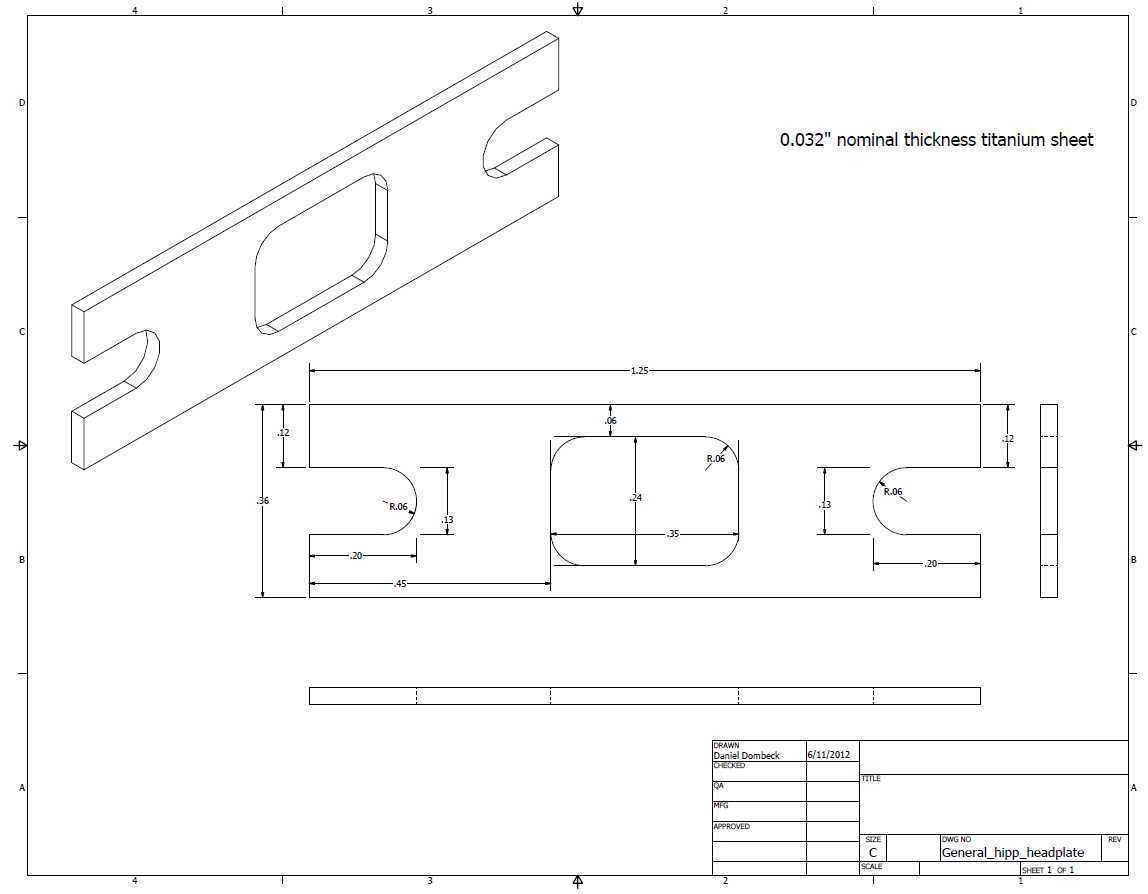
Midbrain viral injections for striatal fiber photometry in mice
Maite Azcorra
Disclaimer
Appropriate ethics approval must be obtained before undertaking these experiments.
Abstract
This protocol describes how to:
-
Inject AAV viruses into the midbrain (SNc: substantia nigra pars compacta, or VTA: ventral tegmental area) of mice
-
Train mice for head-fixed running on a cylindrical treadmill, and to receive rewards and air puffs while head-fixed
-
Implant head plates on mice for head-fixation during behavior (optional)
While this protocol is focused on injections of AAV viruses into the midbrain, it can be easily modified to inject into other brain regions or other viruses.
