One-Flask Synthesis of Cyclic Diguanosine Monophosphate (c-di-GMP)
Barbara L. Gaffney, Barbara L. Gaffney, Roger A. Jones, Roger A. Jones
Abstract
The bacterial signaling molecule cyclic diguanosine monophosphate (c-di-GMP) plays a key role in controlling biofilm formation and pathogenic virulence, among many other functions. It has widespread consequences for human health, and current research is actively exploring its molecular mechanisms. The convenient one-flask, gram-scale synthesis of c-di-GMP described here has facilitated these efforts and has been applied to a variety of analogs. © 2023 The Authors. Current Protocols published by Wiley Periodicals LLC.
Basic Protocol 1:
A number of routes for the synthesis of c-di-GMP have been reported over the years, starting with van Boom's original phosphate triester approach (Ross et al., 1990). Since then, various combinations of amidite and H-phosphonate couplings have been employed (Amiot et al., 2006; Hayakawa et al., 2003; Hyodo & Hayakawa, 2004; Hyodo et al., 2006; Kiburu et al., 2008; Yan & Aguilar, 2007; Zhang et al., 2004). Until now, these methods have given only small amounts of products. In contrast, the method reported here (Gaffney et al., 2010) yields over 1 g of product and can readily be scaled up further. No chromatographic steps are required, and the intermediate tert- butylammonium salt and the final product 5 are purified by simple crystallizations.
NOTE : Reagents and solvents are available from Sigma-Aldrich, and the apparatus from Fisher.
Materials
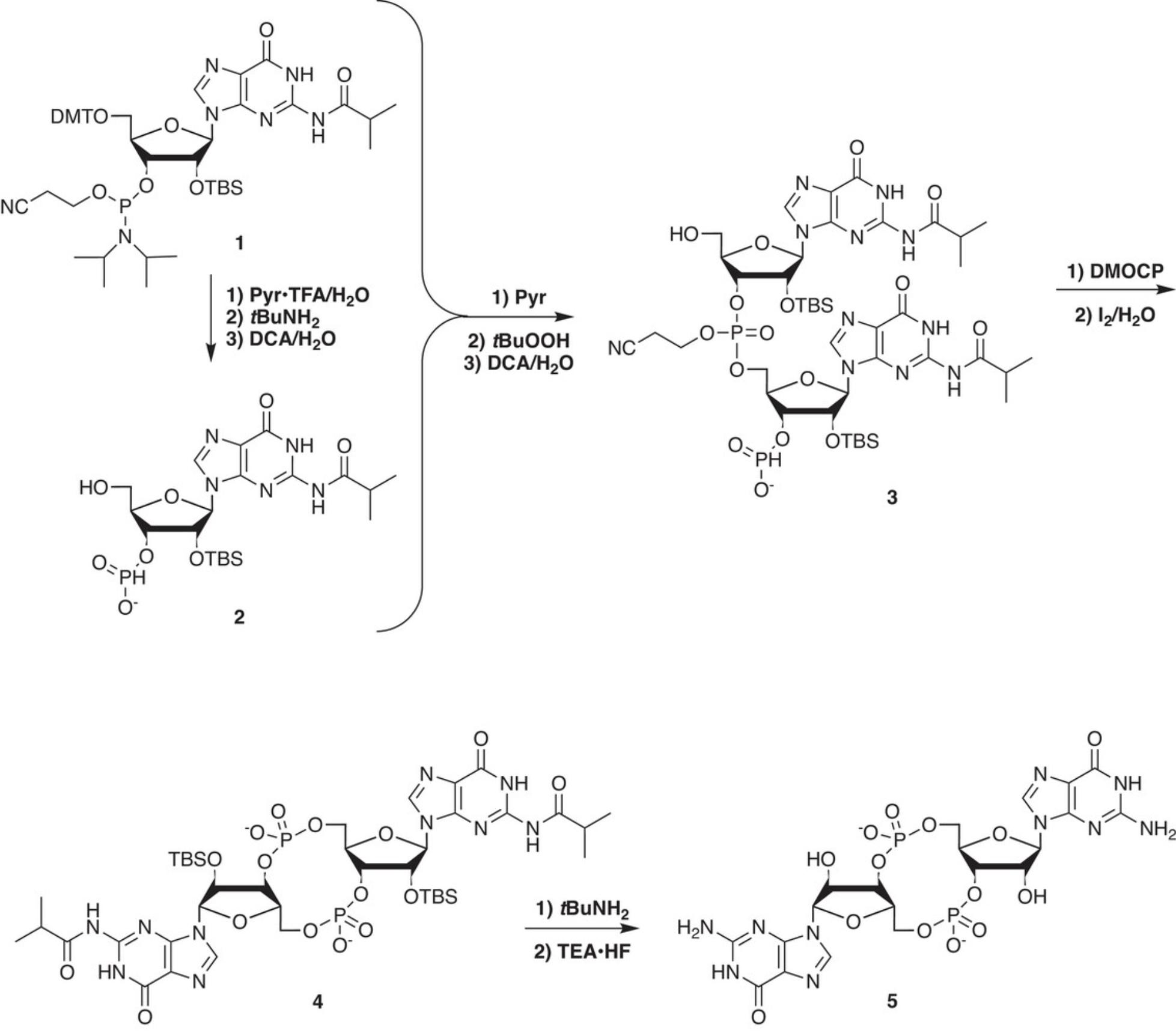
