Phenotypic analysis of root nodules based on paraffin fixation
Manoj-Kumar Arthikala, Kalpana Nanjareddy, Carolina Cervera Torres
Abstract
The mutualistic relationship between leguminous plants and rhizobia bacteria stands out as one of the most crucial, primarily because of its capacity to convert atmospheric nitrogen (N2) into ammonia (NH3). Recent years have witnessed a surge in studies focusing on the regulation and establishment of this symbiosis, leading to the development of protocols aimed at scrutinizing the phenotype of the pivotal structures facilitating nutrient exchange between the symbiotic partners – the nodules. The subsequent protocol outlines a highly effective method for paraffin processing of nodules, enabling a comprehensive phenotypic analysis. This protocol serves as a valuable analytical tool for research endeavors delving into the intricate details of the rhizobia-legume symbiosis.
Steps
Sample collection
Precision in sample collection is crucial to prevent any damage. Submerge the roots in water to facilitate the handling of nodules. Utilizing tweezers and delicate scissors, carefully sever the roots approximately 1-2 cm away from the nodule. This ensures ease of manipulation during processing without disrupting the nodule anatomy.
Fixation
Immerse the tissues in 5mL of FAA fixative solution (10% formalin, 5% glacial acetic acid, 50% ethanol, and 35% ddH2O) or Carnoy's solution (60% ethanol, 30% chloroform, 10% glacial acetic acid, and 1gof ferric chloride).
Place the tissues in the fixative within a vacuum chamber for 20 minutes, breaking the vacuum at 10-minute intervals.
Paraffin embedding
Following fixation, immerse the samples in 5mL of a 1X PBS solution for 10 minutes on ice.
Dehydrate the samples in a 5mL ethanol series at room temperature: 30% ethanol for 30 minutes, 50% ethanol for 30 minutes, 70% ethanol for 1 hour, 85% ethanol for 30 minutes, 95% ethanol for 30 minutes, and 100% ethanol twice for 30 minutes each.
Infiltrate the samples with 5mL of ethanol and xylene substitute (Histochoice) for 30 minutes at room temperature, using the following concentrations: ethanol/xylene substitute (2:1, v/v), ethanol/xylene substitute (1:1, v/v), ethanol/xylene substitute (1:2, v/v), and pure xylene substitute twice.
Infiltrate the samples with 5mL of xylene and paraffin substitute in glass bottles overnight, mixing in an oven at 60°C. Use the following concentrations: xylene/paraffin substitute (2:1, v/v), xylene/paraffin substitute (1:1, v/v), xylene/paraffin substitute (1:2, v/v).
Continue to make 4 changes of paraffin infiltration of tissues with 5 ml of pure paraffin with an interval of 5-8 hrs and incubate in a hot air oven at 60°C.
Preparation of blocks and cuts
Pour approximately 15mL of paraffin into a plastic Petri dish, and carefully empty the tissues from the jars.
Use tweezers to manipulate and arrange the nodules in the desired orientation within the Petri dish. If additional tissue coverage is required, add a small amount of paraffin. Allow the blocks to dry for 24 hours.
Prepare the slides to be used a day before making cuts by immersing them in a 2% gelatin solution.
Mount the paraffin blocks containing tissue onto wooden pegs. Attach these pegs to the microtome for sectioning at the desired thickness.
Place the cut sections onto gelatin-coated slides and position them on a hot plate (50-60°C) until the tissue spreads out evenly. Discard any excess gelatin from the slides.
Leave the slides to dry until the next day.
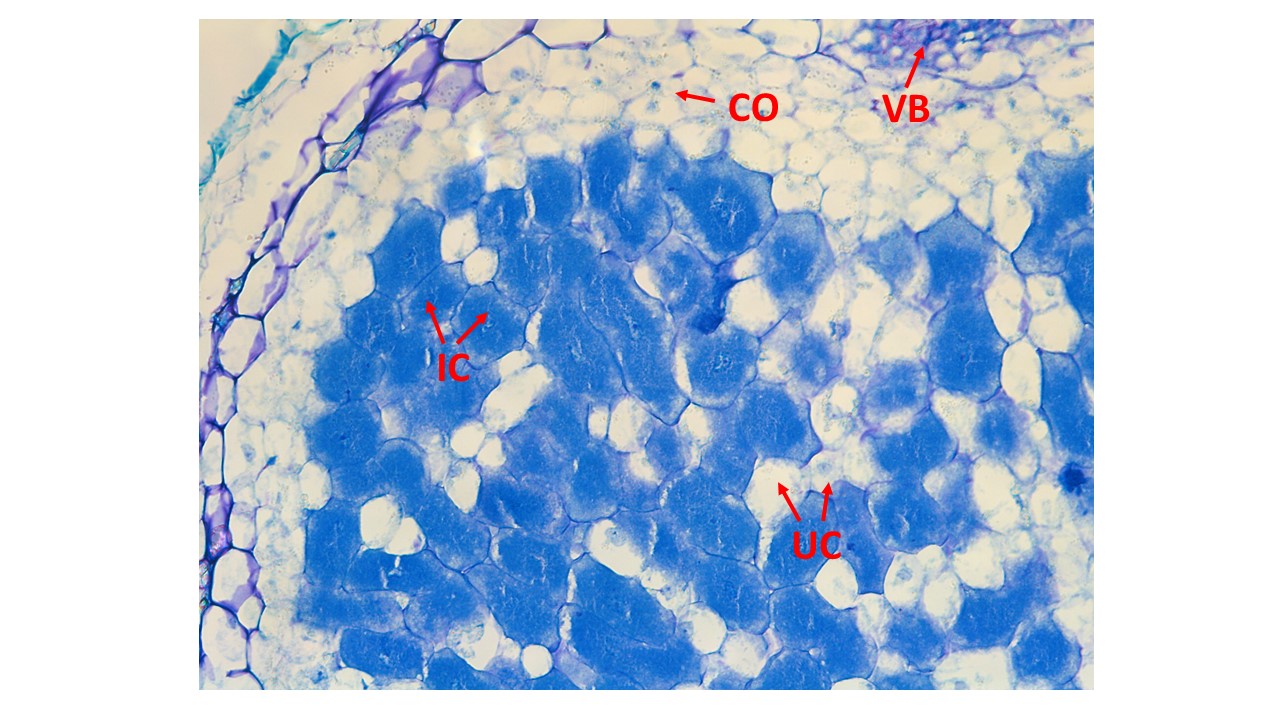
Histochemical staining
The slides were deparaffinized in 150mL of xylene substitute (histochoice) for 15 minutes, gently shaken every 5 minutes. The slides were deparaffinized in 150mLof xylene substitute (histochoice) for 15 minutes, gently shaken every 5 minutes.
Subsequently, the samples were rehydrated using solutions of varying ethanol concentrations (100%, 95%, 70%, 50%, and 30%) in ddH2O with each step lasting 3 minutes at room temperature.
Following rehydration, the samples were placed in a container with ddH2O for an additional 3 minutes.
For staining, a 0.1% Toluidine blue solution in ddH2O was prepared.
Samples were immersed in the staining solution for 20 seconds, followed by three rinses in ddH2O, each lasting 10 seconds.
Dehydration was carried out by briefly treating the samples in 95% ethanol and 100% ethanol for 10 seconds at room temperature.
The final step involved immersing the samples in xylene substitute for 5 minutes.
Approximately 400µL of xylene-based mounting medium was applied to the slide, and the cover glass was carefully lowered, avoiding the formation of any air bubbles that might interfere with obtaining high-quality images.
The slides were left to air dry overnight before observation and photography at the desired magnifications.