Steps
Outline and comments
This protocol is designed to inject a AAV virus into the midbrain (SNc or VTA) of mice to label dopaminergic neurons with fluorescent reporters (GCaMP). It then describes how to implant a head plate during the same surgery that can later be used for head-fixing the mice on a treadmill for acute striatal fiber photometry during behavior (see the protocol "Acute striatal or midbrain fiber photometry in head-fixed mice").
However, other viruses can be injected with minimal modifications - just test for injection volume and titer by doing histology on a few test animals. Injecting into other brain areas only requires editing the injection coordinates. Also, the head plate implantation is optional - this section can be skipped (as described below) if head fixation is not necessary. The injection procedure can be used as is for labelling neurons for histology, for example.
This protocol is designed for mice 2-3 months of age . For younger or older mice, injection coordinates might have to be modified.
For labelling of axons of dopaminergic neurons in SNc or VTA, allow 4 weeks after injection for expression to ramp up.
Injection pipette pulling and beveling
Pull glass pipettes using program 28 on Shutter Instrument Co. laser puller model P-2000 using the following program. This program might have to be adjusted for each puller’s characteristics, with the goal of pulling a pipette with a narrow shaft about 6 mm long (5 mm will go into the brain) and a short tip at the end of about 1.5 mm. Ensure that the puller pulls in two steps plus a third one to break the pipettes apart – if three steps are used, this will result in pipettes with three sections where the third one will be too narrow and thus increase the probability of pipette clogging.
Glass capillaries : 1.2mm OD x 0.6mm ID w/Fiber (FHC 30-31-1, borosilicate tubing x 100mm)
| A | B | C | D | E |
|---|---|---|---|---|
| 540 | 5 | 45 | 200 | 45 |
| 390 | 3 | 50 | 200 | 15 |
| 350 | 3 | 10 | 150 | - |
| 330 | 3 | 9 | 150 | - |
Pipette pulling program on Shutter Instrument Co. laser puller model P-2000
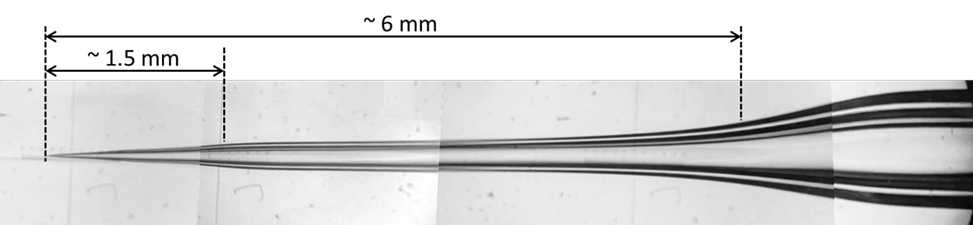
Dry bevel pipette using Shutter Instrument Co. K.T. brown type micro-pipette beveler model BV-10, and Starrett pipette holder/micromanipulator.
Make sure beveler is rotating flat.
Add PBS to the beveler surface to clean tip while beveling.
Hold pipette at 22.5o to the surface of the beveler and perpendicular its direction of rotation (3 o’clock).
Lower pipette with coarse manipulator until it is close to surface, using the pipette’s shadow cast from a light source placed above it to know when this happens.
Use the fine micromanipulator to continue lowering the pipette until it barely touches the grinding surface - this will be indicated by a scratching noise.
Lower the pipette 1 ½ ticks of the fine micromanipulator, wait for 2-5 min for the grinding to take place, then remove the pipette by moving it up with the micromanipulator.
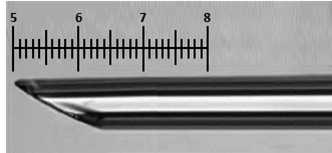
Look at bevel under a light microscope using a 10x objective. The tip should be pointy and not broken, and the bevel should be between 200 and 600 µm long (0.5-1.5 units using the scale on the eyepiece, max of 1.5 units shown below. The image below is used for reference, but does not correspond to the pipette shown previously).
Virus dilution and pipette filling
Defrost virus stock On ice and dilute to a concentration in an order of magnitude of 1012 in PBS (usually 1:1).
