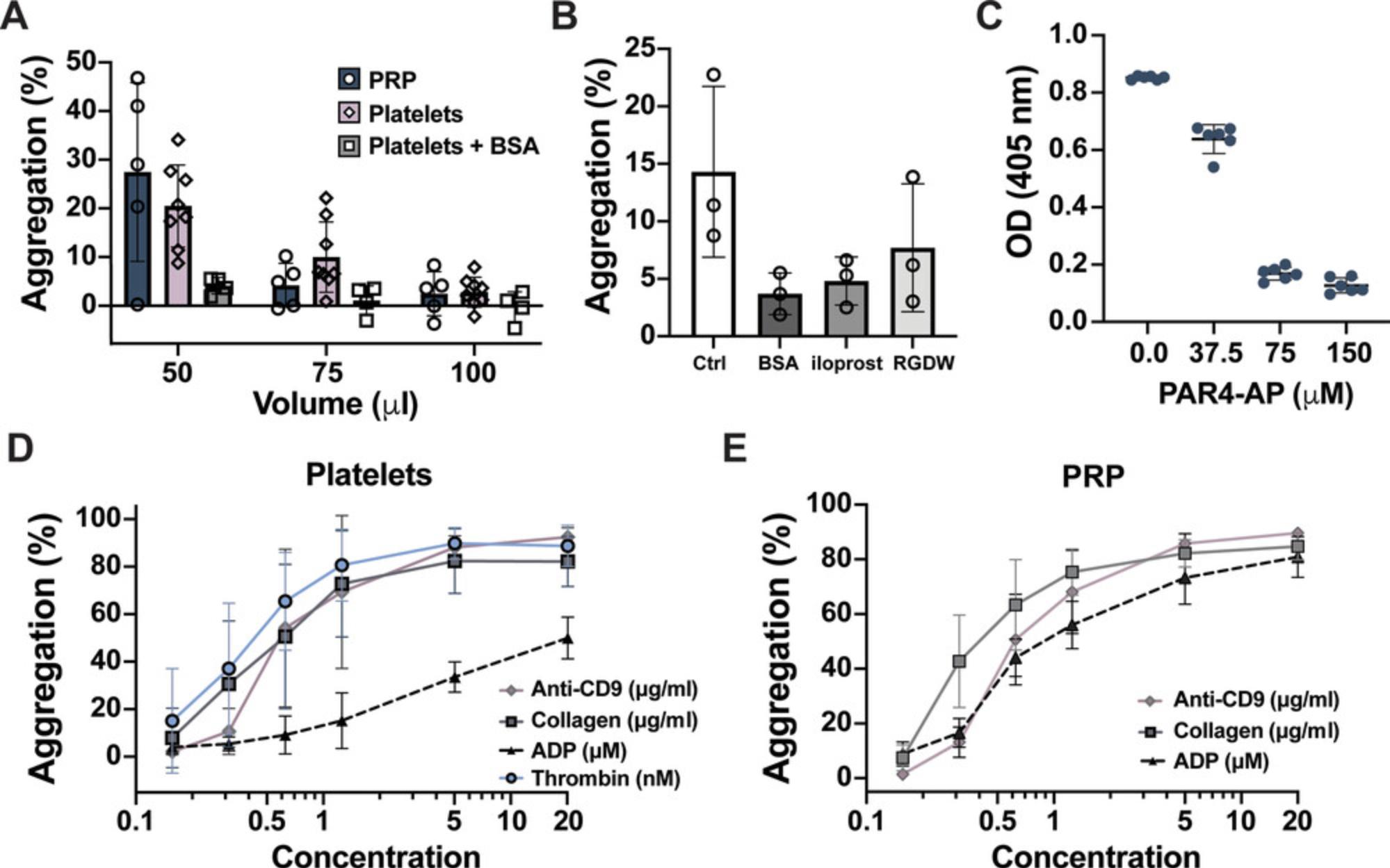
The blood collection and sample handling techniques highlighted in the Support Protocol and the Strategic Planning section have been optimized to provide quiescent platelets at baseline that robustly respond to most agonists and can be inhibited by known pharmacologic antagonists. However, the response of platelets prepared this way to ADP is much less responsive than ADP stimulation of PRP (Fig. 2D,E). Furthermore, the endorsement of this protocol does not invalidate the use of other protocols. Researchers should be conscious of the advantages and limitations that exist for isolation protocols and strive to maintain consistency and provide detailed methodologies on platelet processing in their publications.
