Optimization of Large-Scale Adeno-Associated Virus (AAV) Production
Alina S. Bilal, Alina S. Bilal, Sarah N. Parker, Sarah N. Parker, Victoria B. Murray, Victoria B. Murray, Lauren F. MacDonnell, Lauren F. MacDonnell, Donna J. Thuerauf, Donna J. Thuerauf, Christopher C. Glembotski, Christopher C. Glembotski, Erik A. Blackwood, Erik A. Blackwood
Abstract
Genetic manipulation in vivo is a critical method for mechanistically understanding gene function in disease and physiological processes. To facilitate this, embryonic transgenesis in popular animal models like mice has been developed. Compared to the longer, expensive methods of transgenesis, viral vectors, such as adeno-associated virus (AAV), have grown increasingly in popularity due to their relatively low cost and ease of production, translating to an overall greater versatility as a biological tool. In this article, we describe protocols for AAV production and purification for efficient transduction in vivo. Importantly, our method differs from others in application of a streamlined, more cost-effective approach. From this method, as many as 2 × 1013 genome-containing viral particles (vp), or 200 units, can be produced within 3 to 4 weeks, with a minimal cost of
Basic Protocol 1 : AAV production
Basic Protocol 2 : AAV purification
INTRODUCTION
Adeno-associated virus (AAV) production and purification are integral processes for the production of a biological tool that facilitates mechanistic study of gene function in vivo (Blackwood et al., 2019). AAV production and purification for in vivo studies represent a challenge for large-scale production due to the sheer number of reagents required to facilitate such production. This article describes an optimized approach to AAV production and subsequent purification that facilitates organized, cost-effective, consistent large-scale AAV production in academia. From the described methods, as many as 2 × 1013 genome-containing viral particles (vp) can be produced within 3 to 4 weeks at a cost of
| Item | Catalog number | Price per unit | Quantity to order | Initial cost for supplies | Estimated # of preps worth | Estimated value per 2 preps = ∼2 × 1013 vp, or 200 unitsb |
|---|---|---|---|---|---|---|
| AAVPro-293T cells | Takara, 632273 | $387 | 1 | $387 | Infinite | |
| DMEM/F12 (option 1) | Gibco, 11330032 |
$42.25/500 ml |
5 | $226.25 | 2 preps | $226.25 |
| DMEM/F12 (option 2) | Cytiva, SH30023.01 | $20.42/500 ml | 5 | $102.10 | 2 preps | $102.10 |
| Fetal bovine serum | Omega, FB-02 | $270/500 ml (price will vary greatly) | 1 | $270 | 4-5 preps | $135 |
| 150-mm surface-treated tissue culture dish | Fisher, FB012925 | $98.72/120 | 1 | $98.72 | 4 preps | $49.36 |
| T-175 tissue culture flask, vented cap (option 1) | Corning, 355001 | $359.50/case of 40 | 3 | $1078.50 | 2.5 preps | $862.80 |
| T-175 tissue culture flask, plug seal cap (option 2) | Corning, 353028 | $340.50/case of 40 | 3 | $1021.50 | 2.5 preps | $817.20 |
| T-175 tissue culture flask, vented cap (option 2) | Corning, 354639 | $102/case of 50 | 2 | $205 | 2 preps | $205 |
| PEI MAX - Transfection Grade Linear Polyethylenimine Hydrochloride | Polysciences, 24765-100 | $129/100 mg | 1 | $129 | Infinite | ∼0 |
| pAdDeltaF6c | Addgene, #112867 | Bacterial agar stab | 1 | $75 | Infinite | ∼0 |
| pAAV2/9nc,d | Addgene, #112865 | Bacterial agar stab | 1 | $75 | Infinite | ∼0 |
| Benzonase Nuclease | Sigma, 71205-3 | $273/25 KU | 1 | $273 | 2 preps | $273 |
| OptiSeal 32.4 ml Tube Kit | Beckman, 361662 | $2,271/kit | 1 | $2,271 | Tubes – 6 preps; other kit elements – infinite use (one-time purchase) | $111 |
| OptiPrep | Sigma, D1556 | $332/250 ml | 1 | $332 | 6 preps | $111 |
| Exel International disposable spinal needles | Med Vet International, 26960 | $19.88/needle | 1 | $19.88 | Infinite (one-time purchase) | ∼0 |
| 10-ml sterile syringe | Fisher, 14-955-459 | $22.45/pack of 100 | 1 | $22.45 | 25 preps | ∼0 |
| Weighing scale | Ohaus, 1450-SD | $367.84/scale | 1 | $367.84 | Infinite (one-time purchase) | ∼0 |
| Cast iron support ring stand | Fisher, S13747 | $23/stand | 1 | $23 | Infinite (one-time purchase) | ∼0 |
| Three-prong extension clamps | Fisher, 05-769-7Q | $72.80/clamp | 1 | $72.80 | Infinite (one-time purchase) | ∼0 |
| Lactated Ringer's | NDC, 0990-7953-09 | $9.99/1000 ml | 1 | $9.99 | 3 preps | $6 |
| Vivaspin 20, 100-kDa MWCO | Cytiva, 28-9323-63 | $236.50/pack of 12 | 1 | $236.50 | 3 preps | $158 |
| Cost for 2 preps using option 1 selections | $1808 | |||||
| Cost for 2 preps using option 2 selections | $2092 |
- a These are the costs associated with supplies/reagents unique to AAV production/purification and supplies/reagents that are consumed in high quantities in this method (see culture dishes, fetal bovine serum, and DMEM/F12). Not included are standard supplies/reagents associated with cell culture or animal work.
- b One unit is defined as 1 × 1011 vp, our standard dose per animal.
- c These items require DNA preparation, and its associated costs are not detailed here.
- d This item will vary depending on the serotype desired.
The methods described consist of two protocols. Basic Protocol 1 covers AAVPro-293T cell culture and growth, transfection of AAV plasmids and helper plasmids, and harvest of AAV particles from AAVPro-293T cell lysates. Basic Protocol 2 covers purification of AAV particles from among other cellular contents of AAVPro-293T cell lysates and subsequent solubilization in a solution suitable for in vivo administration.
STRATEGIC PLANNING
To address the challenge of large-scale production of AAV, the following streamlined approach has been developed (Fig. 1), which allows for two AAV preparations over the course of 3 to 4 weeks. A single AAV preparation should yield 80 to 100 units, where a unit is defined as 1 × 1011 vp. Therefore, within 3 to 4 weeks, as many as 2 × 1013 vp or 200 units of AAV can be generated with this approach.
CAUTION : AAV is a Biosafety Level 2 pathogen. Follow all appropriate guidelines and regulations for the use and handling of pathogenic microorganisms.
Basic Protocol 1: AAV PRODUCTION
Here, we describe our protocol for AAV production, which entails the use of AAVPro-293T cell culture, transfection, and cell lysis for AAV particle harvest. Specifically, this protocol encompasses the following major steps: (1) passaging of AAVPro-293T cells; (2) plating of AAVPro-293T cells into T-175 tissue culture (TC) flasks; (3) transfection of AAVPro-293T cells for recombinant AAV production; and (4) harvest of recombinant AAV particles from AAVPro-293T cells. By the end of Basic Protocol 1, cell lysates containing AAV should be ready for subsequent purification, which is described in Basic Protocol 2.