Fill pipette using a micro-pipette filler with at least 2.5µL of virus - less won’t be enough to inject accurately, as the bevel must be in the straight portion of the pipette. Make sure there are no bubbles, as this will likely cause clogging of the pipette. Keep the pipettes On ice until they are needed.
Surgery preparation
Turn on heat sterilizer ahead of time. When hot, sterilize tools:
- Flat spatula
- Coarse forceps
- Large scissors
- Fine scissors
- Fine forceps
Make sure that you have the following equipment/tools (normally in the surgery area) or prepare them:
- Enough isoflurane
- Betadine vial
- Ethanol vial
- Cotton swabs
- Artificial tears
- PBS vial
- Disposable transfer pipette
- Buprenex SR 1mg/ml vial
- Insulin syringes (30 gauge)
- Metabond mixing dish (in freezer), white powder, liquid base, catalyzer, scoop
Anesthesia and mouse preparation
Anesthetize mouse. Redirect the anesthesia system (VetEquip 901806) to the induction chamber (VetEquip 941444) and turn on oxygen flow at 0.8 liters per minute. Turn on isoflurane to 3% for induction, and place mouse inside. Wait until mouse’s breathing has slowed down.
Place mouse on the stereotax. Redirect flow to nose cone on the stereotax (KOPF 1900 + 1940, mounted on a Newport CM-225 air table), take mouse out of the induction chamber and place on nose cone: hold mouse from neck skin, and use flat spatula to open its mouth, pushing down on the tongue and lower teeth. Slide nose cone plate into open mouth until top teeth fit into small hole on plate. Make sure mouse is secure by gently pulling on tail, without mouth slipping off. Slide nose cone over nose and secure, but not too tight yet as it would difficult ear bar placement. Reduce isoflurane (around 1.5/2%, but adjust to keep the mouse anesthetized and breathing consistently).
Cover eyes completely with artificial tears (Puralube Ophthalmic ointment) to prevent the eyes from drying. Use this also to keep whiskers away from surface of head, to prevent cutting them during shaving.
Place temperature probe in mouse’s rear and hold down to table with tape. Turn on the temperature controller (Physitemp TCAT-2LV), which should be set at 35.7°C.
Administer analgesia to the mouse. Inject 0.02mL of Buprenex SR (slow release) 1mg/mL using insulin syringe subcutaneously between mouse’s shoulders.
Make sure mouse is anesthetized by checking the foot pinching reflex. Adjust ear bars into ears , making sure they are centered. Careful not to put them too low (into ear canal, as it will hurt the mouse) or too high (head might slip from bars when pressure is applied). Normally when ear bars are properly placed the 10-mark on the stereotax is near the 6-mark on the bars (on our stereotax). Use coarser forceps to hold ears away for easier visualization. Tighten the nose cone to help fixate the head.
Shave mouse’s head , from a bit before the eyes until between the ears, as wide an area as possible.
- First cut hair off with large scissors.
- Then smear shaving cream (Nair) thoroughly in area, wait
0h 1m 0sand remove all of it with cotton swabs.
Sterilize mouse’s skin first with Betadine and then with EtOh. Use cotton swabs, and always swipe skin in one direction.
Incision, skull leveling and zeroing coordinates
Cut the skin off the top of the skull by pulling up with the coarse forceps and cutting around with small scissors. For head plate implantation do not make a single incision, but instead cut out a whole section of skin, from between the eyes until lambda is exposed. Make the hole as big as possible, to allow the head plate to be securely attached later, but without getting too close to the eyes or ears.
Dip two cotton swabs in PBS and rub off the connective tissue layer above skull, removing out of the way to expose both bregma and lambda. Let skull dry off for a bit, as this will allow better identification of landmarks. Note: if the mouse is not deeply anesthetized, this process can trigger a reflex, moving their paws every time the connective tissue is touched.
Level the mouse's skull:
Use skull leveler tool on the stereotax (KOPF 1905) to level skull surface (bregma and lambda should be in the same plane). Also check right-left level, although it is usually good when the ear-bars are properly in place.