The use of either platelets or PRP is suitable for most of the assays described in this article. Certain advantages and disadvantages exist for using either platelets or PRP. Platelet isolation requires more preanalytical handling than PRP; therefore, washed platelets take longer to acquire and are more susceptible to user manipulation. However, because platelets are resuspended in a buffered medium, they are stable for longer than PRP. PRP requires more assay reagents, including Fluo-4 AM and CHRONO-LUME, than platelets. Furthermore, pharmacologic inhibitors are often bound by plasma proteins, lowering the effective free concentration of antagonist available to bind to platelets. Hence, the concentration of an inhibitor used in PRP is a good approximation for the amount of inhibitor required in whole blood. However, if the assays are being performed with a rare or expensive reagent, the use of isolated platelets might be better to test its functions. A multivalent adhesive ligand capable of crosslinking αIIbβ3 on adjacent platelets, predominantly fibrinogen, in the medium is required for platelet aggregation. Plasma contains 2 to 4 mg/ml fibrinogen to support aggregation mediated in PRP. With washed platelets, enough fibrinogen is released from platelet α-granules upon stimulation to support aggregation with most agonists. However, weak agonists (ADP, serotonin, epinephrine) that activate αIIbβ3 without triggering α-granule secretion will not initiate aggregation without the supplementation of exogenous fibrinogen (100 to 250 μg/ml; see Table 1). α-thrombin is part of the coagulation cascade responsible for converting fibrinogen to fibrin that also causes platelet activation through PARs. The crosslinking of fibrin, physiologically important for clot stabilization, can facilitate the incorporation of unactivated platelets into clumps, which cannot be distinguished from aggregation by OD and thus may give an artificially higher read than otherwise expected. A small peptide, glycine-proline-arginine-proline (GPRP; 1 to 5 mM) can inhibit fibrin crosslinking (Michelson, 1994). Thus, the use of GPRP is recommended with higher concentrations of α-thrombin with platelets but is a requisite to use α-thrombin in PRP.
It is essential to use the appropriate concentration of antagonist and agonist to achieve robust results. Interindividual differences have been observed in platelet activation to threshold concentrations of all agonists (Yee, Sun, Bergeron, Dong, & Bray, 2005). Therefore, rather than using a single agonist dose, a concentration–response curve with a range of agonists is recommended to provide broad phenotype of platelet reactivity under the conditions being tested. Once diluted in a physiologic buffer, some agonists such as collagen are labile, and a noticeable decline in platelet activation is observed after 30 min. As seen in Figure 3A, higher concentrations of agonists require increased amounts of antagonist to achieve inhibition, and a high enough concentration of agonist may bypass the need for a particular protein to achieve full aggregation. Therefore, pharmacologic inhibitors should be tested with submaximal agonist concentrations. Furthermore, pharmacologic inhibitors often have off-target effects at higher concentrations; thus, we recommend performing a dose–response assay of antagonist and using the lowest concentration of inhibitor that caused maximum inhibition (see examples in Fig. 3).
Additionally, a number of factors will influence the extent of aggregation; for example increasing shaking time or speed will enhance the amount of aggregation seen for a given agonist concentration. We have obtained similar lumiaggregation data using a Thermo Scientific LP Vortex Mixer and an Eppendorf ThermoMixer FP. However, since different shakers may have different orbital diameters, mixing patterns, or actual shaking speeds, concentration–response curves should be established for each orbital plate shaker used. Real-time kinetics of platelet aggregation in a 96-well plate reader are often slow and require more agonist for the same effect due to the decrease in shaking speeds in most plate readers and the stoppage time required to read the plate (Vinholt et al., 2017). Therefore, although real-time aggregation data can be collected in a plate reader for most experiments, the added kinetic data are not impactful.