- 3′-O -[(Diisopropylamino)(2-cyanoethoxy)phosphino]-5′-O -(4,4′-dimethoxytrityl)-2′-O -tert -butyldimethylsilyl-N 2-isobutyrylguanosine (1 ; Fig. 1)
- Dry acetonitrile (CH3CN)
- 3-Å molecular sieves
- Pyridinium trifluoroacetate
- tert -Butyl amine (tert -BuNH)
- Dichloroacetic acid (DCA)
- Pyridine
- Ethyl acetate (EtOAc)
- Dry nitrogen or argon
- 5.5 M tert- butyl hydroperoxide in decane
- Sodium bisulfite (NaHSO3)
- Sodium bicarbonate (NaHCO3)
- Dichloromethane (CH2Cl2)
- 95% 5,5-dimethyl-2-oxo-2-chloro-1,3,2-dioxaphosphinane (DMOCP)
- Iodine (I2)
- Diethyl ether (Et2O)
- Methanol (CH3OH)
- Potassium hydroxide (KOH)
- 33% (w/w) methylamine (CH3NH2) in anhydrous ethanol
- Triethylamine (TEA; Et3N)
- Triethylamine trihydrogen fluoride (Et3N·3HF)
- Calcium gluconate gel (Lab Safety Supply)
- 10% (w/w) aqueous potassium carbonate (K2CO3)
- HPLC-grade acetone
-
100-ml pear-shaped flask
-
Rotary evaporator
-
Vacuum pump
-
Septa
-
100-ml, 250-ml, 500-ml, and 2-L round-bottom flasks
-
Stir bars
-
Magnetic stir plates
-
16-G double-tipped needle
-
Disposable syringes and needles
-
Ice bath
-
2-L Erlenmeyer flasks
-
2-L separatory funnels
-
Spatula
-
Filter paper
-
Sintered glass funnels
-
Desiccator
-
50°C oil bath
-
Additional reagents and equipment for analytical high-performance liquid chromatography (HPLC; Sinha & Jung, 2015)
Dry compound 1
1.Place 6.31 g (6.5 mmol) of 1 into a 100-ml pear-shaped flask. Dry the amidite twice by evaporation of 40 ml CH3CN on a rotary evaporator, the last time leaving ∼20 ml. Add ten 3-Å molecular sieves, and a stopper with a septum. Set aside until needed.