Materials
-
AAVPro-293T cells (one frozen vial of 1 ml of 8–10 × 106 AAVPro-293T cells; Takara, cat. no. 632273)
-
70% (v/v) ethanol
-
Culturing medium (see recipe), 37°C
-
Dulbecco's phosphate-buffered saline (DPBS), no calcium, no magnesium (1×; Gibco, cat. no. 14190250)
-
TrypLE Express Enzyme (1×; Gibco, cat. no. 12605010)
-
1× polyethyleneimine (PEI; diluted from 10× PEI, Polysciences, cat. no. 24765-100)
-
DMEM/F12 (1:1; Gibco, cat. no. 11320033, or Cytiva, cat. no. SH30023.FS)
-
pAdDeltaF6 (AAV helper plasmid; Addgene, cat. no. 112867)
-
pAAV2/9n (rep/cap gene expression construct; Addgene, cat. no. 112865)
-
rAAV plasmid (backbone vectors are available from Addgene)
-
Transfection medium (see recipe)
-
AAV lysis buffer (see recipe)
-
Dry ice
-
Methanol
-
37°C water bath
-
Serological pipets, sterile (VWR or equivalent)
-
50-ml conical centrifuge tubes (Corning or equivalent)
-
Centrifuge with swinging-bucket rotor (Eppendorf or equivalent)
-
150-mm TC dishes (Fisher, cat. no. FB012925, or equivalent)
-
TC microscope (ECHO or equivalent)
-
Hemocytometer
-
175-cm2 (T-175) TC flasks, vented cap (Corning, cat. no. 355001, or equivalent)
-
Vortex
NOTE : Apart from centrifugation and microscopic visualization, ensure all cell culture steps are performed in a laminar flow hood (Labconco Purifier BSC Class II or equivalent). All solutions and equipment coming into contact with cells must be sterile, and proper sterile technique should be used accordingly.
NOTE : All culture incubations are performed in a 37°C water-jacketed incubator with 5% CO2 (Thermo Scientific) unless otherwise specified.
Passaging AAVPro-293T cells
1.Thaw one frozen vial of 1 ml of 8–10 × 106 AAVPro-293T cells quickly in a 37°C water bath. Once thawed, spray vial with 70% ethanol before placing it in a laminar flow hood.
2.Add 1 ml warm culturing medium dropwise to thawed cells in vial and then collect cells with a serological pipet and add, dropwise, to 3 ml culturing medium in a 50-ml conical centrifuge tube.
3.Centrifuge 5 min at 1500 × g in a centrifuge with a swinging-bucket rotor.
4.During centrifugation, add 10 ml culturing medium to one 150-mm TC dish.
5.After centrifugation of the cells, aspirate the supernatant and resuspend the cell pellet in 5 ml culturing medium using a 5-ml serological pipet, resuspending gently but thoroughly by pipetting up and down 3 to 4 times.
6.Add the 5 ml cells to the 150-mm TC dish dropwise. Rock 3 to 4 times and incubate at 37°C, 5% CO2 for 16 to 24 hr.
7.Visually inspect cells under a TC microscope the next day to ensure that they are at least ∼70% confluent and then proceed to the next step. If they have not reached the required confluence, maintain them in the incubator until ∼70% confluency is reached (Fig. 2).
8.Begin passaging cells by aspirating medium, adding 10 ml DPBS to the dish, rocking 1 to 2 times, and aspirating DPBS. Then, add 1 ml TrypLE Express Enzyme and incubate at room temperature for 2 to 3 min.
9.Rock 3 to 4 times to ensure that the cells are no longer adherent to the dish.
10.Add 4 ml culturing medium to collect trypsinized cells into a 50-ml conical centrifuge tube and centrifuge 5 min at 1500 × g.
11.During centrifugation of the cells, wash the 150-mm TC dish with 7 to 10 ml DPBS and aspirate.
12.To this dish and each of three new 150-mm TC dishes, add 14 ml culturing medium. After centrifugation of the cells, aspirate supernatant from the 50-ml conical tube and resuspend cell pellet in 8 ml culturing medium, using a 5- or 10-ml serological pipet to resuspend the cell pellet gently but thoroughly by pipetting up and down 3 to 4 times.
13.Add 1 ml cells to each of the four 150-mm TC dishes and rock 3 to 4 times for even plating. Place at 37°C, 5% CO2 for 72 hr.
14.After 72 hr, visually inspect cells to ensure all four 150-mm TC dishes are ≥70% confluent. Passage the cells by trypsinization, as described in steps 8 to 13, but use a 1:2 split, i.e., cells from four 150-mm TC dishes should be resuspended in 8 ml culturing medium and then plated onto eight 150-mm TC dishes (1 ml each). Incubate 16 to 24 hr at 37°C, 5% CO2.
15.Repeat step 14 daily for two more days, resulting in 32 dishes.
Plating AAVPro-293T cells into T-175 TC flasks
16.Trypsinize all the cells, as previously described in steps 8 to 13, but after centrifugation, resuspend the cells in 30 ml culturing medium.
17.Perform a 1:10 dilution by adding 100 µl cells to 900 µl culturing medium and perform a cell count by adding 10 µl of the diluted cells to a hemocytometer.
18.Resuspend the cells to a concentration of 11 × 106 cells/ml with culturing medium, i.e., in ∼50 ml, depending on the exact count.
19.Add 17 ml culturing medium to 48 T-175 TC flasks. Then, add 1 ml cells (i.e., 11 × 106 cells from step 18) to each flask. Rock and then incubate at 37°C, 5% CO2 for 16 to 24 hr.
20.If another AAV preparation is desired, as outlined in Figure 1, proceed to steps 21 and 22.If not, skip to step 23.
| Flask size | Culturing/transfection medium (ml) | AAVPro-293T cell density (million/ml) | # flasks for a single AAV prep |
|---|---|---|---|
| T-175 | 18 | 11 | 48 |
| T-182 | 19 | 12 | 46 |
| T-225 | 23 | 14 | 38 |
21.To one 150-mm TC dish, add 8–10 × 106 cells (there should be enough cells left over after step 19) and bring up to 15 ml with culturing medium. Incubate at 37°C, 5% CO2 for 16 to 24 hr.
22.On the next day, perform a 1:8 split of the single 150-mm TC dish from step 21, resulting in four dishes, as described in steps 8 to 13.
Transfecting AAVPro-293T cells for recombinant AAV production
23.On the next day, prepare six 50-ml conical centrifuge tubes. In three of the tubes, dilute the transfection agent, 1× PEI, with DMEM/F12 according to the volumes listed in Table 3.In the remaining three tubes, dilute the helper plasmids (pAdDeltaF6 and pAAV2/9n) and the recombinant AAV (rAAV) plasmid with DMEM/F12 according to the volumes listed in Table 3. Allow to sit at room temperature for 5 min.
