Nanopore Library Preparation for R10 Ligation Sequencing Kit
Guan Jie Phang
Abstract
Protocols for Nanopore R10 flow cell Ligation Sequencing library construction.
Steps
Step1. End-prep
In a 200 µL PCR tube, mix the following chemicals:
| A | B |
|---|---|
| DNA | 50 µL |
| KAPA HyperPrep End Repair & A-tailing Buffer | 7 µL |
| KAPA HyperPrep End Repair & A-tailing Enzyme | 3 µL |
Incubate in a thermocycler at 20°C for 0h 30m 0s and 65°C for 0h 30m 0s. Hold at 4°C
Step2. Adapter ligation
Thaw the Ligation Adapter (LA) and Ligation Buffer (LNB). Vortex and spin down the tubes, and place on ice immediately afterwards.
Spin down the KAPA HyperPrep Ligase and place on ice immediately.
In a 1.5 mL tube, add the following chemicals:
| A | B |
|---|---|
| End-prep product | 60 µL |
| Ligation adapter | 5 µL |
| ddH2O | 5 µL |
| Ligation Buffer | 30 µL |
| Ligase | 10 µL |
Incubate overnight at 20Room temperature .
Step3. Cleanup
Thaw either Long Fragment Buffer (LFB) or Short Fragment Buffer (SFB) at room temperature on a cooling block, mix by vortexing, spin down and place on ice.
Resuspend the AMPure XP beads by vortexing.
Add 0.45X AMPure XP Beads (AXP) to the tube and mix by flicking the tube.
Incubate for 5 minutes at Room temperature.
Spin down the sample and pellet on a magnet. Keep the tube on the magnet, and pipette off the supernatant.
Wash the beads by adding either 250 µL Long Fragment Buffer (LFB) or 250 µL Short Fragment Buffer (SFB). Flick the beads to resuspend, spin down, then return the tube to the magnetic rack and allow the beads to pellet.
Remove the supernatant using a pipette and discard.
.
Spin down and place the tube back on the magnet. Pipette off any residual supernatant. Allow to dry for 5~30 seconds.
Remove the tube from the magnetic rack and resuspend the pellet in 15 µL Elution Buffer (EB). Spin down and incubate for 10 minutes at 37°C.
Pellet the beads on a magnet until the eluate is clear and colourless, for at least 1 minute.
Leave the tube aside, and go to the priming steps.
Step4. Priming and loading the SpotON flow cell
Thaw the flow cell, Sequencing Buffer (SQB), Loading Solution (LIS), and one tube of Flush mixture (FB & FLT) at room temperature on cooling block.
After opening the priming port, check for a small air bubble under the cover. Draw back a small volume to remove any bubbles:
-
Set a P1000 pipette to 800 μl.
-
Insert the tip into the priming port.
-
Turn the wheel until the dial shows 820–230 µl, or until you can see a small volume of buffer entering the pipette tip.
Load 800 μl of the priming mix into the flow cell via the priming port by turning the pipet wheel, avoiding the introduction of air bubbles. Wait for 5 minutes. During this time, prepare the library for loading by following the steps below.
Aspirate 12 µL of the eluted prepared library into a new 1.5 mL microtube.