Perform hydrolysis, β-elimination, and detritylation of second portion of 1 to give 2
2.Place another portion of 1 (4.85 g, 5.0 mmol) into a 500-ml round-bottom flask with a stir bar. Add 1.16 g of pyridinium trifluoroacetate (6.0 mmol, 1.2 equiv) and rinse down the sides of the flask with 25 ml CH3CN. Add 0.18 ml H2O (10 mmol, 2 equiv) and stir for 1 min. Add 25 ml tert- BuNH2 and let the solution stir for 10 min. Place the flask on a rotary evaporator and concentrate to a foam. Evaporate twice with 50 ml CH3CN, each time to give a foam.
3.Add 60 ml CH2Cl2 and mix until the oil is dissolved. Add 0.90 ml H2O (50 mmol, 10 equiv) and then 60 ml of 6% dichloroacetic acid (DCA; 44 mmol, 8.8 equiv) in CH2Cl2.
4.After 10 min, quench with 7.0 ml pyridine (87 mmol, 2 equiv relative to DCA). Concentrate the solution to ∼20 ml and then evaporate three times with 40-ml portions of dry CH3CN, the last time leaving about 12 ml. Check by HPLC (HPLC conditions are described in the Critical Parameters section; retention times are given in Table 1) before the last concentration to be sure detritylation is complete. (If not, concentrate, evaporate several times with ethyl acetate to remove pyridine, and repeat step 3 with less acid, depending on how much tritylated material was left.) Stopper the flask with a septum.
| Reverse-phase HPLC retention time (min) | |
|---|---|
| S.1 | 11.7a |
| S.2 | 5.4a |
| S.3 | 9.2/9.5a |
| S.4 | 7.2a |
| S.5 | 7.3/7.6a |
| S.6 | 6.2a, 9.7b |
| S.7 | 3.2b |
- a
Gradient of 2% to 100% CH3CN and 0.1 M TEAA.
- b
Gradient of 2% to 40% CH3CN and 0.1 M TEAA.
Perform linear coupling of 1 with 2 and detritylation to give 3
5.Transfer the dried 1 to 2 through a 16-G double-tipped needle by inserting one end of the needle through the septum of the flask containing 1 all the way to the bottom, and the other end through the septum of the flask containing 2 but above the solution. Place a vent needle in the flask with 2. Place a needle-tipped line from a source of dry nitrogen or argon into the flask with 1 , thereby causing the solution of 2 to flow into the flask with 1.
6.Immediately inject a 1-ml rinse of dry CH3CN into the flask that had contained 1 (from step 1), rotate the flask quickly to rinse the sides of the flask, and transfer this rinse through the double-tipped needle into the flask with 2. Repeat this rinse one more time with 1 ml fresh CH3CN. Allow the mixture of 1 and 2 to stir for 2 min, and then take a sample for HPLC analysis (HPLC conditions are described in the Critical Parameters section; retention times are given in Table 1).
7.As soon as the HPLC sample is removed, add 2.73 ml anhydrous 5.5 M tert- butyl hydroperoxide in decane (15 mmol, 3 equiv). Let the solution stir for 30 min and then take an HPLC sample. Once the HPLC sample is removed, place the flask in an ice bath and add 1.25 g NaHSO3 dissolved in 2.5 ml H2O. After 5 min of stirring, concentrate to an oil.
8.Add 80 ml CH2Cl2 to the flask and mix until the oil is dissolved. Add 0.90 ml H2O (50 mmol, 10 equiv) and then 80 ml of 6% dichloroacetic acid (58 mmol, 11.6 equiv) in CH2Cl2.
9.After 10 min, quench the reaction with 15 ml pyridine. Take an HPLC sample, add 35 ml more pyridine, and concentrate to ∼20 ml. Add 150 ml pyridine and concentrate to 100 ml. Stopper the flask with a septum.
Perform cyclization of 3 to give 4
10.Add 3.40 g of 95% 5,5-dimethyl-2-oxo-2-chloro-1,3,2-dioxaphosphinane (DMOCP; 17.5 mmol, 3.5 equiv) and stir for 10 min.