Once leveled, re-check by using the fine drill stereotax tool (KOPF 1911): move it until it is centered on bregma, and touching the skull. Zero the coordinates at this point. Move the drill towards lambda and check the leveling with the coordinates (should be under +-0.1mm off).
Use this chance to write down bregma-lambda distance for the mouse, as this can help adjust coordinates during any future troubleshooting.
Craniotomy
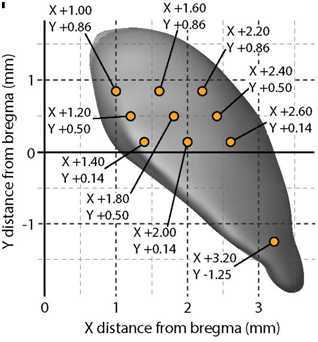
Move the drill to the (X,Y) injection coordinates (see below for SNc and VTA) and mark the location by drilling just slightly into the skull using the fine drill tool (KOPF 1911). The marking must be deep enough so that you can see the center of the drill mark (because the skull is not flat, initially only a half-circle drill mark will appear).
| A | B | C | D | E | F | G |
|---|---|---|---|---|---|---|
| SNc | ±1.55 mm | -3.25 mm | -3.80 mm | -4.10 mm | -4.30 mm | -4.70 mm |
| VTA | ±0.30 mm | -3.20 mm | -3.70 mm | -4.00 mm | -4.20 mm | -4.60 mm |
Injection coordinates from bregma for SNc and VTA
Using the hand-held dental drill (Midwest Tradition 282-9205), first indent the skull around the drill mark, outlining it. This is the most important step for correct injection location, as the injection will then be centered to the craniotomy, so it must be perfectly centered around the coordinate-precise drill mark. See diagram below. This will result in a marking of about 1mm in diameter.
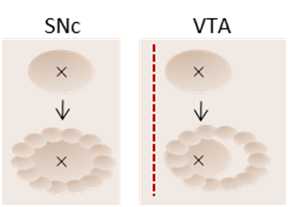
Once the craniotomy has been outlined, use the hand drill to deepen the outline, slowly and consistently throughout. Keep going until the skull is thinned down. This might be indicated by the central bone moving slightly every time the drill touches the skull, or by a slight cracking of the skull.
Use the fine scalpel to poke around the outline, both making sure that the bone is thin enough all around and weakening the area so that it is easier to remove the center. If an area is too thick, drill again.
Use the thin scalpel as a lever to pull up the central bone from one side, separating it from the brain. Once it sticks out, use the fine forceps to pull it off.
If at any point the craniotomy starts bleeding, clean it with PBS and dabbing with kimwipes until bleeding stops, making sure the craniotomy is clean from blood.
Injection
Attach the virus-containing pipette to the injection tubbing (which is connected to the pressure manometer and a syringe, see setup image) and hold it on the stereotax’s pipette holder (KOPF 1975).
Focus the microscope to the tip of the pipette, and clean it with PBS by pushing a drop half way out of the transfer pipette and moving it over the glass tip.
Turn on the pressure meter and switch the units to psi.
Press slowly on the syringe while looking at the pipette tip until you see a drop of virus leave the tip. Immediately release the pressure so no more virus leaks out, and clean the drop off with PBS.
Make sure the craniotomy is completely dry so that the dura is clearly visible, and lower the pipette until it touches the surface of the brain centered to the craniotomy (or to one side for VTA). Zero the Z coordinate at this point to measure depth from dura.
Slowly start lowering the pipette to pierce the dura, and then cover the craniotomy with PBS to prevent the brain from drying. Keep lowering the pipette slowly until the first injection depth, at an approximate speed of 500 µm per minute. Injection depths for SNc and VTA:
| A | B | C | D | E | F | G |
|---|---|---|---|---|---|---|
| SNc | ±1.55 mm | -3.25 mm | -3.80 mm | -4.10 mm | -4.30 mm | -4.70 mm |
| VTA | ±0.30 mm | -3.20 mm | -3.70 mm | -4.00 mm | -4.20 mm | -4.60 mm |
Injection coordinates from bregma for SNc and VTA
Once you reach the first injection depth, wait 0h 5m 0s for the tissue to settle.