| Prepare the transfection reagent by diluting with DMEM/F12 as follows: | |||
|---|---|---|---|
|
Per 16 T-175 flasks (add listed volumes to each of three 50-ml conical tubes) |
Per 12 T-182 flasks (add listed volumes to each of three 50-ml conical tubes) |
Per 19 T-225 flasks (add listed volumes to each of two 50-ml conical tubes) |
|
|
1× polyethylenimine (PEI); dilute 10× PEI stock (5.17 mg/ml) to 1× with molecular biology–grade water |
2.56 ml | 2.56 ml | 3.84 ml |
|
1:1 DMEM/F12, no antibiotic |
16 ml | 16 ml | 24 ml |
| Prepare the helper and recombinant AAV plasmids by diluting with DMEM/F12 as follows: | |||
|
Per 16 T-175 flasks (add listed volumes to each of three 50-ml conical tubes) |
Per 12 T-182 flasks (add listed volumes to each of three 50-ml conical tubes) |
Per 19 T-225 flasks (add listed volumes to each of two 50-ml conical tubes each) |
|
| rAAV plasmid | 80 µg | 80 µg | 120 µg |
| pAdDeltaF6 | 160 µg | 160 µg | 240 µg |
| pAAV2/9n | 80 µg | 80 µg | 120 µg |
|
1:1 DMEM/F12, no antibiotic |
16 ml | 16 ml | 24 ml |
|
Allow the diluted PEI and plasmids to sit separately for ∼5 min at room temperature. Then, combine the diluted PEI with the diluted plasmids and vortex vigorously for 1 min. Add to transfection medium as follows: |
|||
| Flask size |
Transfection medium (for each 50-ml conical tube of PEI/plasmid mixture) |
# flasks (for each 50-ml conical tube of PEI/plasmid mixture) |
|
| T-175 | 270 | 16 | |
| T-182 | 273 | 12 | |
| T-225 | 385 | 19 | |
24.Combine the diluted PEI from one tube with the diluted plasmids from another tube. Repeat for the remaining two pairs of tubes. Vortex vigorously for 1 min. Incubate mixture at room temperature for 30 min and then combine the contents of one tube (∼18 ml PEI/plasmid mixture) with 270 ml transfection medium (see Table 3).
25.Aspirate culturing medium from 16 T-175 flasks from step 19 and replace with 18 ml of the transfection medium/PEI/plasmid mixture from step 24.Rock 4 to 5 times before placing in a 37°C, 5% CO2 incubator.
26.Repeat steps 24 and 25 for the remaining two tubes and 32 T-175 flasks. Incubate all 48 T-175 flasks at 37°C, 5% CO2 for 3 to 4 days.
Harvesting recombinant AAV particles from AAVPro-293T cells
27.Three or four days later (Monday or Tuesday, if following the Strategic Planning outline in Fig. 1), set up 24 tubes (i.e., 50-ml conical centrifuge tubes) in a laminar flow hood.
28.Move 12 of the 48 T-175 flasks from the 37°C, 5% CO2 incubator into the laminar flow hood. Collect all of the medium from two T-175 flasks with a serological pipet and transfer into one 50-ml conical centrifuge tube. Repeat for the remaining 10 T-175 flasks.
29.To each bare T-175 flask, add 3 ml TrypLE, rock 3 to 4 times to evenly coat the surface, and place back in the 37°C, 5% CO2 incubator for ∼10 min.
30.During the 10-min incubation, repeat steps 28 and 29 with another 12 T-175 flasks. By the time this second set of flasks has TrypLE and are ready to incubate in the 37°C, 5% CO2 incubator, move the first set of flasks back into the laminar flow hood and rock 3 to 4 times to ensure the cells have been efficiently trypsinized from the surface of the flask. Using a 10-ml serological pipet, add ∼10 ml of the collected medium from one tube to each of two flasks and wash the surface of each flask to collect the trypsinized cells. Add the cells from the two flasks to the same tube from step 28.Repeat for the remaining 10 flasks.
31.Repeat step 30 for the second set of T-175 flasks.
32.Centrifuge the 12 tubes from steps 30 and 31 for 10 min at 300 × g at room temperature, with gentle handling. Remove the supernatant from the pellet with a 25-ml serological pipet and place into a biohazard waste collection container. Add 3 ml AAV lysis buffer to each tube, resuspend with a 10-ml serological pipet, and combine cell pellets from six tubes into one. Place these tubes on ice.
33.Repeat steps 28 to 32 for the remaining 24 T-175 flasks.
34.Next, perform three rounds of freeze-thaw on the four tubes of cell lysate by placing lysates in a bath of dry ice and methanol for 20 to 30 min and then thawing in a 37°C water bath for 20 to 30 min. After three rounds of freeze-thaw, store at −80°C until ready for Basic Protocol 2.
Basic Protocol 2: AAV PURIFICATION
Here, we describe our basic protocol for AAV purification, which entails two major steps: (1) iodixanol gradient centrifugation to purify the AAV particles from other cellular contents of the AAVPro-293T cell lysates and (2) concentration of AAV particles using an ultrafiltration and concentrator device that also facilitates the replacement of iodixanol with Lactated Ringer's solution. By the end of Basic Protocol 2, high-titer AAV in a safe, injectable solution will be ready for administration in vivo.
Materials
-
Four 50-ml conical centrifuge tubes of cell lysate containing AAV particles (Basic Protocol 1)
-
250 U/µl Benzonase Nuclease (Millipore Sigma, cat. no. 71205-3)
-
1 M MgCl2 (Sigma, cat. no. M1208)
-
15%, 25%, 40%, and 60% (w/v) iodixanol solutions (see recipes; make 60% fresh)
-
AAV lysis buffer (see recipe)
-
70% (v/v) ethanol
-
4× Laemmli buffer (Bio-Rad, cat. no. 1610747)
-
β-mercaptoethanol (Sigma-Aldrich, cat. no. M6250)
-
4%-12% Bis-Tris protein gel (Bio-Rad, cat. no. 3450124)
-
1× MES buffer (from 20×; Bio-Rad, cat. no. 1610789)
-
Pre-stained protein ladder (Precision Plus Protein Kaleidoscope Prestained Protein Standards, Bio-Rad, cat. no. 1610375)
-
Coomassie blue stain (Instant Blue , Abcam, cat. no. ab119211)
-
Lactated Ringer's solution (NDC 0990-7953-09)
-
DPBS, no calcium, no magnesium (1×; Gibco, cat. no. 14190250)
-
AAV of known titer (Addgene or equivalent)
-
37°C water bath
-
Centrifuge with swinging-bucket rotor (Eppendorf or equivalent)
-
OptiSeal ultracentrifuge tubes (32.4 ml; OptiSeal ultracentrifuge tube kit, Beckman, cat. no. 361662)
-
Serological pipets, sterile (VWR or equivalent)
-
Spinal needles (18G × 3.5 in.; Med Vet International, cat. no. 29660)
-
10-ml syringes, sterile (Fisher, cat. no. 14-955-459, or equivalent)
-
18G hypodermic needles (BD, cat. no. 305195, or equivalent)
-
Weighing scale (Ohaus, cat. no. 1450-SD)
-
Type 70 Ti fixed-angle rotor (Beckman, cat. no. 337922)
-
Optima XE-90 ultracentrifuge (Beckman, cat. no. A9983), 16°C
-
Metal support stand with burette clamp
-
2.0-ml tubes, sterile (Fisher-Scientific, cat. no. 02-681-375)
-
5-ml syringes, sterile (Fisher, cat. no. 14-955-458, or equivalent; optional)
-
95°C heat block
-
Electrophoresis system (Criterion cell and PowerPac power supply, Bio-Rad, cat. no. 1656019)
-
50-ml conical centrifuge tubes (Corning or equivalent)
-
Vivaspin 20 ultrafiltration devices (100-kDa MWCO; Sartorius, cat. no. 28-9323-63)
-
1.5-ml microcentrifuge tubes (VWR or equivalent)
-
Microcentrifuge (Thermo Scientific or equivalent)
-
ImageJ
NOTE : Apart from centrifugation and weighing of ultracentrifuge tubes, ensure all AAV handling is performed in a laminar flow hood (Labconco Purifier BSC Class II or equivalent).