Platelet counts exists in a linear relationship with ODs in the microtiter wells (Fig. 1E). Beside platelet concentration, the OD of a given sample is dependent on the shape, size, and granularity of the platelets, which may all contribute to overall changes in OD observed following addition of agonist. Aggregation monitors bulk changes in platelet aggregation; one important limitation is its inability to detect small or microaggregates. Another important reminder is that OD is on a log scale; therefore, an OD of 1 corresponds to 10% light transmittance, whereas an OD of 2 corresponds to just 1% light transmittance. Therefore, to obtain robust data, OD readings under 1 are preferred for aggregation.
Troubleshooting
To help address the most commonly encountered problems associated with these protocols, the main troubleshooting strategies are discussed in Table 2.
| Problem | Possible cause | Solution |
|---|---|---|
| Platelet pellet will not resuspend | Not enough ACD, PGE1, or apyrase used during centrifugation of PRP | Ensure appropriate amount of ACD, PGE1, and apyrase were used |
| Spontaneous activation of washed platelet | Inappropriate sample processing or nonspecific platelet interaction with 96-well plate surface | Coat 96-well plate with BSA to prevent surface activation |
| Spontaneous activation of PRP | Inappropriate blood draw or sample processing | Review best practices for blood draw and handling in the Strategic Planning section |
| No platelet activation to AA | Donor took COX inhibitor (aspirin, NSAID) within the last 7 days | Do not use sample as results will be invalid; during donor recruitment, remind donors to not take aspirin at time of blood collection, and confirm donors have not taken a COX inhibitor |
| >100% aggregation | PPP or Tyrode's blank may be contaminated | Respin PPP to remove any potential cellular contaminants |
| Inconsistent OD reads | Air bubbles in well | Dip a pipette tip in alcohol (ethanol, methanol, or isopropanol), and then touch bubbles |
| Unstable Flou-4 AM baseline in unstimulated platelets | Fluo-4 AM concentration may be too high | Decrease amount of Fluo-4 AM used |
- AA, arachidonic acid; ACD, acid citrate dextrose; BSA, bovine serum albumin; COX, cyclooxygenase; NSAID, nonsteroidal anti-inflammatory drug; OD, optical density; PGE1, prostaglandin E1; PPP, platelet-poor plasma; PRP, platelet-rich plasma.
Understanding Results
Techniques capable of monitoring platelet reactivity in vitro are invaluable to better understand the molecular mechanism regulating platelet activation and the physiologic processes underlying hemostatic plug or clot formation. The assays in these protocols are primarily designed to evaluate pharmacologic agents known or hypothesized to inhibit platelet activation. To this end, we recommend using multiple agonists that signal through GPCRs and ITAM-containing receptors. The pathways leading from Ca2+ mobilization to aggregation or dense-granule secretion have redundant and distinct signaling components; therefore, these three assays were selected to help broadly characterize pharmacologic inhibitors. Importantly, there is positive predictive value in using platelet functional assays to identify novel pharmaceutical antiplatelet therapies, which makes these assays a great tool to screen pharmacologic libraries. However, caution should be used in directly extrapolating the findings of in vitro experiments to the role a drug may have on physiologic hemostatic plug or thrombus formation. Complementary studies, including ex vivo whole-blood microfluidic and in vivo mouse models, should be performed with any novel inhibitors identified by these techniques (May & Heptinstall, 2004). Lastly, the assays are readily adaptable to address other experimental questions. After data collection, the plate can be stored to perform additional biochemical assays with the samples, including western blot or ELISA.
There are limitations to conventional and 96-well-based platelet aggregation. Due to the feed-forward amplification mechanisms inherent to platelets, once a threshold level of activation has been reached with most agonists, roughly >30% aggregation will proceed to full >70% aggregation. Therefore, for most agonists, aggregation is typically a binary response of either low aggregation (<30%) or full aggregation. In composite data, at threshold concentrations of agonists, a typical dose response more often reflects the proportion of responders and nonresponders. Using single-endpoint reads to monitor final aggregation allows the simultaneous evaluation of multiple platelet signaling pathways (Fig. 2D,E). However, with some weak agonists such as ADP, platelets can aggregate to >30% and then disaggregate back to near baseline levels. Therefore, the final aggregation may not reflect the achieved maximal aggregation. In conventional aggregometry, immediately following the addition of most agonists, there is a paradoxical increase in OD that has often been attributed to platelet shape change but may also reflect the formation of small platelet aggregates (Maurer-Spurej & Devine, 2001).
