Microbe/Phage Wastewater DNA/RNA Concentration and Extraction (Nanotrap® and NucleoMag® RNA Water)
Brett Rasile, Kendra Maas, Archana Anand, Michael Hajkowski
Abstract
Wastewater epidemiology is a method used to study the diseases affecting a population. Conventional methods of wastewater collection for composite samples have involved the usage of autosamplers that require installation in sewers with appropriate expertise and certification. Tampon-based wastewater sampling is a cost-effective and simple way to collect wastewater to study microbial pathogens. It is also a safer alternative as tampons can be deployed and retrieved without opening manhole covers. In this protocol we detail a wastewater sampling strategy using tampons to obtain composite samples.
Next, wastewater-based epidemiology has predominantly been targeted, where scientists have looked for specific pathogens and use biomarkers for the same. Notably, traditional wastewater filtration methods (i.e. skim milk filtrations and membrane filtration) require large sample sizes with varying nucleic acid yields (Ahmed 2022). Recently, Nanotrapcopyright technology has been shown to have a higher nucleic acid yield using a smaller sample size when compared to traditional membrane filtration techniques, making it a better tool for scientists to concentrate microbial nucleic acids in wastewater for wastewater epidemiology. Specifically, the Ceres Nanotrapcopyright technology was developed to trace severe acute respiratory syndrome (SARS-CoV-2) in public wastewater and Nanotrapcopyright particles have been used specifically to track viral pathogenic shedding in wastewater of: Endogenous pepper mottle virus (PMMoV), Influenza A, CrAssphage, and monkey pox. However, there is little known about the success rates of different downstream analysis techniques to concentrate microbes in wastewater in an untargeted fashion. Therefore, it remains unclear whether one method will work better than the other if one were to be interested in characterizing the microbial community composition and abundance in a wastewater sample. To this end, in this protocol we detail a wastewater processing method involving Ceres Nanotrapcopyright technology and NucleoMag® RNA Water.
Steps
Virus Capture & Concentration
Create a 1:100 dilution of Zoetis Bovine Rhinotracheitis-Parainfluzena-Respiratory Syncytial Virus (BRSV) Vaccine in H2O.
Spike the500mL wastewater sample with 500µL of 1:100 BSRV to achieve a 1µL/mL concentration of 1:100 BSRV .
Mix the added 1:100 BRSV into the wastewater sample by aggressively inverting the bottle several times or shaking.