11.Add 3.2 ml H2O (175 mmol, 10 equiv relative to DMOCP). Immediately add 1.65 g I2 (6.5 mmol, 1.3 equiv) and stir for 5 min. Take an HPLC sample, immediately pour the mixture into a 2-L Erlenmeyer flask containing 700 ml H2O and 1.0 g NaHSO3, and stir 5 min.
12.Slowly add 20 g NaHCO3 (238 mmol) and stir for 5 min.
13.Add 800 ml of 1:1 (v/v) EtOAc/Et2O and stir for 5 min. Pour the mixture into a 2-L separatory funnel and shake thoroughly. Drain the aqueous layer and pour the organic layer into a 2-L round-bottom flask. Extract the aqueous layer again with an additional 200 ml of 1:1 EtOAc/Et2O. Drain the aqueous layer and pour the second organic layer into the round-bottom flask. Concentrate to remove most of the ether, and check by HPLC.
Perform deprotection of 4 to give 5
14.Continue concentrating the solution of 4 to less than 50 ml. Transfer the solution into a 250-ml round-bottom flask. Add 20-ml portions of ethyl acetate and evaporate three times to help remove excess pyridine.
15.Add a stir bar and 25 ml CH3CN. Stir to mix, add 25 ml of tert- BuNH2, and stir for 10 min. Concentrate the solution to dryness. Add 25 ml CH3CN, scrape the sides of the flask with a spatula, and concentrate to dryness. Repeat two more times. Dissolve the solid in 25 ml CH3OH and check by HPLC. Filter (using a filter paper) the solution into a 100-ml round-bottom flask, removing the stir bar. Concentrate the solution to a foam.
16.Add 25 ml CH2Cl2 to the foam and immediately swirl vigorously until the solid dissolves. Stopper the flask with a septum, let it sit until crystal deposition has slowed, and then refrigerate overnight.
17.Collect the white crystals of the tert- butyl ammonium salt in a preweighed sintered glass funnel and wash them three times with no more than 2 ml CH2Cl2 each time, stirring thoroughly. Cover the funnel with filter paper and dry it in a desiccator over KOH overnight. Weigh it and check a sample by HPLC.
18.Transfer the above into a 250-ml round-bottom flask. For each 1.00 mmol (1.20 g), add 100 ml CH3NH2 (800 equiv) in anhydrous ethanol (33% by weight). Add a stir bar, cap with a septum, and stir for 90 min. Check completion of the reaction by HPLC. Concentrate to an oil. Add 4 ml pyridine and 2 ml Et3N, swirl to mix, and concentrate to an oil. Repeat this procedure two more times.
19.Add 4 ml pyridine to the flask, cap with a septum, and insert a vent needle. Place the flask in an oil bath at 50°C in a hood and stir. For every 1.00 mmol (1.20 g), draw up 14 ml Et3N and 8.3 ml Et3N·3HF (150 equiv F–) into separate syringes and insert them both through the septum of the flask. Add the two reagents simultaneously over ∼1 min. Stir the mixture for 1 hr at 50°C, occasionally rotating the flask at an angle to dissolve solids on the sides.
20.Remove the flask from the oil bath and start it stirring on a different stir plate. Remove the septum, and for every 1.00 mmol, add 112 ml HPLC-grade acetone in a slow, steady stream over 1 min. Continue to stir the mixture as the product crystallizes. After 10 min, collect the crystals by filtration in a sintered glass funnel and wash them five times using 5-ml portions of acetone. Stir thoroughly between each wash. If the product is sticky, use a spatula to repeatedly smear it against the sides of the funnel, thereby removing trapped impurities. Cover the funnel with filter paper and dry it in a desiccator over KOH overnight. Weigh it and check a sample by HPLC. Analyze 5 by 1H and 31P NMR.