Iodixanol gradient centrifugation
1.Thaw the four 50-ml conical centrifuge tubes of cell lysate containing AAV particles from Basic Protocol 1 in a 37°C water bath for 20 to 30 min. Once thawed, add 11 µl of 250 U/µl Benzonase Nuclease and 20 µl of 1 M MgCl2 to each of the four tubes. Incubate in a 37°C water bath for 30 min.
2.Centrifuge 20 min at 3200 × g at room temperature in a centrifuge with a swinging-bucket rotor to collect the supernatant.
3.Set up eight OptiSeal ultracentrifuge tubes for iodixanol gradient centrifugation in a laminar flow hood. To each ultracentrifuge tube, add 15%, 25%, 40%, and 60% iodixanol phases according to the volumes listed in Table 4.Begin by adding the 15% iodixanol phase to the bottom of each ultracentrifuge tube with a 5-ml serological pipet. Then, use a spinal needle and 10-ml syringe to set up each subsequent phase, placing the needle all the way at the bottom of the tube so that each phase is layered below the other (Fig. 5A and 5B). Then, layer the AAV9-containing cell lysate from step 2 (∼10 ml/ultracentrifuge tube) carefully on top of the 15% phase using an 18G hypodermic needle and 10-ml syringe. Bring the cell lysate layer up to the base of the tube's neck with AAV lysis buffer.
| Iodixanol (OptiPrep) | Volume |
|---|---|
| 15% iodixanol | 7.3 ml |
| 25% iodixanol | 4.9 ml |
| 40% iodixanol | 4 ml |
| 60% iodixanol | 4 ml |
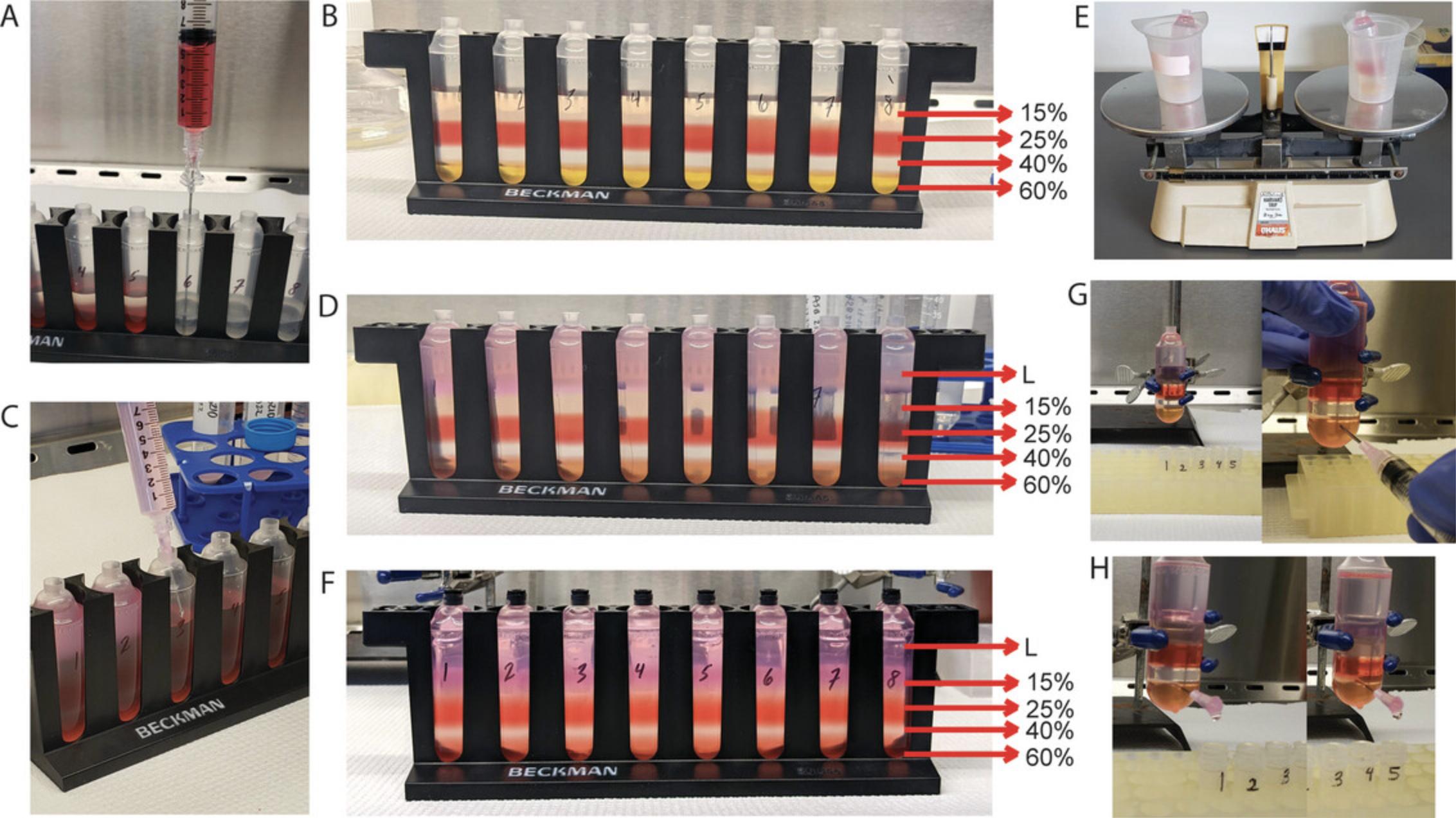
4.Balance the ultracentrifuge tubes using a weighing scale (Fig. 5E) by placing a pair on the balance together and removing/adding lysate between the tubes until they are balanced.
5.Seal the tubes with the plastic corks provided in the OptiSeal ultracentrifuge tube kit. Press down firmly on caps to ensure closure.
6.Centrifuge in a Type 70 Ti fixed-angle rotor in an Optima XE-90 ultracentrifuge for 1 hr at 69,000 rpm, 16°C, with maximum acceleration and coast (no brake) on deceleration.
7.Prepare a metal support stand with a burette clamp, as depicted in Figures 5G and 5H, in a laminar flow hood. Wipe each ultracentrifuge tube with 70% ethanol. Remove the cap on top of a tube, being mindful not to apply too much force that would disturb the layers. Gently secure one iodixanol gradient tube onto the burette clamp so that it is held in place. For collection of the 40% phase and the 40%-to-60% interface in subsequent steps, set up five 2.0-ml tubes with caps off in a rack underneath the iodixanol gradient tube, as shown in Figures 5G and 5H.
8.Attach a 5- or 10-ml syringe to an 18G hypodermic needle and use the syringe to guide insertion of the needle into the tube ∼2 mm below the 40%-to-60% interface. While applying pressure as the needle is inserted, twist the needle clockwise and counterclockwise until a drop appears from the tube and the needle breaks through the tube into the 60% iodixanol phase. Ensure that the needle is positioned bevel side up inside the tube.
9.Once the needle is fully inserted into the tube, unfasten the syringe from the needle. As soon as the syringe is unfastened, the iodixanol solution will quickly begin to flow through the needle hub.