In that new tube, prepare the library for loading as follows:
| A | B |
|---|---|
| Sequencing Buffer (SB) | 37.5 µL |
| Library Solution (LIS) | 25.5 µL |
Complete the flow cell priming:
-
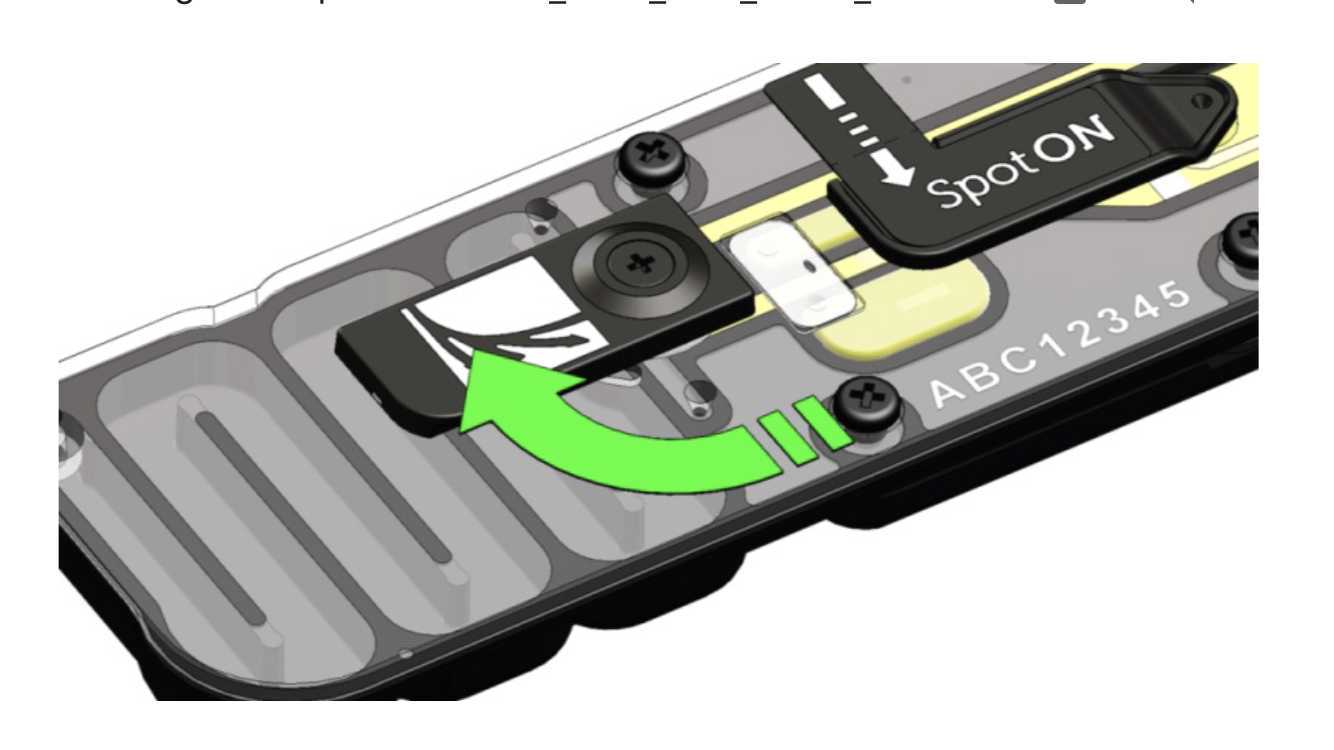
Gently lift the SpotON sample port cover to make the SpotON sample port accessible.
-
Load 200 μl of the priming mix into the flow cell via the priming port (not the SpotON sample port), avoiding the introduction of air bubbles.
Mix the prepared library gently by pipetting up and down in the tip using a P200 pipet just prior to loading.
Add the library to the flow cell via the SpotON sample port in a dropwise fashion. Ensure each drop flows into the port before adding the next.
Quantify 1 µL of eluted sample and the original DNA library using Qubit.
Step5. Flow Cell Wash and Storage
Stop or pause the sequencing experiment in MinKNOW, and leave the flow cell in the device.
Place the tube of Wash Mix (WMX) on ice. Do not vortex the tube.
Thaw one tube of Wash Diluent (DIL) at room temperature on a cooling block.
In a clean 1.5 mL microtube, prepare the following Flow Cell Wash Mix:
| A | B |
|---|---|
| Wash Mix (WMX) | 1 µL |
| Wash Diluent (DIL) | 199 µL |
Lift the sensory cover.