COMMENTARY
Background Information
The bacterial signaling molecule cyclic diguanosine monophosphate (c-di-GMP) plays key roles in controlling biofilm formation, organelle formation for motility, cell-cycle differentiation, and pathogenic virulence, among many other functions (Cotter & Stibitz, 2007; Hengge, 2009; Romling & Amikam, 2006; Schirmer & Jenal, 2009; Tamayo et al., 2007). It acts by relaying extracellular signals to internal receptors, including PilZ proteins (Ko et al., 2010), as well as two different classes of riboswitches (Kulshina et al., 2009; Lee et al., 2010; Smith et al., 2009, 2011; Sudarsan et al., 2008). It has widespread consequences for human health, and current research is actively exploring its molecular mechanisms. The convenient one-flask, gram-scale synthesis of c-di-GMP described here and shown in Figure 1 has facilitated these efforts and has been applied to a variety of analogs (Bartsch et al., 2022; Chen, 2023; Chen et al., 2023; Gao, Ascano, Wu, et al., 2013; Gao, Ascano, Zillinger, et al., 2013; Meehan et al., 2016; Sun et al., 2023; Yan, 2021; Zhang et al., 2013, 2023).
Critical Parameters
The detritylations in steps 3 and 8 are potentially reversible upon quenching and again upon concentration, in spite of the water added to prevent it. The concentrated oils of detritylated 2 and 3 should be used immediately, and not stored.
Conditions for the initial amidite coupling between 1 and 2 should be as dry as possible.
HPLC is useful for following the course of the synthesis. We used a Waters Atlantis C18 column, 4.6 mm × 50 mm, 3 μm, with gradients of 2% to 100% CH3CN and 0.1 M triethylamine acetate (TEAA; pH 6.8) for steps 1 to 17, and 2% to 40% at the end of step 18. However, many steps are time dependent, and the procedure must be continued immediately after taking samples, without waiting to see the results. Note that compounds with a cyanoethyl group may elute as two peaks because there are two diastereomers.
The linear dimer is best cyclized and oxidized as soon as it is made. Therefore, steps 1 to 13 should be performed on the same day.
Because of the danger of using Et3N·3HF, HPLC should not be performed to monitor progress of the final desilylation. Once the solids are in solution (there may be two layers), the reaction should go to completion in 1 hr.
Anticipated Results
The yield for crystallization of the intermediate tert -butylammonium salt should be ∼35% from 1 , and the yield for crystallization of the final product, 5 , should be ∼30% from 1. Both are white powders that are homogeneous by HPLC-mass spectrometry (HPLC-MS).
Time Considerations
The entire sequence of reactions can be done in 4 days, with final NMR analysis taking longer. Steps 1 to 13 should be performed all in 1 day (8 to 10 hr) or the yield will suffer. Steps 14 to 16 take a second day (2 to 3 hr), and step 17 takes only a few minutes of a third day. Steps 18 to 20 take a fourth day (6 to 8 hr).
Acknowledgment
This work was supported by US National Institutes of Health grant GM 79760.
Author Contributions
Barbara L. Gaffney : Investigation, writing—original draft, writing—review and editing; Roger A. Jones : Conceptualization, funding acquisition, project administration, writing—original draft, writing—review and editing.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
No new data were created or analyzed during the writing of this protocol.
Literature Cited
- Amiot, N., Heintz, K., & Giese, B. (2006). New approach for the synthesis of c-di-GMP and its analogues. Synthesis , 24, 4230–4236.