Incubate spiked wastewater samples for a minimum of 0h 10m 0s at room temperature (RT) to allow large aggregates to sediment at the bottom of the sample bottle.
Use a 25mL serological pipette to transfer top 40mL of spiked wastewater to a 50mL conical tube.
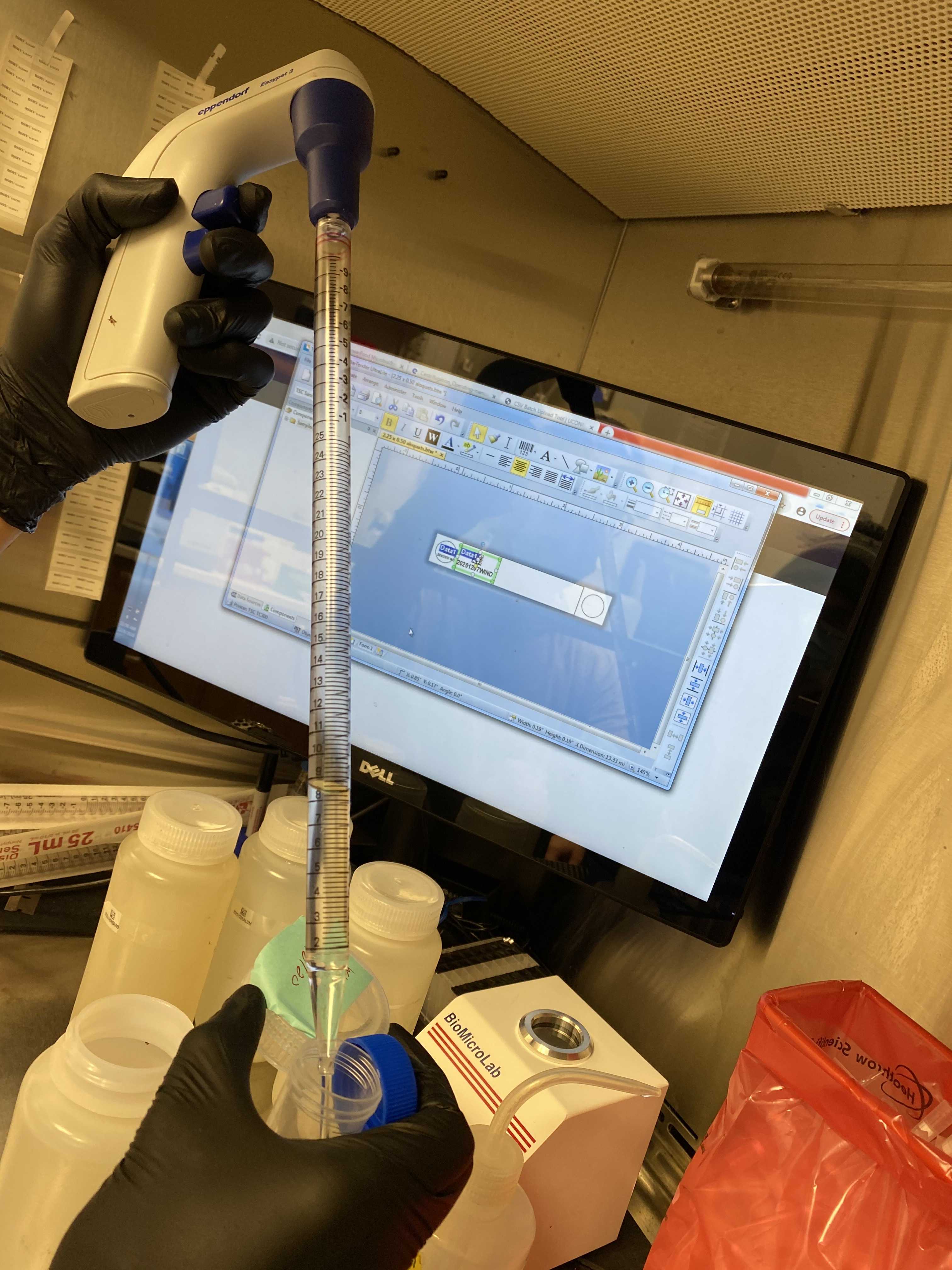
Add600µLof Ceres Magnetic Nanotrap® particles to the 40mL spiked wastewater aliquot.
Invert the wastewater samples several times to incorporate the Magnetic Nanotrap® particles and incubate for 20 minutes at RT.
Place conical tubes into custom magnet racks and allow magnets to attract Magnetic Nanotrap® particles for a minimum of 20 minutes at RT.

Keeping the conical tubes fixed to the magnets, pour off the supernatant carefully as to not disturb the pellet of Magnetic Nanotrap® particles at the bottom of the conical tubes.
Viral RNA Extraction (Nucleomag® RNA Water)
Add 500µL of Buffer MWA1 to the falcon tubes.
Vortex to resuspend the Magnetic Nanotrap® pellets in the Buffer MWA1.
Incubate samples for 0h 10m 0s at RT.
Place conical tubes on the custom magnet racks to separate the Magnetic Nanotrap® particles.
With the tubes on the magnets, transfer 450µL of lysate to a 2mL deep well plate.

Add 475µL of Buffer MWA2 and 25µL of NucleoMag® B-Beads to the lysate in the deep well plate.
Shake the sample plate for 0h 5m 0s at 1400rpm , 56 .
Place the sample plate on a plate magnet for at least 0h 5m 0s to separate NucleoMag® B-beads.
Use a multichannel pipette to remove the supernatant from each well.
Add850µL of Buffer MWA3 to each well.
Shake the sample plate for 0h 2m 0s at 1400rpm , 56 .
Place the sample plate on a plate magnet for at least 0h 2m 0s to separate NucleoMag® B-beads.
Use a multichannel pipette to remove the supernatant from each well.
Add850µL of Buffer MWA3 to each well.
Shake the sample plate for 0h 2m 0s at 1400rpm , 56 .
Place the sample plate on a plate magnet for at least 0h 2m 0s to separate NucleoMag® B-beads.
Use a multichannel pipette to remove the supernatant from each well.
Add850µL of Buffer MWA4 to each well.
Shake the sample plate for 0h 2m 0s at 1400rpm , 56 .
Place the sample plate on a plate magnet for 0h 2m 0s to separate NucleoMag® B-beads.
Use a multichannel pipette to remove the supernatant from each well.
Place plate on shaker at 56°C for approximately 30 minutes to allow beads to air dry.
Add 60µL of RNase-free H2O 2O to each well.
Shake the sample plate for 0h 5m 0s at 500rpm , 56 .
Place the sample plate on a plate magnet for 0h 2m 0s to separate NucleoMag® B-beads.
Transfer eluted RNA to a 96-well elution plate for further processing.


