ONT Flongle Flowcell Loading with Q20+ (V12) Chemistry
Stephen Douglas Russell
Abstract
Overview: This protocol describes the steps used to load a Flongle flowcell utilizing the Q20+ (V12) Ligation Sequencing Kit from ONT.
This protocol has been tested with Flongle R9.4.1 flowcells.
Time required: 10 minutes
Steps
Starting out
Before starting, watch the first 13 minutes of this video: https://vimeo.com/651243660
It covers all of the vital aspects of this protocol.
Begin by restarting your computer. This will help to ensure there are no performance issues during your run or other programs running that may conflict with the software.
Attach the USB cable from the computer to the MinION device. Place the Flongle module with the white configuration cell into the MinION device. The top portion of the Flongle should slide underneath the clip and it should gently sit on the top of the device.
Open MinKNOW on the computer and perform a hardware check on the configuration cell. (Start -> Hardware Check) The hardware check should pass. You can validate this on the "System Messages" tab.
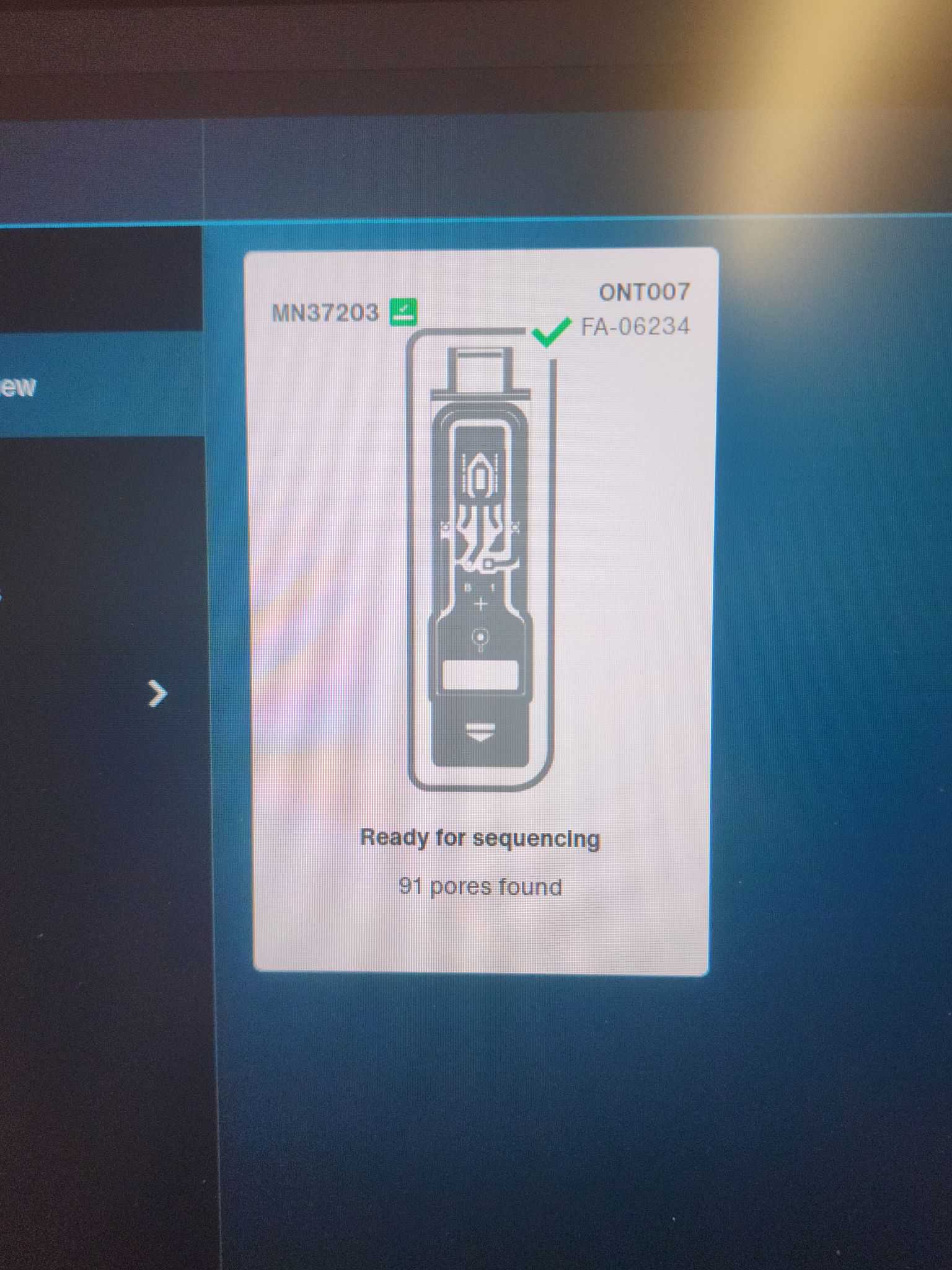
Wearing gloves, remove a flowcell from the fridge and remove the outer packaging. Remove the configuration cell and place the flowcell into the Flongle device on the MinION. Be sure not to touch any of the electrodes on the back of the cell or the contact pads on the top of the Flongle. It should snap into place with a click. Place the configuration module into the empty pouch from the Flongle until the run is completed. Run a flowcell check within the MinKNOW software. (Start -> Flow cell check) There should be at least 60 useable pores.