A dose response to assess an agonist's dynamic, submaximal activation concentrations is recommended to detect inhibition of platelet activation. For most applications, a dose response starting with a concentration that results in <20% aggregation should be performed with an additional three doses in the linear or submaximal response and two to four potent agonist concentrations above the concentration known to cause the maximal response. To determine the potency of investigational new drugs, we recommend comparing them to known antiplatelet therapies under similar conditions. In these studies, we used 20 nM iloprost (a potent platelet inhibitor), cangrelor (a medium potency P2Y12 receptor inhibitor), and aspirin (a COX inhibitor with mild inhibitory effects) in the 96-well plate format (Figs. 3 and 4E). Vehicle controls are always required when studying inhibitors to ensure they do not have inhibitor activity. Ideally, DMSO and ethanol should be kept to ≤0.1%.
The luminescence generated by platelets treated with CHRONO-LUME is proportional to the amount of ATP released from dense granules (Fig. 4B). The amount of luminescence is typically reported as maximum RLU since the luminescence generated by each plate reader experiment can vary. Interestingly, differences in ATP secretion can be observed between higher agonist concentrations with the same amount of aggregation. Similar to aggregation, there is interdonor variability in granule secretion. Therefore, to reduce variability between donors, background levels of luminescence can be subtracted and data reported as fold-change relative to control, or ATP can be quantified as nmol per 1 × 108 platelets by using an ATP standard curve. Furthermore, we have used microtiter-based ATP secretion in whole blood to help screen for the appropriate amount of inhibitor in whole-blood microfluidic devices. Since other cellular components of blood are present, it is essential to note that even if platelet-specific agonists are used, other cellular sources may contribute to the levels of released ATP.
A hierarchy of platelet functions depends on the concentration of intracellular Ca2+, with increasing threshold levels of Ca2+ inducing different platelet functions, including shape change, integrin activation, secretion, and procoagulant activity (Versteeg et al., 2013). Using nonratiometric fluorescent dyes such as Fluo-4 AM, Ca2+ mobilization is typically reported as maximum fluorescence intensity.
Ratiometric Ca2+-sensitive dyes such as FURA-2 AM can be used for absolute quantification of Ca2+. The baseline fluorescence intensity levels are dependent on the time and temperature used to incubate Fluo-4 AM with platelets. Different types or concentrations of agonists can elicit transient (PAR1-AP) or prolonged (thrombin) Ca2+ mobilization (Fig. 5B). This is important because some agonists like PAR1-AP are unsuitable for measuring Ca2+ mobilization as an endpoint read (Fig. 5C). It should be noted that for most agonists, maximum Ca2+ mobilization will not be captured, and the assays are probably best used to detect broad changes in Ca2+ mobilization. Even in real-time assays, the peak Ca2+ release for thrombin occurs in seconds, especially at high concentrations, and the maximum may be missed because of the time it takes from the addition of agonist until the first read. Maximum Ca2+ mobilization occurs typically within the 5 min following agonist stimulation. However, there is a lag time from adding some agonists, such as anti-CD9 antibodies or collagen, before a response commences. When performing Ca2+ mobilization assays with these agonists, peak Ca2+ release is more easily observed.
Time Considerations
Preparation of platelets from whole blood takes ∼50 min. Consenting and collecting whole blood from donors typically takes 15 min or less. The centrifugation step to obtain PRP from whole blood takes about 20 min, and isolating platelets from PRP takes an additional 15 min to pellet and resuspend the platelets. Preparation of platelets takes a total hands-on time of about 10 min. Following resuspension, platelets should be allowed to rest for 30 min to recover. To save time during the recovery step, count platelets and, if necessary, begin treatment with antiplatelet agents. If you are new to preparing platelets, you should test platelets for spontaneous activation before preparing antagonists or agonists. However, if you are confident in your platelet isolation ability, then preparing reagents needed for experiments while platelets are resting can save time. Preparing reagents, inhibitors, or agonists can take 15 to 20 min. Serial dilutions of agonists are typically prepared in a 96-well plate and transferred to platelets with a multichannel pipette.