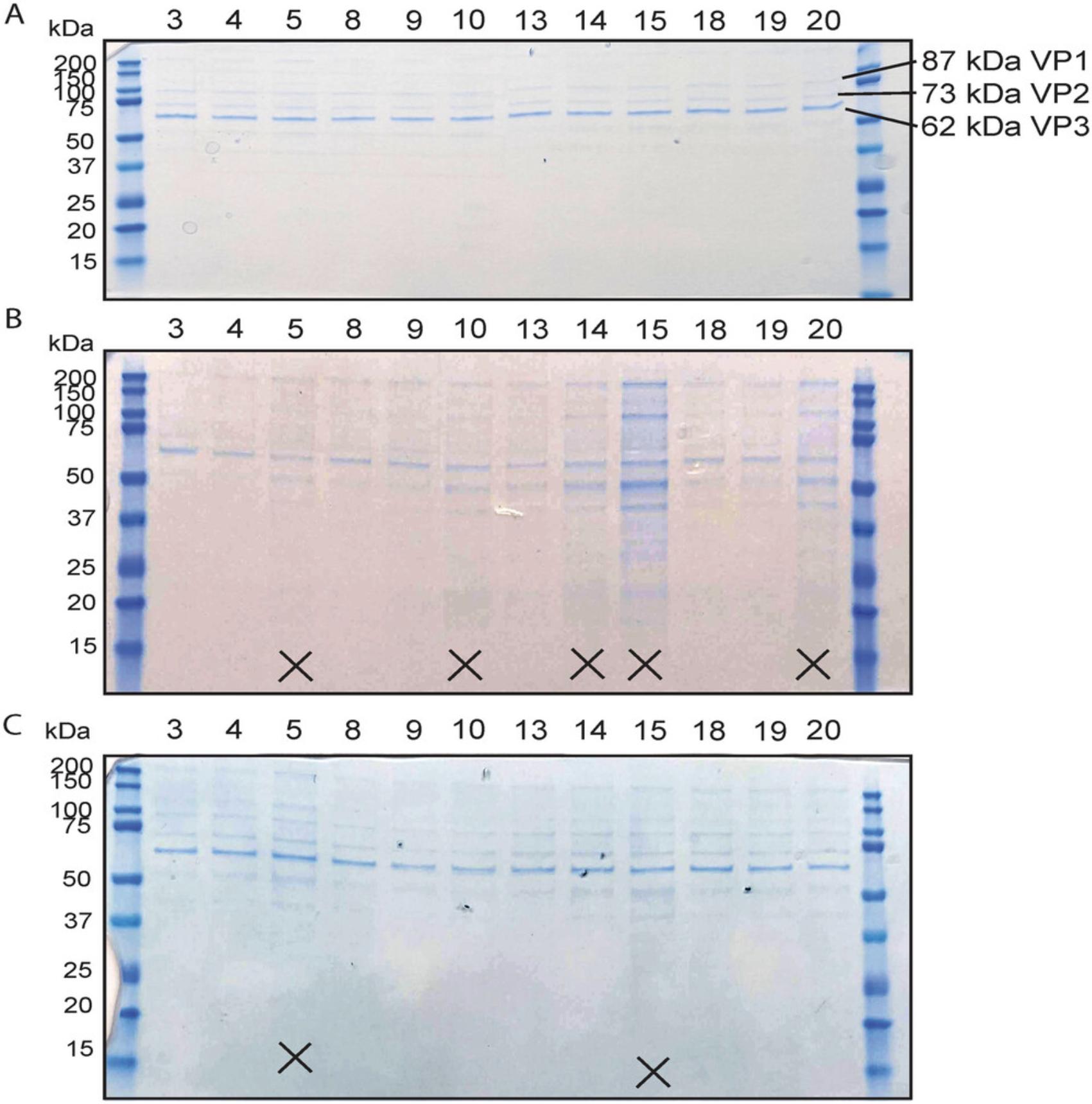
10.Load a sample from each 2.0-ml tube on a Bis-Tris protein gel to determine successful generation of AAV and purity of the AAV after iodixanol gradient ultracentrifugation. Begin by taking 18 µl from each 2.0-ml tube and supplementing with 6 µl of 4× Laemmli buffer and 0.3 µl β-mercaptoethanol and boil for 5 min at 95°C in a heat block. Load on a 4%-12% Bis-Tris protein gel with 1× MES buffer. Include a lane with 4 µl pre-stained protein ladder. Run the gel at 200 V for 40 min using an electrophoresis system. Then, stain with Coomassie blue stain for ≥15 min to visualize the viral capsid proteins VP1, VP2, and VP3, whose molecular weights are 87, 73, and 62 kDa, respectively (Kimura et al., 2019).
Concentration of AAV particles
11.Discard any samples with obvious contamination from the 25% phase (Fig. 6). Combine all pure samples into two 50-ml conical centrifuge tubes, with about 10 to 15 ml sample in each tube. Bring the volume of each tube to ≥40 ml with Lactated Ringer's solution.
12.Wash four Vivaspin 20 ultrafiltration devices by adding 10 ml Lactated Ringer's solution and centrifuging for 5 min at 200 × g in a swinging-bucket rotor at room temperature.
13.Add 20 ml of the diluted sample from step 11 to each of the four Vivaspin 20 devices and centrifuge 10 to 15 min at 3000 × g at room temperature, until the volume of sample has reached between 1 and 5 ml, as indicated on the Vivaspin 20 device. Discard the effluent.
14.Repeat step 13 if there is any remaining sample from step 11.Bring the volume to 20 ml with Lactated Ringer's solution.
15.Then, fill the Vivaspin 20 device up to 20 ml with Lactated Ringer's solution and centrifuge 10 to 15 min at 3000 × g at room temperature, until the volume is between 1 and 5 ml. Discard the effluent.
16.Repeat step 15 two more times.
17.Perform a final centrifugation with 20 ml Lactated Ringer's solution at 3000 × g at room temperature. Run the centrifuge until the volume of sample reaches the 0.2-ml mark on each device.
18.Unscrew the caps of the Vivaspin 20 devices, and with a 200-µl pipet, collect all traces of sample from inside the columns and combine into one 1.5-ml microcentrifuge tube. Separate 16 µl of the virus into each of two 1.5-ml tubes (8 µl/tube). For the remaining sample, measure and take note of the total volume and then aliquot the virus into four 1.5-ml tubes. Store at −80°C.
19.To each tube with 8 µl virus, add 4 µl DPBS and then 4 µl of 4× Laemmli buffer and 0.3 µl β-mercaptoethanol. Set aside.
20.Prepare a dilution series of an AAV of known titer, i.e., the standard, by adding 16 µl standard to 4 µl Lactated Ringer's solution in a 1.5-ml tube. From this tube, take 10 µl and add to 10 µl DPBS in a new tube, and so on, until five tubes are prepared in total. Prepare a technical replicate of two.
21.Boil the samples from steps 19 and 20 for 5 min at 95°C in a heat block. Let cool at room temperature for 1 min and then centrifuge 1 min at >12,000 × g. Load all samples on a 4%-12% Bis-Tris gel with 1× MES running buffer, including 4 µl pre-stained protein ladder. Run at 200 V for 40 min. Stain with Coomassie blue stain for 1 hr or overnight to obtain the most sensitive detection of the viral capsid proteins. Wash the gel several times with water to wash off the background stain.
22.Perform densitometry analysis of VP3 of the standard AAV and the new AAV sample preparation using ImageJ.
