NGS workflow with rRNA depletion for viral RNA sequencing from animal tissue specimens
Yiqiao Li, Magda Bletsa, Ine Boonen, Philippe Lemey
Disclaimer
DISCLAIMER – FOR INFORMATIONAL PURPOSES ONLY; USE AT YOUR OWN RISK
The protocol content here is for informational purposes only and does not constitute legal, medical, clinical, or safety advice, or otherwise; content added to protocols.io is not peer reviewed and may not have undergone a formal approval of any kind. Information presented in this protocol should not substitute for independent professional judgment, advice, diagnosis, or treatment. Any action you take or refrain from taking using or relying upon the information presented here is strictly at your own risk. You agree that neither the Company nor any of the authors, contributors, administrators, or anyone else associated with protocols.io, can be held responsible for your use of the information contained in or linked to this protocol or any of our Sites/Apps and Services.
Abstract
This NGS workflow describes how to prepare libraries from total RNA with a rRNA depletion step to increase the yield of non-host RNA transcripts.
This workflow was initially designed with the aim of generating full-length hepaciviruses, but can also be used for sequencing any other RNA viral sequences or RNA-related metagenomes.
Steps
1. Pre quantity QC
The quantity of total RNA is evaluated using Qubit RNA BR assay kit (following manufacturer's protocol)
2. Pre quality QC
The quality of total RNA is evaluated using the Agilent RNA 6000 Nano kit in Bioanalyzer (following the manufacturer's protocol)
(based on previous Qubit results and the bioanalyzer kit range, prepare samples dilution 1:10 or else for bioanalyzer)
Samples with RIN>2 can proceed to the next step of rRNA depletion
3. rRNA depletion
Adjust your starting material, which can be anything between 5 ng–1 µg total RNA (DNA free), according to the Qubit results in a 12 µl total volume reaction with nuclease-free water, and follow the manufacturer's manual of NEBNext rRNA Depletion kit (Human/Mouse/Rat) to remove total rRNA.
This kit removes any rRNA while retaining the viral RNA. Upon incubation, we evaluate our results using a custom qPCR assay targeting the 12S rRNA and the NS3 genomic region of hepaciviruses.
Analogous evaluation is recommended while working with other viruses.
4. Post quantity/quality QC
Upon rRNA depletion, the RNA product is quantified and qualified using the Qubit RNA BR assay kit (or Qubit RNA HS assay kit based on the results) and the Agilent RNA 6000 Pico kit following manufacturer's protocol.
5. Library preparation
Based on the Qubit results obtained in step 4, set the total amount of rRNA-depleted RNA input to anything between ~1ng - 100 ng.
Adjust to 14µL with nuclease-free water.
Follow the protocol of NGS library preparation using the NEXTFLEX Rapid Directional RNAseq kit (NOVA-5138-08) for tissue samples.
(will be released soon, current private link:
https://www.protocols.io/private/46D9A030792211ECB5450A58A9FEAC02)
5. Library quality/quantity QC
Check the fragment size of your libraries using Bioanalyzer with the Agilent High Sensitivity DNA kit
(following manufacturer's protocol, starting material: 1 µL)
The ideal fragment size for Illumina sequencing should range between 350-500bp.
Check the total amount of prepared libraries for pooling and confirm whether the adaptors are well ligated. This can be tested using a qPCR assay for each library with the complete Rox Low kit with Illumina general primers (catalogue number KK4873).
Prepare dilutions of your DNA libraries in 1:10, 1:1000, and 1:8000 with TET-buffer/nuclease-free water.
Prepare the master mix as follows:
KAPA Master Mix 12 µl
H2O 4 µl
Diluted DNA 4 µl
(total volume 20 µl)
Load in 96-well plate
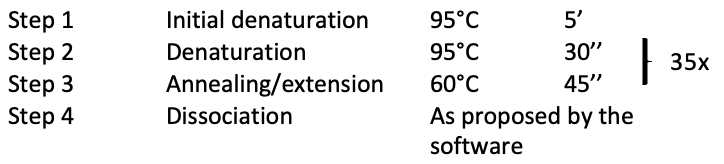
The PCR cycling conditions are listed below :
6. Library pooling
Pool libraries according to the library requirements of your sequencing platform.
Pool together 8-12 libraries (differs according to customers' demands for deep or ultra-deep sequencing) and adjust the volume using the same reagent as the one used for the elution in the library preparation step.
Ready for sequencing!