My first flowcell test had 87 useable pores. Future tests up to 6 weeks after receipt still passed with 70+ useable pores.
Prepare the loading solution
Thaw the Sequencing Buffer II (SBII), Loading Beads (LBII), Flush Buffer (FB), and Flush Tether (FLT) at room temperature.
FLT is found in the
All of the remainder are in the
Original full Ligation Sequencing Kit (Q20+) protocol can be found here:amplicons-by-ligation-sqk-lsk112-ACDE_9142_v112_revE_01Dec2021-minion.pdf
Mix the Sequencing Buffer II (SBII), Flush Buffer (FB) and Flush Tether (FLT) tubes by vortexing and spin down at room temperature.
In a 1.5uL tube, mix 117uL of the Flush Buffer (FB) with 3uL of the Flush Tether (FLT) and mix by pipetting.
Peel back the seal tab from the Flongle flow cell to the point where the sample port is exposed and hold the seal tab open by using the adhesive on the tab to stick it to the lid of the MinION.
Video of the process: https://youtu.be/zlkTfRf8g7I (2 minutes)
Using a 200uL pipette, bring up the FB/FLT mix into the tip. This is your priming solution.
VERY IMPORTANT: there should be NO air bubble in the tip of the pipette. Introducing any air bubbles will destroy any nanopores that the air bubble contacts.
If there is any air in the tip, turn the dial of the pipette clockwise (towards 0) to move the air bubble out of the tip.
Place the tip of the pipette securely into the sample port. Turn the dial of the pipette clockwise a time or two and you should see a small amount of yellow-green fluid come into the tip. This helps to ensure there are no air bubbles present.
Once confirmed, slowly dispense the priming fluid into the flowcell by slowly turning the dial of the pipette clockwise (towards 0). Do not push down on the plunger to eject the fluid.
You should be able to see the storage liquid entering the waste port as you enter it into the flowcell.
STOP LOADING before the liquid in the tip reaches the bottom. You do not want to push any air into the flowcell
Prepare Library
In a new 1.5uL tube, add the reagents below in the order they are listed.
Note: The LBII settles quickly, so mix it thoroughly before removing it from the tube.
Reagent Volume
Sequencing Buffer II (SBII) 13.5uL
Loading Beads II (LBII) 11uL
3 - 20 fMol of the DNA library 5.5uL
Total 30uL
Mix the solution by gently pipetting up and down.
Bring the entire volume into the tip of a pipette.
VERY IMPORTANT: Once again make sure there are no air gaps.
Insert the tip of the pipette into the sample port and slowly dispense the library into the flowcell by slowly turning the dial of the pipette clockwise (towards 0). Do not push down on the plunger to eject the fluid.
STOP LOADING before the liquid in the tip reaches the bottom. You do not want to push any air into the flowcell
Seal the Flongle cell be folding the tab back down, using the adhesive on the seal tab. Ensure that the wheel section covers the loading port and the two dots cover each waste port. Gently press over the tab to ensure a good seal. (Do not do a wiping press, just an up-and-down press.)