Once you have platelets or PRP, each experiment can take an additional 15 to 30 min to perform, depending on the number of samples and type of experiment being performed. For example, lumiaggregometry performed with an inhibitor would take 5 to 10 min for antagonist treatment, 5 to 10 min to add agonist and stimulate platelets, and 5 min to set up and read the plate. Therefore, the total time to isolate platelets and perform lumiaggregation will take ∼2 hr. Real-time dense-granule secretion and Ca2+ mobilization assays have lower throughput for some agonists and may take ∼20 min per run with 5 to 10 min to treat platelets with antagonists and 5 to 10 min to measure platelet activation.
Acknowledgments
This work was funded in part through the National Institutes of Health grant R00 HL136784 (BET), National Institute of Diabetes and Digestive and Kidney Diseases Cooperative Centers of Excellence in Hematology (U54DK126108), and Trustee Grant from Cincinnati Children's Hospital (BET).
Author Contributions
Hem Kumar Tamang : methodology, visualization, writing–original draft, writing–review and editing; Emily N. Stringham : formal analysis, investigation, writing–original draft, writing–review and editing; Benjamin E. Tourdot : data curation, formal analysis, funding acquisition, investigation, methodology, supervision, validation, visualization, writing–original draft, writing–review & editing.
Conflict of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data are available from corresponding author upon reasonable request.
Literature Cited
- Armstrong, P. C., Dhanji, A.-R., Truss, N. J., Zain, Z. N. M., Tucker, A. T., Mitchell, J. A., & Warner, T. D. (2009). Utility of 96-well plate aggregometry and measurement of thrombi adhesion to determine aspirin and clopidogrel effectiveness. Thrombosis and Haemostasis , 102(10), 772–778. doi: 10.1160/TH09-04-0215
- Born, G., & Cross, M. (1963). The aggregation of blood platelets. The Journal of Physiology , 168(1), 178–195. doi: 10.1113/jphysiol.1963.sp007185
- Born, G. V. R. (1962). Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature , 194(4832), 927–929. doi: 10.1038/194927b0
- Braune, S., Küpper, J.-H., & Jung, F. (2020). Effect of prostanoids on human platelet function: An overview. International Journal of Molecular Sciences , 21(23), 9020. doi: 10.3390/ijms21239020
- Bye, A. P., Unsworth, A. J., & Gibbins, J. M. (2018). Screening and high-throughput platelet assays. Methods in Molecular Biology , 1812, 81–94. doi: 10.1007/978-1-4939-8585-2_5
- Cattaneo, M., Cerletti, C., Harrison, P., Hayward, C. P. M., Kenny, D., Nugent, D., … Michelson, A. D. (2013). Recommendations for the standardization of light transmission aggregometry: A consensus of the working party from the platelet physiology subcommittee of SSC/ISTH. Journal of Thrombosis and Haemostasis , 11(6), 1183–1189. doi: 10.1111/jth.12231
- Cattaneo, M., Hayward, C., Moffat, K., Pugliano, M., Liu, Y., & Michelson, A. D. (2009). Results of a worldwide survey on the assessment of platelet function by light transmission aggregometry: A report from the platelet physiology subcommittee of the SSC of the ISTH. Journal of Thrombosis and Haemostasis , 7(6), 1029. doi: 10.1111/j.1538-7836.2009.03458.x
- Cazenave, J. P., Ohlmann, P., Cassel, D., Eckly, A., Hechler, B., & Gachet, C. (2004). Preparation of washed platelet suspensions from human and rodent blood. Methods in Molecular Biology , 272, 13–28. doi: 10.1385/1-59259-782-3:013