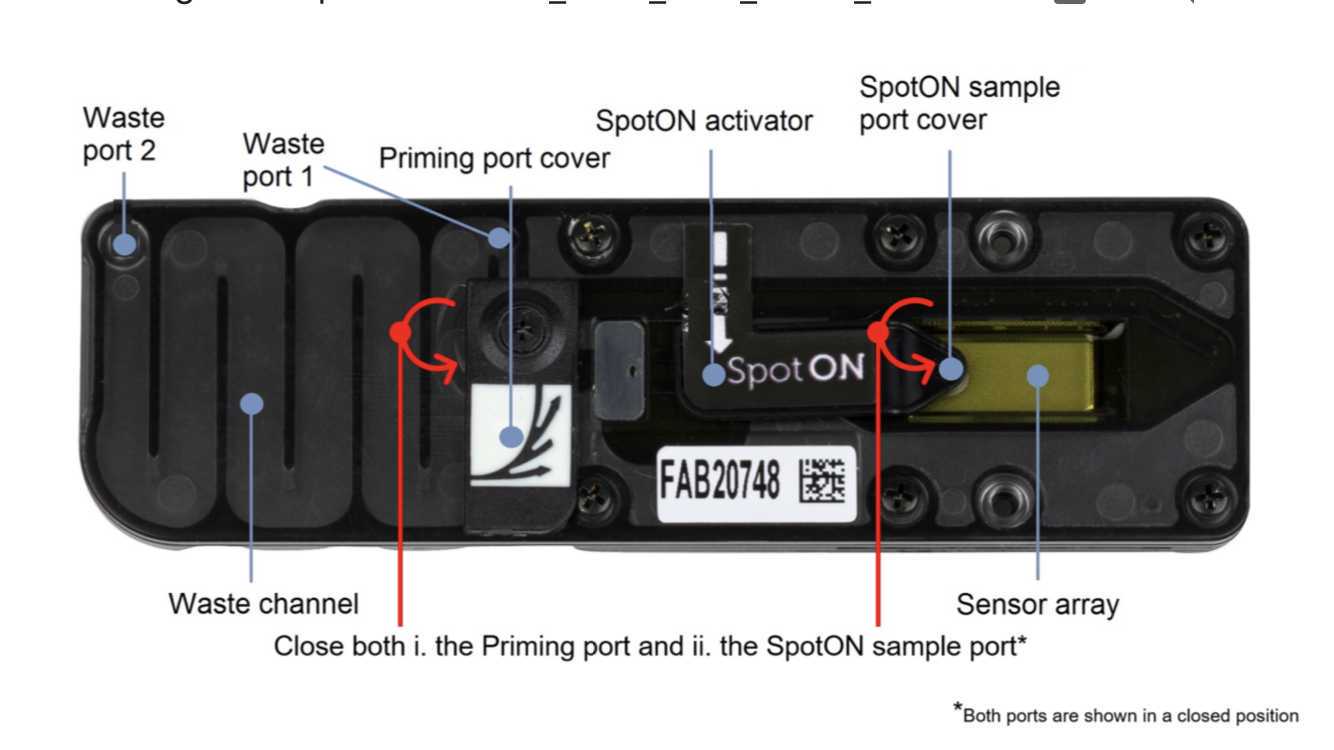
Rotate the flow cell priming port cover clockwise so that the priming port is visible.
After opening the priming port, check for a small air bubble under the cover. Draw back a small volume to remove any bubbles:
-
Set a P1000 pipette to 800 μl.
-
Insert the tip into the priming port.
-
Turn the wheel until the dial shows 820–830 μl, or until you can see a small volume of liquid entering the pipette tip.
Load 200 μl of the prepared Flow Cell Wash Mix into the flow cell via the priming port, avoiding the introduction of air.
Close the priming port and keep into 4°C for 2 days.
Take out the MinION device from 4°C fridge.
Thaw one tube of Storage Buffer (S) at room temperature on a cooling block.
Mix contents thoroughly by pipetting and spin down briefly.
Rotate the flow cell priming port cover clockwise so that the priming port is visible. Open the device lid.