Close the MinION lid and start your sequencing run.
Start -> Start Sequencing
Run Name: Ex - SeventhRun Flow cell type: FLO-FLG001
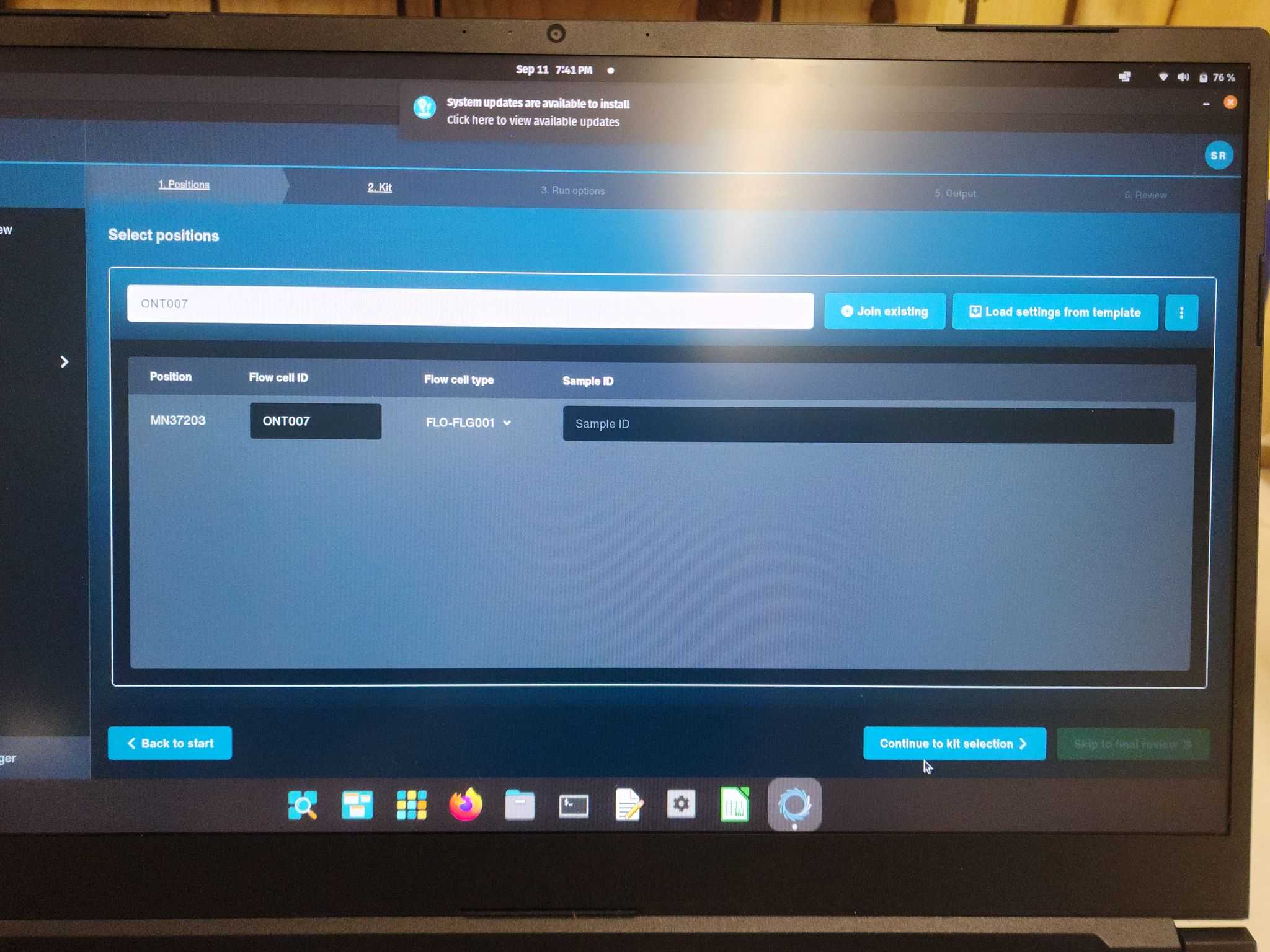
Step 1: Positions
Flow cell ID: Ex - ONT07 Sample ID: Ex - SDR007 Continue to kit selection >
Step 2: Kit
Sample Type: DNA PCR-free: PCR Multiplexing: Yes
These three options just filter the kits to select from.