- Chan, M. V., Armstrong, P. C., Papalia, F., Kirkby, N. S., & Warner, T. D. (2011). Optical multichannel (optimul) platelet aggregometry in 96-well plates as an additional method of platelet reactivity testing. Platelets , 22(7), 485–494. doi: 10.3109/09537104.2011.592958
- Chan, M. V., Leadbeater, P. D., Watson, S. P., & Warner, T. D. (2018). Not all light transmission aggregation assays are created equal: Qualitative differences between light transmission and 96-well plate aggregometry. Platelets , 29(7), 686–689. doi: 10.1080/09537104.2018.1466388
- Fernández, D. I., Provenzale, I., Cheung, H. Y., van Groningen, J., Tullemans, B. M. E., Veninga, A., … Heemskerk, J. W. M. (2022). Ultra-high-throughput Ca2+ assay in platelets to distinguish ITAM-linked and G-protein-coupled receptor activation. iScience , 25(1), 103718. doi: 10.1016/j.isci.2021.103718
- Fratantoni, J. C., & Poindexter, B. J. (1990). Measuring platelet aggregation with microplate reader: A new technical approach to platelet aggregation studies. American Journal of Clinical Pathology , 94(5), 613–617. doi: 10.1093/ajcp/94.5.613
- French, S. L., Thalmann, C., Bray, P. F., Macdonald, L. E., Murphy, A. J., Sleeman, M. W., & Hamilton, J. R. (2018). A function-blocking PAR4 antibody is markedly antithrombotic in the face of a hyperreactive PAR4 variant. Blood Advances , 2(11), 1283–1293. doi: 10.1182/bloodadvances.2017015552
- Hechler, B., Dupuis, A., Mangin, P. H., & Gachet, C. (2019). Platelet preparation for function testing in the laboratory and clinic: Historical and practical aspects. Research and Practice in Thrombosis and Haemostasis , 3(4), 615–625. doi: 10.1002/rth2.12240
- Ho, C. H., & Chan, I. H. (1995). The influence of time of storage, temperature of storage, platelet number in platelet-rich plasma, packed cell, mean platelet volume, hemoglobin concentration, age, and sex on platelet aggregation test. Annals of Hematology , 71(3), 129–133. doi: 10.1007/BF01702648
- Hsu, H., Chan, M. V., Armstrong, P. C., Crescente, M., Donikian, D., Kondo, M., … Rabbolini, D. J. (2022). A pilot study assessing the implementation of 96-well plate-based aggregometry (Optimul) in Australia. Pathology , 54(6), 746–754. doi: 10.1016/j.pathol.2022.03.012
- Iyú, D., Jüttner, M., Glenn, J. R., White, A. E., Johnson, A. J., Fox, S. C., & Heptinstall, S. (2011). PGE1 and PGE2 modify platelet function through different prostanoid receptors. Prostaglandins & Other Lipid Mediators, 94(1-2), 9–16. doi: 10.1016/j.prostaglandins.2010.11.001
- Jarvis, G. E. (2004). Platelet aggregation: Turbidimetric measurements. Methods in Molecular Biology , 272, 65–76. doi: 10.1385/1-59259-782-3:065
- Kattlove, H. E., & Alexander, B. (1971). The effect of cold on platelets. I. Cold-induced platelet aggregation. Blood , 38(1), 39–48. doi: 10.1182/blood.V38.1.39.39
- Mannuß, S. (2020). Influence of different methods and anticoagulants on platelet parameter measurement. Journal of Laboratory Medicine , 44(5), 255–272. doi: 10.1515/labmed-2020-0037
- Martins Lima, A., Bragina, M. E., Burri, O., Chapalay, J. B., Costa-Fraga Fabiana, P., Chambon, M., … Stergiopulos, N. (2019). An optimized and validated 384-well plate assay to test platelet function in a high-throughput screening format. Platelets , 30(5), 563–571. doi: 10.1080/09537104.2018.1514106
- Maurer-Spurej, E., & Devine, D. V. (2001). Platelet aggregation is not initiated by platelet shape change. Laboratory Investigation , 81(11), 1517–1525. doi: 10.1038/labinvest.3780365