After opening the priming port, check for a small air bubble under the cover. Draw back a small volume to remove any bubbles:
-
Set a P1000 pipette to 800 μl.
-
Insert the tip into the priming port.
-
Turn the wheel until the dial shows 820–830 μl, or until you can see a small volume of liquid entering the pipette tip.
Add 500 μl of Storage Buffer (S) through the priming port of the flow cell by turning the pipet wheel.
Close the priming port.
Using a P1000, remove all fluids from the waste channel through Waste port 2. As both the priming port and SpotON sample port are closed, no fluid should leave the sensor array area.
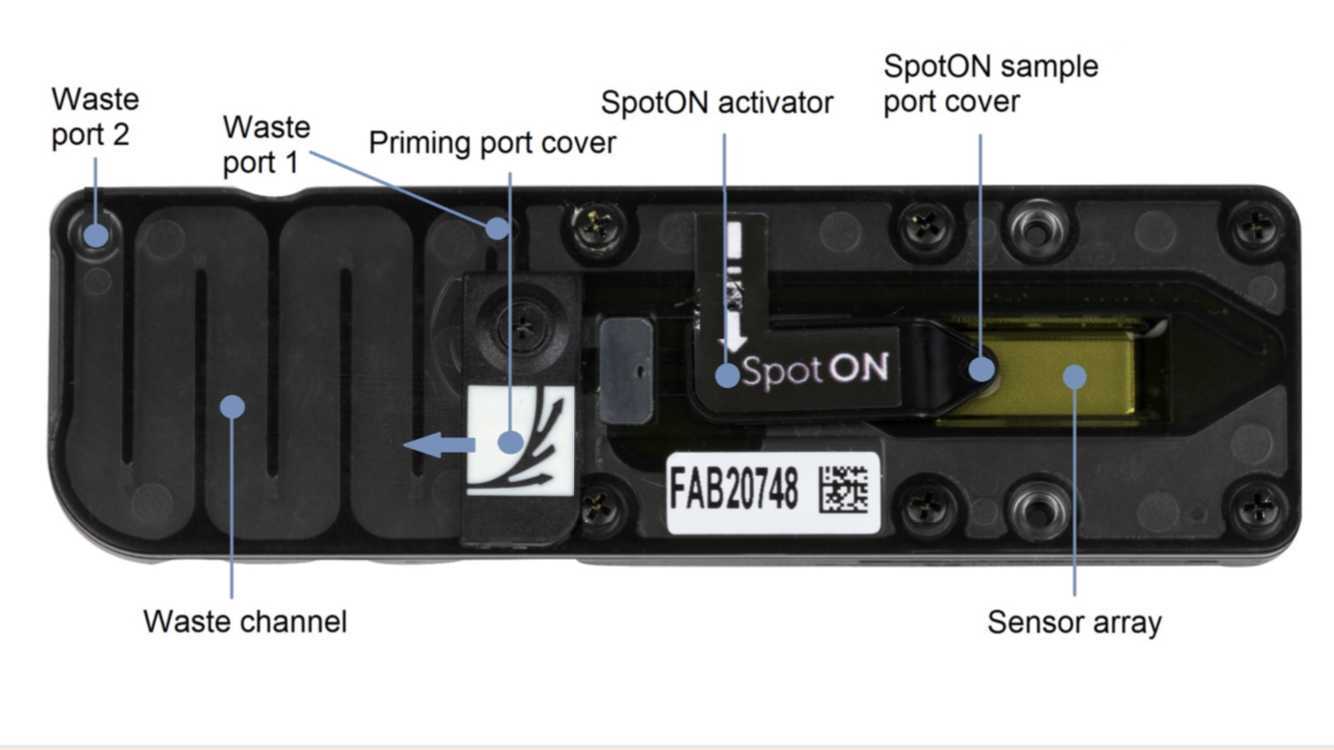
Perform a Flow Cell Check.
The flow cell can now be stored at 4°C.