REAGENTS AND SOLUTIONS
AAV lysis buffer
- 150 mM NaCl
- 50 mM Tris base
- Molecular biology–grade H2O
- Adjust pH to 8.5 with HCl
- Optional: Filter-sterilize with Nalgene sterile disposable filter unit with PES membrane (Thermo Scientific, cat. no. 5670020, or equivalent) and Nalgene sterile disposable 250- or 500-ml receiver (Thermo Scientific, cat. no. 455-0250 or 455-0500, or equivalent)
- Store ≤6 months at room temperature
Culturing medium
- DMEM/F12 (1:1; Gibco, cat. no. 11320033, or Cytiva, cat. no. SH30023.FS)
- 10% (v/v) fetal bovine serum (FBS; Omega or equivalent)
- 1× penicillin-streptomycin-glutamine (PSG; from 100×; Gibco, cat. no. 10378016)
- 0.8 µg/ml amphotericin B (from 10 mg/ml; Sigma, cat. no. A9528)
- Filter-sterilize with Nalgene sterile disposable filter unit with PES membrane (Thermo Scientific, cat. no. 5670020, or equivalent) and Nalgene sterile disposable 250- or 500-ml receiver (Thermo Scientific, cat. no. 455-0250 or 455-0500, or equivalent)
- Store ≤6 months at 4°C
Two liters will be needed for one AAV preparation. Preparation should be conducted in a laminar flow hood to maintain sterility.
Iodixanol, 15%
- 45 ml OptiPrep (Sigma-Aldrich, cat. no. 1556)
- 60 ml 3 M NaCl (see recipe)
- 36 ml 5× TD buffer (see recipe)
- 39 ml molecular biology–grade H2O
- Store ≤3 months at room temperature
This recipe is for 180 ml. This will be sufficient for three sets of eight ultracentrifuge tubes. Do not filter-sterilize, but preparation should be conducted in a laminar flow hood.
Iodixanol, 25%
- 50 ml OptiPrep (Sigma-Aldrich, cat. no. 1556)
- 24 ml 5× TD buffer (see recipe)
- 46 ml molecular biology–grade H2O
- 300 µl 0.5% phenol red (Sigma-Aldrich, cat. no. P4758)
- Store ≤3 months at room temperature
This recipe is for 120 ml. This will be sufficient for three sets of eight ultracentrifuge tubes. Do not filter-sterilize, but preparation should be conducted in a laminar flow hood.
Iodixanol, 40%
- 68 ml OptiPrep (Sigma-Aldrich, cat. no. 1556)
- 20 ml 5× TD buffer (see recipe)
- 12 ml molecular biology–grade H2O
- Store ≤3 months at room temperature
This recipe is for 100 ml. This will be sufficient for three sets of eight ultracentrifuge tubes. Do not filter-sterilize, but preparation should be conducted in a laminar flow hood.
Iodixanol, 60%
- 36 ml OptiPrep (Sigma-Aldrich, cat. no. 1556)
- 90 µl 0.5% phenol red (Sigma-Aldrich, cat. no. P4758)
- Prepare fresh immediately before use
This recipe is for one set of eight ultracentrifuge tubes. Do not filter-sterilize, but preparation should be conducted in a laminar flow hood.
NaCl, 3 M
- 43.83 g NaCl
- Molecular biology–grade H2O to 250 ml
- Optional: Filter-sterilize with Nalgene sterile disposable filter unit with PES membrane (Thermo Scientific, cat. no. 5670020, or equivalent) and Nalgene sterile disposable 250- or 500-ml receiver (Thermo Scientific, cat. no. 455-0250 or 455-0500, or equivalent)
- Store ≤6 months at room temperature
TD buffer, 5×
- 5× PBS
- 5 mM MgCl2
- 12.5 mM KCl
- Molecular biology–grade H2O
- Optional: Filter-sterilize with Nalgene sterile disposable filter unit with PES membrane (Thermo Scientific, cat. no. 5670020, or equivalent) and Nalgene sterile disposable 250- or 500-ml receiver (Thermo Scientific, cat. no. 455-0250 or 455-0500, or equivalent)
- Store ≤6 months at room temperature
Transfection medium
- DMEM/F12 (1:1; Gibco, cat. no. 11320033, or Cytiva, cat. no. SH30023.FS)
- 2% (v/v) FBS (Omega or equivalent)
- 1× penicillin-streptomycin-glutamine (PSG; from 100×; Gibco, cat. no. 10378016)
- 0.8 µg/ml amphotericin B (from 10 mg/ml; Sigma, cat. no. A9528)
- Filter-sterilize with Nalgene sterile disposable filter unit with PES membrane (Thermo Scientific, cat. no. 5670020, or equivalent) and Nalgene sterile disposable 250- or 500-ml receiver (Thermo Scientific, cat. no. 455-0250 or 455-0500, or equivalent)
- Store ≤6 months at 4°C
One liter will be needed for one AAV preparation. Preparation should be conducted in a laminar flow hood to maintain sterility.
COMMENTARY
Background Information
AAV generation is used as a method for genetic manipulation in vivo , in addition to or in place of traditional forms of genetic manipulation, namely transgenesis (Kumar et al., 2009). AAV is a popular alternative to transgenesis due to its relatively low cost of production and comparatively quick production, qualities that together add to the overall greater versatility of AAV as a tool. As popular means of genetic manipulation in vivo , procedures for generating high titers of recombinant AAV have grown in popularity, with industrial labs devoted to its production in high-throughput procedures. The method detailed here suits an academic lab/institution that seeks to generate their own AAV without specialized equipment and high costs.
Critical Parameters
The most critical parameters when generating robust AAV yields are rooted in Basic Protocol 1, where low plasmid integrity is the most common cause of low AAV yield. Ensure that helper plasmids are primarily supercoiled for efficient transfection and that recombinant AAV plasmid has intact internal terminal repeats (ITRs). A common method to check for ITR integrity is to perform a restriction digest with SmaI. An AAV plasmid should have two ITRs, and within each is a SmaI restriction site. Therefore, at least two bands should be generated from a SmaI restriction digest, with more if internal SmaI sites exist. Ensure that you know your sequence well to predict the result of the restriction digest and confirm intact ITRs.