- Maurer-Spurej, E., Pfeiler, G., Maurer, N., Lindner, H., Glatter, O., & Devine, D. V. (2001). Room temperature activates human blood platelets. Laboratory Investigation , 81(4), 581–592. doi: 10.1038/labinvest.3780267
- May, J. A., & Heptinstall, S. (2004). Effects of anticoagulants used during blood collection on human platelet function. Methods in Molecular Biology , 272, 3–11. doi: 10.1385/1-59259-782-3:003
- Michelson, A. (1994). Platelet activation by thrombin can be directly measured in whole blood through the use of the peptide GPRP and flow cytometry: Methods and clinical applications. Blood Coagulation & Fibrinolysis, 5(1), 121–131. doi: 10.1097/00001721-199402000-00014
- Mindukshev, I., Fock, E., Dobrylko, I., Sudnitsyna, J., Gambaryan, S., & Panteleev, M. A. (2022). Platelet hemostasis reactions at different temperatures correlate with intracellular calcium concentration. International Journal of Molecular Sciences , 23(18), 10667. doi: 10.3390/ijms231810667
- Munnix, I., Van Oerle, R., Verhezen, P., Kuijper, P., Hackeng, C. M., Hopman-Kerkhoff, H. I. J., … Henskens, Y. M. C. (2021). Harmonizing light transmission aggregometry in the Netherlands by implementation of the SSC-ISTH guideline. Platelets , 32(4), 516–523. doi: 10.1080/09537104.2020.1771549
- Noetzli, L. J., French, S. L., & Machlus, K. R. (2019). New insights into the differentiation of megakaryocytes from hematopoietic progenitors. Arteriosclerosis, Thrombosis, and Vascular Biology , 39(7), 1288–1300. doi: 10.1161/ATVBAHA.119.312129
- Østerud, B., & Brox, J. (1983). The clotting time of whole blood in plastic tubes: The influence of exercise, prostacyclin and acetylsalicylic acid. Thrombosis Research , 29(4), 425–435. doi: 10.1016/0049-3848(83)90246-3
- Stalker, T. J., Traxler, E. A., Wu, J., Wannemacher, K. M., Cermignano, S. L., Voronov, R., … Brass, L. F. (2013). Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood , 121(10), 1875–1885. doi: 10.1182/blood-2012-09-457739
- Sun, B., Tandon, N., Yamamoto, N., Yoshitake, M., & Kambayashi, J.-I. (2001). Luminometric assay of platelet activation in 96-well microplate. Biotechniques , 31(5), 1174–1181. doi: 10.2144/01315dd02
- Tiffany, M. L., & Henry, R. L. (1983). Technical considerations for platelet aggregation and related problems. Critical Reviews in Clinical Laboratory Sciences , 19(1), 27–69. doi: 10.3109/10408368309165759
- Tourdot, B. E., & Holinstat, M. (2017). Targeting 12-lipoxygenase as a potential novel antiplatelet therapy. Trends in Pharmacological Sciences , 38(11), 1006–1015. doi: 10.1016/j.tips.2017.08.001
- van der Meijden, P. E., & Heemskerk, J. W. (2019). Platelet biology and functions: New concepts and clinical perspectives. Nature Reviews Cardiology , 16(3), 166–179. doi: 10.1038/s41569-018-0110-0
- Versteeg, H. H., Heemskerk, J. W., Levi, M., & Reitsma, P. H. (2013). New fundamentals in hemostasis. Physiological Reviews , 93(1), 327–358. doi: 10.1152/physrev.00016.2011
- Vinholt, P. J., Nybo, M., Nielsen, C. B., & Hvas, A. M. (2017). Light transmission aggregometry using pre-coated microtiter plates and a Victor X5 plate reader. PLoS One , 12(10), e0185675. doi: 10.1371/journal.pone.0185675
- von Papen, M., Gambaryan, S., Schütz, C., & Geiger, J. (2013). Determination of ATP and ADP secretion from human and mouse platelets by an HPLC assay. Transfusion Medicine and Hemotherapy , 40(2), 109–116. doi: 10.1159/000350294
- Yee, D. L., Sun, C. W., Bergeron, A. L., Dong, J. F., & Bray, P. F. (2005). Aggregometry detects platelet hyperreactivity in healthy individuals. Blood , 106(8), 2723–2729. doi: 10.1182/blood-2005-03-1290