- Bartsch, T., Becker, M., Rolf, J., Rosenthal, K., & Lütz, S. (2022). Biotechnological production of cyclic dinucleotides—Challenges and opportunities. Biotechnology and Bioengineering , 119(3), 677–684. https://doi.org/10.1002/bit.28027
- Chen, C. (2023). 10th anniversary of discovering cGAMP: Synthesis and beyond. Organic Chemistry Frontiers , 10, 1086–1098. https://doi.org/10.1039/D2QO02033E
- Chen, L.-Y., Pang, X.-Y., Chen, C., & Xu, H.-G. (2023). NF-κB regulates the expression of STING via alternative promoter usage. Life Sciences , 314, 121336. https://doi.org/10.1016/j.lfs.2022.121336
- Cotter, P. A., & Stibitz, S. (2007). c-di-GMP-mediated regulation of virulence and biofilm formation. Current Opinion in Microbiology , 10, 17–23. https://doi.org/10.1016/j.mib.2006.12.006
- Gaffney, B. L., Veliath, E., Zhao, J., & Jones, R. A. (2010). One-flask syntheses of c-di-GMP and the [R p,R p] and [R p,S p] thiophosphate analogues. Organic Letters , 12, 3269–3271. https://doi.org/10.1021/ol101236b
- Gao, P., Ascano, M., Wu, Y., Barchet, W., Gaffney, B. L., Zillinger, T., Serganov, A. A., Liu, Y., Jones, R. A., Hartmann, G., Tuschl, T., & Patel, D. J. (2013). Cyclic [G(2′,5′)pA(3′,5′)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell , 153(5), 1094–1107. https://doi.org/10.1016/j.cell.2013.04.046
- Gao, P., Ascano, M., Zillinger, T., Wang, W., Dai, P., Serganov, A. A., Gaffney, B. L., Shuman, S., Jones, R. A., Deng, L., Hartmann, G., Barchet, W., Tuschl, T., & Patel, D. J. (2013). Structure-function analysis of STING activation by c[G(2',5')pA(3',5')p] and targeting by antiviral DMXAA. Cell , 154(4), 748–762. https://doi.org/10.1016/j.cell.2013.07.023
- Hayakawa, Y., Nagata, R., Hirata, A., Hyodo, M., & Kawai, R. (2003). A facile synthesis of cyclic bis(3′-5′)diguanylic acid. Tetrahedron , 59, 6465–6471. https://doi.org/10.1016/S0040-4020(03)01045-7
- Hengge, R. (2009). Principles of c-di-GMP signalling in bacteria. Nature Reviews Microbiology , 7, 263–273. https://doi.org/10.1038/nrmicro2109
- Hyodo, M., & Hayakawa, Y. (2004). An improved method for synthesizing cyclic bis(3′-5′)diguanylic acid (c-di-GMP). Bulletin of the Chemical Society of Japan , 77, 2089–2093. https://doi.org/10.1246/bcsj.77.2089
- Hyodo, M., Sato, Y., & Hayakawa, Y. (2006). Synthesis of cyclic bis(3′-5′)diguanylic acid (c-di-GMP) analogs. Tetrahedron , 62, 3089–3094. https://doi.org/10.1016/j.tet.2006.01.025
- Kiburu, I., Shurer, A., Yan, L., & Sintim, H. O. (2008). A simple solid-phase synthesis of the ubiquitous bacterial signaling molecule, c-di-GMP and analogues. Molecular Biosystems , 4, 518–520. https://doi.org/10.1039/b719423d
- Ko, J., Ryu, K.-S., Kim, H., Shin, J.-S., Lee, J.-O., Cheong, C., & Choi, B.-S. (2010). Structure of PP4397 reveals the molecular basis for different c-di-GMP binding modes by Pilz domain proteins. Journal of Molecular Biology , 398, 97–110. https://doi.org/10.1016/j.jmb.2010.03.007
- Kulshina, N., Baird, N. J., & Ferré-D'Amaré, A. R. (2009). Recognition of the bacterial second messenger cyclic diguanylate by its cognate riboswitch. Nature Structural & Molecular Biology, 16, 1212–1217.