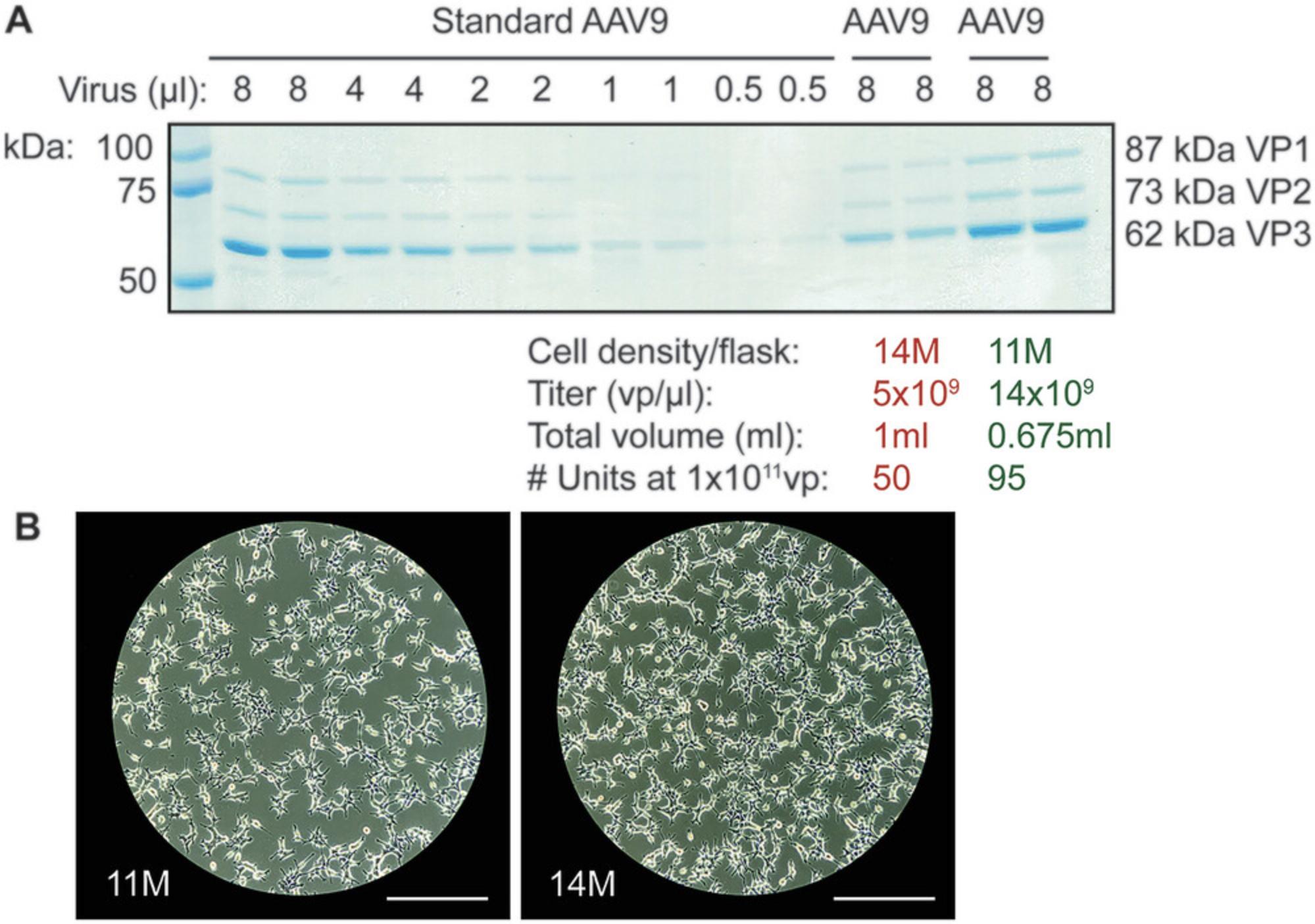
In addition, cell confluency in T-175 flasks at the time of transfection is a crucial factor in obtaining high AAV titers (Fig. 3). Lastly, the section on harvest of AAV at the end of Basic Protocol 1 details the use of TrypLE to harvest cells, and using TrypLE to gently detach cells from the flask is optimal for obtaining high yields of AAV. Using cell lifters/cell scrapers can result in significant loss of AAV, likely due to the disruptive nature of cell lysis (unpub. observ.). Therefore, to most efficiently retrieve AAV produced from AAVPro-293T cells, gentle trypsinization is highly recommended.
Troubleshooting
For common problems and suggested troubleshooting, see Table 5.
| Problem | Possible cause | Solution |
|---|---|---|
| Low AAV titer (<60 units from 48 T-175 flasks); unit: 1 × 1011vp | Poor DNA plasmid quality | The most likely reason for low AAV yields is poor DNA plasmid quality. Check plasmid integrity for all plasmids by gel electrophoresis. On a 1% agarose gel, run uncut helper plasmid pAdDeltaF6 and uncut pAAV2/9n to ensure use of primarily supercoiled DNA. For the rAAV genome plasmid, run SmaI-digested plasmid to ensure integrity of ITRs. |
| Low AAV titer due to low volume retrieval from Vivaspin device (<400 µl), typically associated with unusually quick flow through Vivaspin columns (<8 min/wash) | Faulty Vivaspin column, leading to uncontrollable flow through the column and potential loss of AAV | Unusually quick flow through the column will be evident by the first wash with Lactated Ringer's, if not earlier. Reduce speed to 2000 × g. If issue persists, immediately replace columns. |
| Unusually slow flow through Vivaspin columns (>25 min) | Iodixanol was not diluted enough or centrifugation was at 4°C instead of room temperature |
If the iodixanol is too concentrated (e.g., you collected more of the 60% phase than intended), the flow through the Vivaspin will take significantly longer at the packing step and may damage the Vivaspin column. Immediately add more Lactated Ringer's to dilute. If the iodixanol is sufficiently diluted, ensure that the centrifuge is at room temperature. The colder the centrifuge, the slower the flow through the Vivaspin. |
Understanding Results
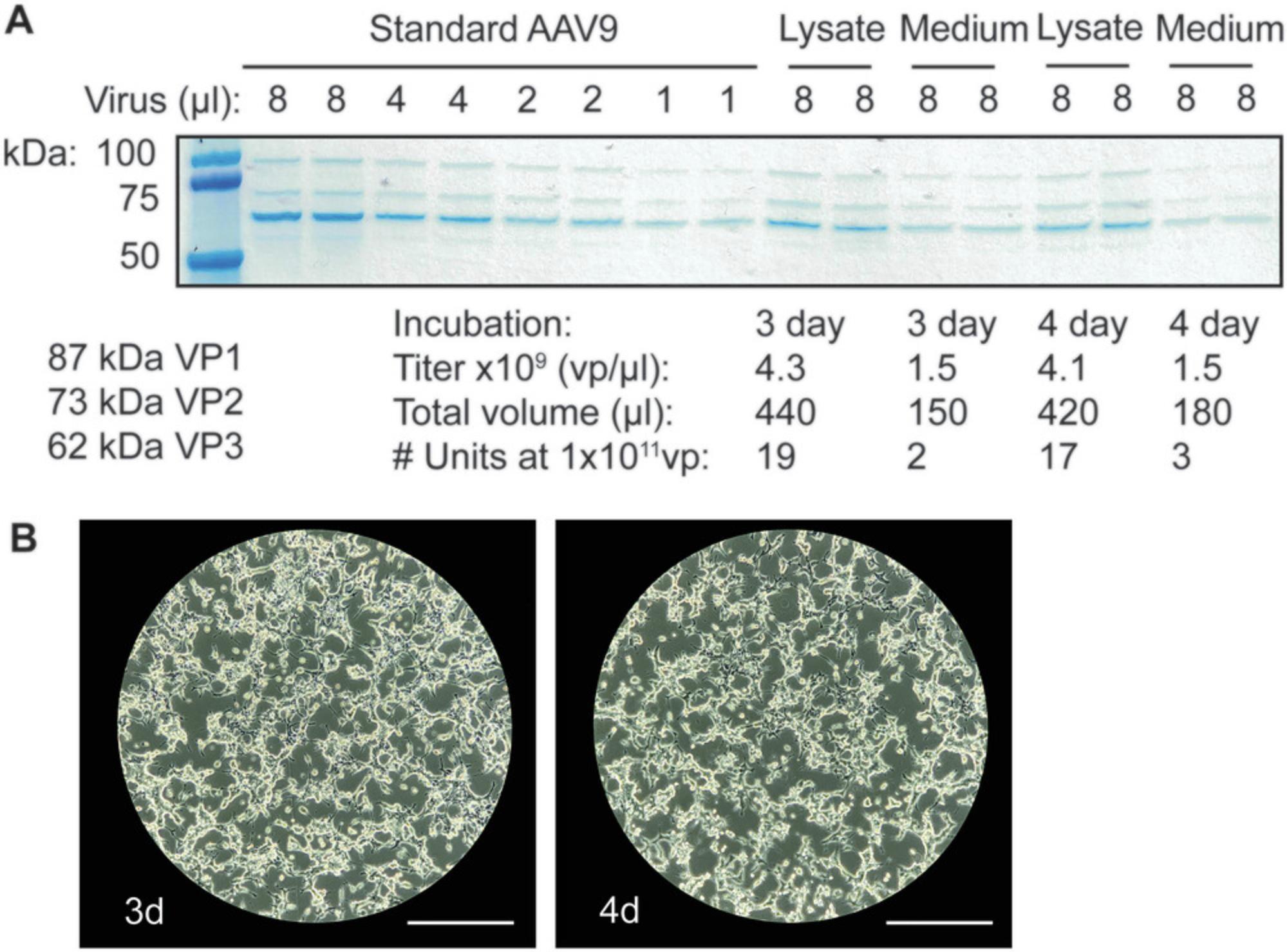
The recombinant AAV obtained as a result of Basic Protocols 1 and 2 will be suitable for use in animals (in vivo). Step 10 of Basic Protocol 2 involves loading a sample from the collected 40% phase of each iodixanol gradient to determine the purity of the AAV particles generated. This step is also the first step that indicates whether recombinant AAV generation was successful. Bands corresponding to VP1, VP2, and VP3 should be clear by 15 min of application of Coomassie blue stain (Fig. 6). Because genome containing AAV particles migrates to the 40%-to-60% interface and 40% phase of the iodixanol gradient, whereas empty AAV capsids sediment at the 25%-to-40% interphase (Kimura et al., 2019; Zolotukhin et al., 1999), viral capsid protein bands are a good indicator for AAV genome–containing particle titer, assuming proper collection technique. Alternatively, qPCR of the recombinant AAV genome can be performed to ensure AAV genome–containing particles are present. However, our method has consistently produced titers that, when injected into the tail vein in vivo , result in efficient transduction of the AAV at 1–3 × 1011 vp.
