Mollusk pedal mucus effects on epilithic biofilms
Ramiro Logares, Clara Maria Arboleda-Baena, Claudia Pareja, Isadora Pla, Rodrigo De La Iglesia, Sergio navarrate
Disclaimer
DISCLAIMER – FOR INFORMATIONAL PURPOSES ONLY; USE AT YOUR OWN RISK
The protocol content here is for informational purposes only and does not constitute legal, medical, clinical, or safety advice, or otherwise; content added to protocols.io is not peer reviewed and may not have undergone a formal approval of any kind. Information presented in this protocol should not substitute for independent professional judgment, advice, diagnosis, or treatment. Any action you take or refrain from taking using or relying upon the information presented here is strictly at your own risk. You agree that neither the Company nor any of the authors, contributors, administrators, or anyone else associated with protocols.io, can be held responsible for your use of the information contained in or linked to this protocol or any of our Sites/Apps and Services.
Abstract
Protocol used in the article “Hidden interactions in the intertidal rocky shore: variation in pedal mucus microbiota among marine grazers that feed on epilithic biofilm communities”. PeerJ
Steps
We cultured epilithic biofilms in K medium (Keller et al. 1987) on a cover glass slide inside a Polycarbonate cell culture plate of 6-Wells, for one week.
During nocturnal low tides, we collected animals of each mollusk species from wave-exposed platforms, brought them to the laboratory in coolers, and then placed them to acclimatize for a week in separate aquariums by circulating seawater and constant aeration.
The pedal mucus was collected under a laminar flow cabinet (Connor 1986). Animals were carefully removed from their containers, washed in filtered seawater (0.2 µm pore-size filters), and then each of them was placed on an individual inclined sterile glass slide (21 x 7 cm).

As they moved through the glass, filtered seawater was added to the animal pedal to stimulate individual mucus production. The animals moved in the glass slides for a maximum of five minutes and then were removed.
The mucus on the glass slides was removed with a sterile scalpel and put in individual cryovials with filtered seawater.
Then, the experiment consisted of placing the pedal mucus collected from individuals of each species in separate replicated wells with cover glass slides cultured with biofilm. The control wells received no mucus, and pedal mucus treatments were randomly assigned to the cell culture plates.
After one week, we took the cover slides from the cell culture plates separately and stained them with 200 μl 0.01% acridine orange for 3 minutes. The acridine orange is a fluorescent compound that emits red fluorescence when attached to single-stranded templates (RNA) and green fluorescence if the nucleic acids are double-stranded (DNA) (Rigler 1966). It can estimate the total amount of bacterial cells in the biofilm.
After 3 min incubation in the dark, the staining solution was removed, and the plate was washed twice with 500 μl of PBS solution.
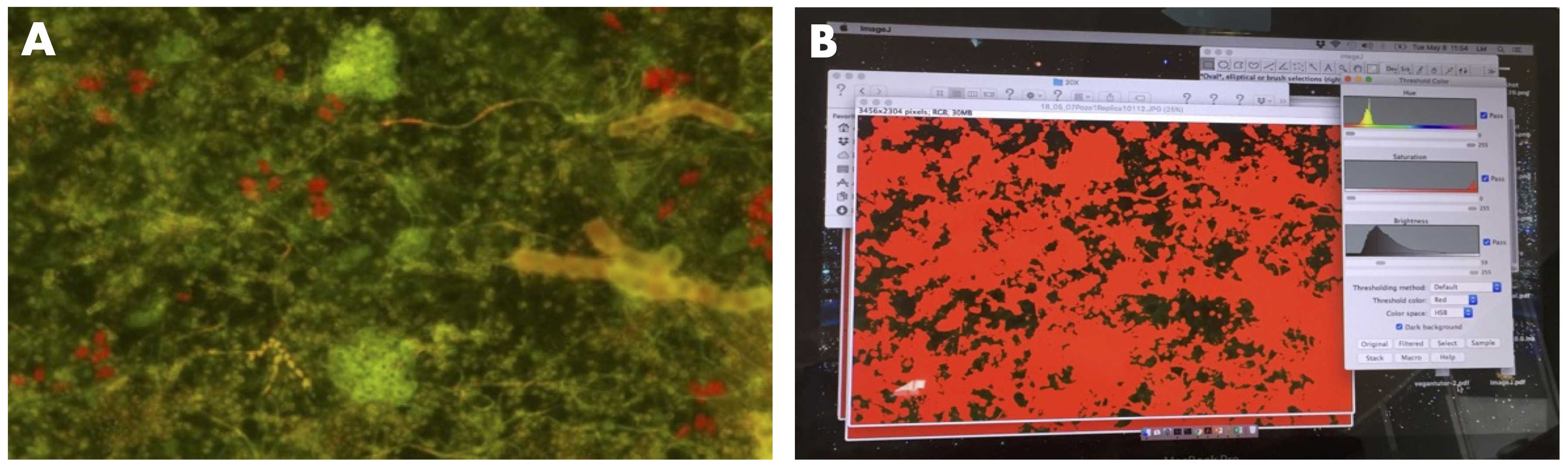
We took five photographs of each cover glass slide in different fields under a Fluorescent Carl Zeiss AXIO Scope A1 Microscope using an excitation filter (FS38) 470/40 nm and an emission filter 525/50 nm.
Then, we measured the cover of the photosynthetic epilithic biofilm using the software Image J and the following workflow:
a. Upload the image
b. Select: Image > Adjust > Color Threshold.
Parameters: Thresholding method = Default;
Threshold color = Red;
Color space = HSB;
Dark background (active).
c. Move the Brightness values until you select only the biofilm, not the background.
d. Select: Analyze >Measure, and copy the results in a table.
Cover results were analyzed with one-way ANOVA with grazer species as a fixed factor. A Tukey post hoc test was performed to determine the pattern of differences.
LITERATURE CITED
Connor, V. M. 1986. The use of mucous trails by intertidal limpets to enhance food resources. The Biological Bulletin 171: 548–564.
Keller, M. D., R. C. Selvin, W. Claus, and R. R. Guillard. 1987. Media for the culture of oceanic ultraphytoplankton 1, 2. Journal of phycology 23: 633–638.
Rigler, R. J. 1966. Microfluorometric characterization of intracellular nucleic acids and nucleoproteins by acridine orange. Acta physiologica Scandinavica 67: 1–122.