- Lee, E. R., Baker, J. L., Weinberg, Z., Sudarsan, N., & Breaker, R. R. (2010). An allosteric self-splicing ribozyme triggered by a bacterial second messenger Science , 329, 845–848. https://doi.org/10.1126/science.1190713
- Meehan, R. E., Torgerson, C. D., Gaffney, B. L., Jones, R. A., & Strobel, S. A. (2016). Nuclease-resistant c-di-AMP derivatives that differentially recognize RNA and protein receptors. Biochemistry , 55, 837–849. https://doi.org/10.1021/acs.biochem.5b00965
- Romling, U., & Amikam, D. (2006). Cyclic di-GMP as a second messenger. Current Opinion in Microbiology , 9, 218–228. https://doi.org/10.1016/j.mib.2006.02.010
- Ross, P., Mayer, R., Weinhouse, H., Amikam, D., Huggirat, Y., Benziman, M., de Vroom, E., Fidder, A., Paus, P. D., Sliedregt, L. A. J. M., van der Marel, G. A., & van Boom, J. H. (1990). The cyclic diguanylic acid regulatory system of cellulose synthesis in Acetobacter xylinum. Journal of Biological Chemistry , 265, 18933–18943. https://doi.org/10.1016/S0021-9258(17)30606-3
- Schirmer, T., & Jenal, U. (2009). Structural and mechanistic determinants of c-di-GMP signalling. Nature Reviews Microbiology , 7, 724–735. https://doi.org/10.1038/nrmicro2203
- Sinha, N. D., & Jung, K. E. (2015). Analysis and purification of synthetic nucleic acids using HPLC. Current Protocols in Nucleic Acid Chemistry , 61, 10.5.1–10.5.39. https://doi.org/10.1002/0471142700.nc1005s61
- Smith, K. D., Lipchock, S. V., Ames, T. D., Wang, J., Breaker, R. R., & Strobel, S. A. (2009). Structural basis of ligand binding by a c-di-GMP riboswitch. Nature Structural & Molecular Biology, 16, 1218–1223.
- Smith, K. D., Shanahan, C. A., Moore, E. L., Simon, A. C., & Strobel, S. A. (2011). Structural basis of differential ligand recognition by two classes of bis-(3′-5′)-cyclic dimeric guanosine monophosphate-binding riboswitches. Proceedings of the National Academy of Sciences of the United States of America , 108, 7757–7762. https://doi.org/10.1073/pnas.1018857108
- Sudarsan, N., Lee, E. R., Weinberg, Z., Moy, R. H., Kim, J. N., Link, K. H., & Breaker, R. R. (2008). Riboswitches in eubacteria sense the second messenger cyclic di-GMP. Science , 321, 411–413. https://doi.org/10.1126/science.1159519
- Sun, X., Yu, X., Zhao, Y., Xing, L., Na, L., Chen, Z., Xiao, Z., Dai, H., Yu, J., Long, S., Wang, Q., Shi, X., Guan, Z., Lei, M., & Yang, Z. (2023). Cyclic diguanylate analogues: Facile synthesis, STING binding mode and anti-tumor immunity delivered by cytidinyl/cationic lipid. European Journal of Medicinal Chemistry , 247, 115053. https://doi.org/10.1016/j.ejmech.2022.115053
- Tamayo, R., Pratt, J. T., & Camilli, A. (2007). Roles of cyclic diguanylate in the regulation of bacterial pathogenesis. Annual Review of Microbiology , 61, 131–148. https://doi.org/10.1146/annurev.micro.61.080706.093426
- Yan, H., & Aguilar, A. L. (2007). Synthesis of 3′,5′-cyclic diguanylic acid (cdiGMP) using 1-(4-chlorophenyl)-4-ethoxypiperidin-4-yl as a protecting group for 2′-hydroxy functions of ribonucleosides. Nucleosides, Nucleotides, and Nucleic Acids , 26, 189–204. https://doi.org/10.1080/15257770601112762
- Yan, H., & Chen, W. (2021). The promise and challenges of cyclic dinucleotides as molecular adjuvants for vaccine development. Vaccines (Basel) , 9(8), 917. https://doi.org/10.3390/vaccines9080917
- Zhang, X., Shi, H., Wu, J., Zhang, X., Sun, L., Chen, C., & Chen, Z. J. (2013). Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Molecular Cell , 51(2), 226–235. https://doi.org/10.1016/j.molcel.2013.05.022
- Zhang, Z., Gaffney, B. L., & Jones, R. A. (2004). c-di-GMP displays a monovalent metal ion-dependent polymorphism. Journal of the American Chemical Society , 126, 16700–16701. https://doi.org/10.1021/ja0449832
- Zhang, L., Tang, X., Xi, Z., & Chattopadhyaya, J. (2023). Cyclic dinucleotides. John Wiley.