Using our method, each preparation of 48 T-175 flasks generates enough AAV for about 80 to 120 mice at a dose of 1 × 1011 vp. It is highly recommended that a dose-response study is done in vivo to determine AAV-based gene expression at 1 and 3 × 1011 vp, as, in our experience, different recombinant AAV preparations express differently, depending on the promoter and the desired tissue to be transduced. However, multiple preparations of the same recombinant AAV genome have produced highly consistent transduction at the same dose; therefore, a dose-response study needs only be performed once for each new recombinant AAV.
Additionally, using an AAV with a fluorescent reporter is a convenient way to visualize gene transduction efficiency at the histological and microscopic levels. Another approach is to apply an AAV-driving Cre recombinase to a Cre-dependent fluorescent mouse model, for example the mT/mG mice provided by the Jackson Laboratory (stock no. 007676). These methods provide important information as to the AAV transduction efficiency and the strength of the promoter of the recombinant AAV generated.
Time Considerations
For Basic Protocol 1, about 10 to 12 hr per week can be expected if one person performs the protocol. This time can be reduced significantly with recruitment of additional personnel. The most time-consuming steps in this protocol are plating AAVPro-293T cells in 48 T-175 flasks and AAV harvest from AAVPro-293T cell lysates. If following the Strategic Planning outline in Figure 1, these steps fall on Thursday and Monday, respectively.
For Basic Protocol 2, about 12 to 13 hr per week can be expected if one person performs the protocol. Consider that ∼3 hr of the 12 to 13 hr consists of centrifugation time. The most time-consuming step in this protocol is setting up and running the iodixanol gradient centrifugation. If following the Strategic Planning outline in Figure 1, this step falls on a Tuesday.
If expecting to perform two or more continuous AAV preps, it is recommended that at least two individuals be recruited for this method, with one person devoted to Basic Protocol 1 and the other to Basic Protocol 2. This ensures consistency in the protocols and manages time efficiently if the personnel are expected to perform other unrelated tasks, as is often the case in an academic lab.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health to EAB (HL140850) and CCG (HL135893; HL141463; HL149931). This work was also supported by grants and fellowships from the University of Arizona RII, Sarver Heart Center, Bio5 Institute, and Valley Research Partnership Program and a New Investigator Award from the Arizona Biomedical Research Centre (RFGA2022-010-10) to EAB and by a grant from the University of Arizona College of Medicine – Phoenix Translational Cardiovascular Research Center to CCG.
Author Contributions
Alina S. Bilal : Conceptualization, data curation, formal analysis, methodology, project administration, supervision, validation, writing – original draft, writing – review & editing; Sarah N. Parker : Data curation, methodology, validation, writing – review & editing; Victoria B. Murray : Data curation, methodology, validation, writing – review & editing; Lauren F. MacDonnell : Data curation, methodology, validation, writing – review and editing; Donna J. Thuerauf : Conceptualization, methodology, project administration, supervision, validation; Christopher C. Glembotski : Project administration, supervision, writing – review and editing; Erik A. Blackwood : Conceptualization, funding acquisition, methodology, project administration, supervision, writing – original draft, writing – review and editing.
Conflict of Interest
The authors have no conflict of interest to disclose.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.
Literature Cited
- Bish, L. T., Morine, K., Sleeper, M. M., Sanmiguel, J., Wu, D., Gao, G., Wilson, J. M., & Sweeney, H. L. (2008). Adeno-associated virus (AAV) serotype 9 provides global cardiac gene transfer superior to AAV1, AAV6, AAV7, and AAV8 in the mouse and rat. Human Gene Therapy , 19(12), 1359–1368. https://doi.org/10.1089/hum.2008.123
- Blackwood, E. A., Hofmann, C., Santo Domingo, M., Bilal, A. S., Sarakki, A., Stauffer, W., Arrieta, A., Thuerauf, D. J., Kolkhorst, F. W., Muller, O. J., Jakobi, T., Dieterich, C., Katus, H. A., Doroudgar, S., & Glembotski, C. C. (2019). ATF6 regulates cardiac hypertrophy by transcriptional induction of the mTORC1 activator, Rheb. Circulation Research , 124(1), 79–93. https://doi.org/10.1161/CIRCRESAHA.118.313854
- Inagaki, K., Fuess, S., Storm, T. A., Gibson, G. A., Mctiernan, C. F., Kay, M. A., & Nakai, H. (2006). Robust systemic transduction with AAV9 vectors in mice: Efficient global cardiac gene transfer superior to that of AAV8. Molecular Therapy , 14(1), 45–53. https://doi.org/10.1016/j.ymthe.2006.03.014
- Kimura, T., Ferran, B., Tsukahara, Y., Shang, Q., Desai, S., Fedoce, A., Pimentel, D. R., Luptak, I., Adachi, T., Ido, Y., Matsui, R., & Bachschmid, M. M. (2019). Production of adeno-associated virus vectors for in vitro and in vivo applications. Scientific Reports , 9, 13601. https://doi.org/10.1038/s41598-019-49624-w
- Kumar, T. R., Larson, M., Wang, H., McDermott, J., & Bronshteyn, I. (2009). Transgenic mouse technology: Principles and methods. In O. K. Park-Sarge & T. Curry (Eds.), Molecular endocrinology (Vol. 590), Humana Press. https://doi.org/10.1007/978-1-60327-378-7_22
- Lopez-Gordo, E., Kohlbrenner, E., Katz, M. G., & Weber, T. (2019). AAV vectors for efficient gene delivery to rodent hearts. In M. Castle (Ed.), Adeno-associated virus vectors (Vol. 1950). Humana Press. https://doi.org/10.1007/978-1-4939-9139-6_19
- Zolotukhin, S., Byrne, B., Mason, E., Zolotukhin, I., Potter, M., Chesnut, K., Summerford, C., Samulski, R. J., & Muzyczka, N. (1999). Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Therapy , 6, 973–985. https://doi.org/10.1038/sj.gt.3300938
- INTERNET RESOURCES.
- https://imagej.net/software/fiji/
- Software for densitometry analysis, as described in Basic Protocol 2, step 22.