Inject of virus per depth 0.1µL of virus per depth (0.4µL total per hemisphere, for 4 depths as shown above).
Switch the microscope’s eye pieces to a calibrated set with higher magnification, turn to the max microscope zoom and focus on the pipette’s bevel, aligning it with the calibrated scale perpendicularly. When the bevel moves 1 division on the scale this corresponds to 0.00625 µl ( 0.1 µl = 16 div ). Therefore, inject 16 divisions per location.
With the pressure meter on and on psi units, slowly press the syringe while looking at the bevel through the microscope. Keep pressing until the bevel starts moving ever so slightly, then lock the pressure. If the bevel is moving too fast, release and restart. The bevel should move very slightly, taking 1-2 min to inject 16 divisions/0.1 µl. Release the pressure once the appropriate volume has been injected.
Wait 0h 1m 0s for the virus to start diffusing, then go down to the next location again at an approximate speed of 500 µm per minute.
Inject another 0.1µL at this depth as above, wait another minute, move to the next step and repeat until the last depth.
Once all injections have been completed, wait 0h 10m 0s for the virus to completely diffuse before bringing the pipette up, to prevent it from sucking up the virus away from the intended location.
Move the pipette up until it exits the brain at an approximate speed of 500 µm per minute, then remove from the pipette holder.
Inject a total of of virus per hemisphere, per location. 0.4µL of virus per hemisphere, 0.1µL per location.
Head-plate implant and final markings
ALTERNATIVELY if a head-plate will not be implanted, close the incision by an approve method (i.e. surgical suture or staples) and skip to the section on "Recovery from Anesthesia".
Dry up the skull . You can use air from a pneumatic drill or just wait for it to dry own its own. This is very important, as the Metabond will not dry properly on a wet skull and thus will not atatch the head plate properly.
Place the head plate on the skull. It must be straight but mainly leveled to bregma-lambda (use the ear-bars for reference), so that when the mouse is head restrained the fibers will go down perpendicularly.
Fix the head plate with Metabond . Mix white Metabond (Parkell) in the cold mixing dish: 1 scoop of powder (Parkell Radiopaque L-powder), 4 drops of liquid base (Parkell “B” quick base) and 1 drop of catalyst (Parkell “C” universal TBB catalysis). Quickly mix it with the back of a cotton swab and apply it to the skull, covering all the exposed bone and joining it to the head plate so that no gaps are left, and noTry to apply a thinner layer around the area when the future craniotomy will be for drilling ease. Clean the mixing dish before it dries.
When the white Metabond is dry, add a thin layer of black Metabond . Mix black Metabond - same recipe as white Metabond above but add half a scoop of activated charcoal powder. Cover the white Metabond with a very thin layer of black Metabond. This serves to reduce light scattering off the head plate.
When the Metabond is dry, mark the locations of future craniotomies with the stereotax drill, making sure the markings are deep enough that they are easily distinguishable but not enough to expose the skull. To facilitate their future identification, use a sharpie to draw over the markings, and draw a cross centered on it to locate its center once the new craniotomy is made. Also draw a large number to identify the animal easily.
Recovery from anesthesia
Remove the mouse from the stereotax. Remove the ear bars and the temperature probe (remember to turn of the thermometer too), and push back the nose cone. To remove the mouse from the nose cone, use the flat spatula again, introducing it in between the plate and the mouse’s top jaw. Push up slightly until the top teeth come out of the hole, and pull the mouse off. Turn off the isoflurane and oxygen flow.
Recovery from anesthesia. Return the mouse to its cage, leaving it lying on its belly. Be careful to hold the mouse’s tail throughout the whole process: although usually it takes a few minutes for them to wake up, they can sometimes jump out of your hand. Keep watch over the mouse until it starts to move. If for any reason it is taking more than a few minutes, place a hot mat under the cage to keep the mouse warm until it does wake up, and to provide extra care.