Select: Ligation Sequencing Kit (LSK-110) Continue to run options >
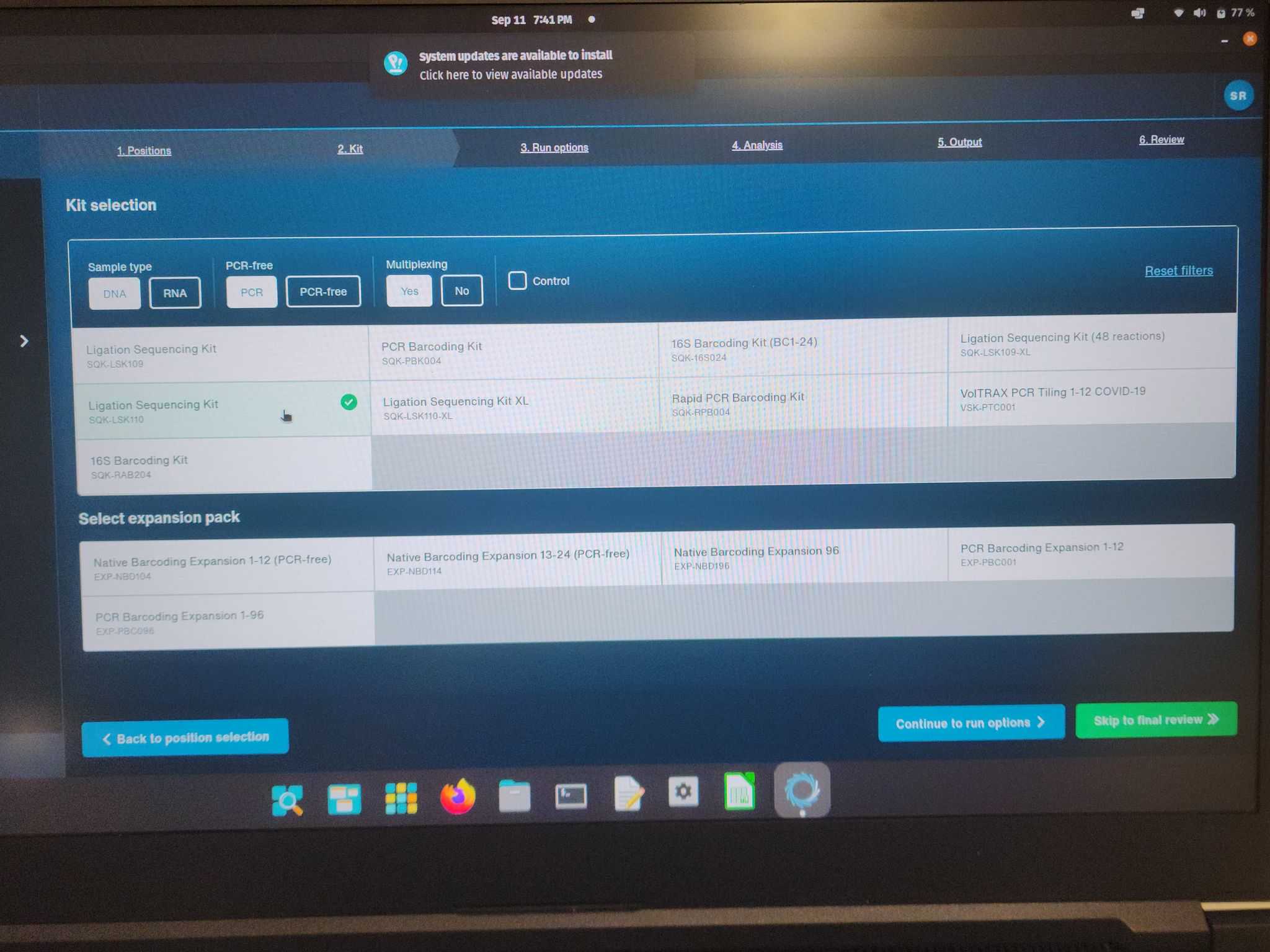
Step 3: Run options
(All should be at defaults)
Run Duration: 24 hours
Minimun read length: 200 base pairs
Active Channel Selection: On
Time between pore scans: 1.5 hours
Reserve pores: off
Continue to analysis >
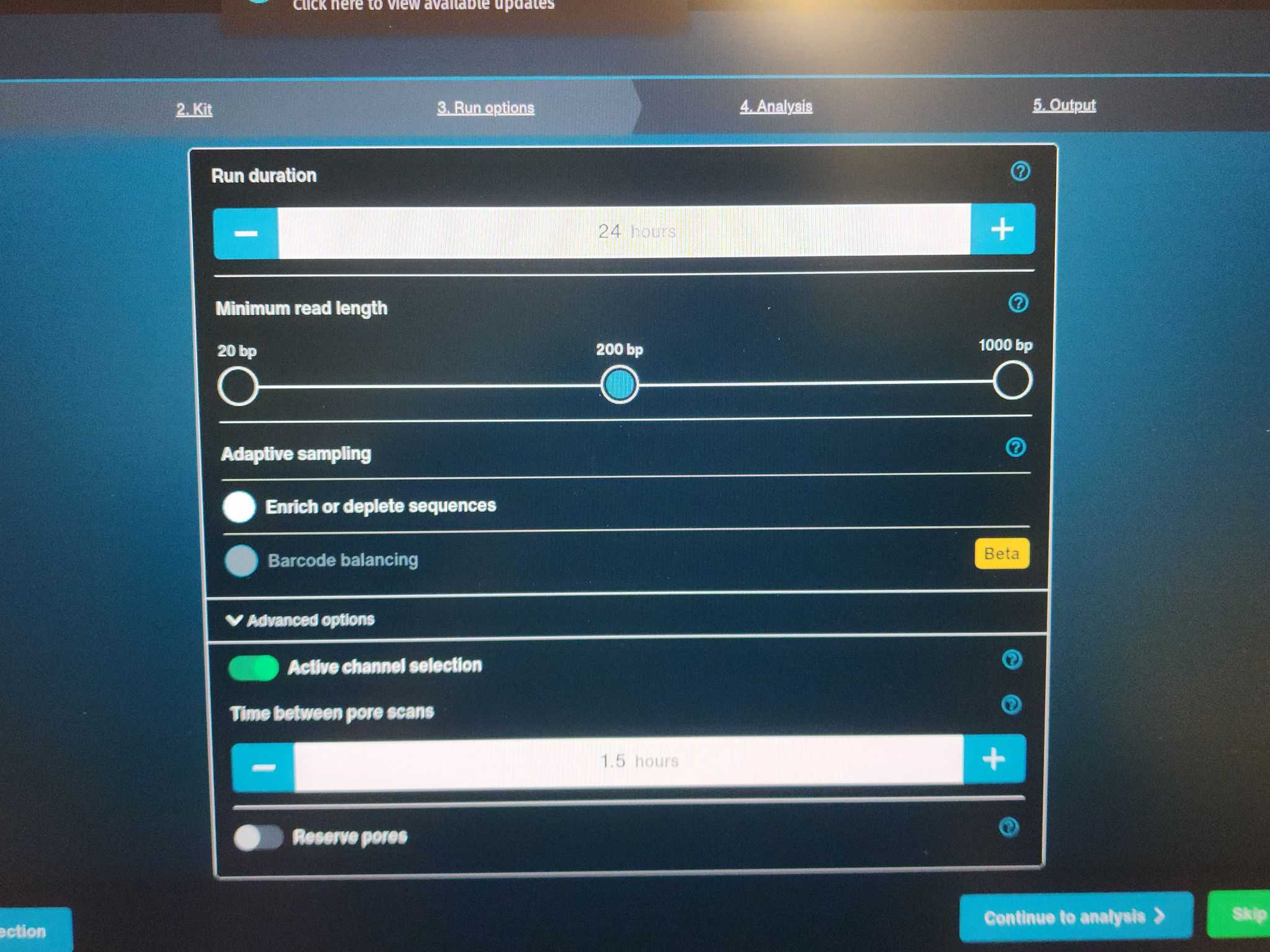
Step 4: Analysis
Basecalling: Off
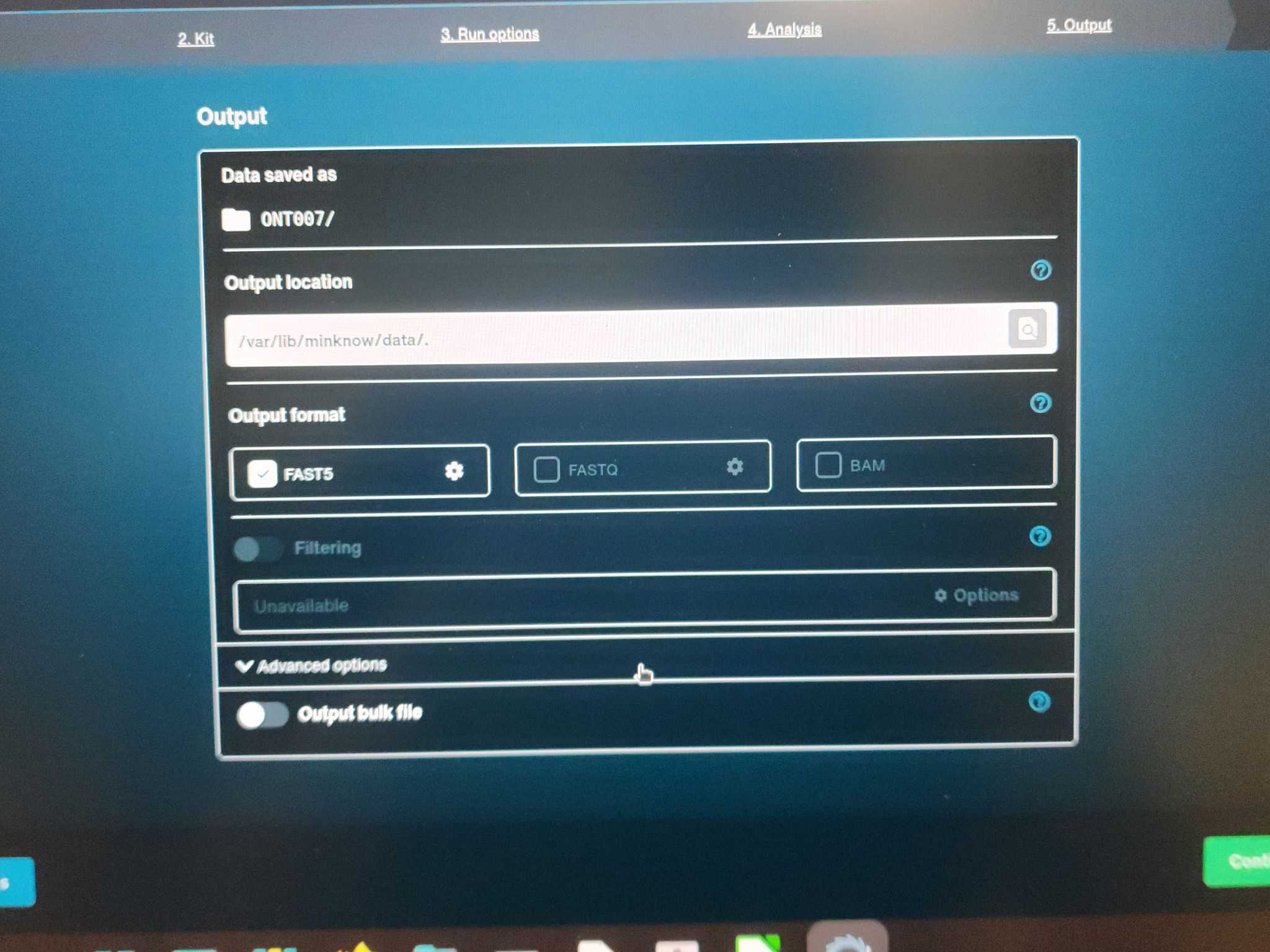
Step 5: Output
(All should be defaults)
Output location: /var/lib/minknow/data/.
Output format: fast5
Output bulk file: on
Continue >