Ligated intestinal loop mouse model protocol.
Pablo Castro-Córdova, Maria José Mendoza-León, Daniel Paredes-Sabja
Abstract
In the present protocol, we provide a step-by-step protocol with all the critical information to perform an intestinal ligated loop in a mouse model. Using this technique, we describe the infection of intestinal loop with C. difficile spores and immunofluorescence of whole mounted intestinal tissue to study the interaction of C. difficile spores with the intestinal mucosa. Also, this work provides information of the processing and mounting of the tissues to acquire high-resolution confocal images, and that may be used to quantify the spore adherence and internalization to the intestinal mucosa.
Steps
Solutions
10× Phosphate buffered saline (PBS) : Stock solution; Calculate the reagents required to prepare 1L of 1.37Molarity (M) NaCl, 27millimolar (mM) KCl, 100millimolar (mM) Na2HPO4, 18millimolar (mM) KH2PO4, and dissolve it in 800mL of Milli-Q water. Then the pH was adjusted to 7.5 with HCl and fill with Milli-Q water to 1L. Sterilize by autoclaving for 0h 20m 0s at121°Cwith 1 Bar, and store at 121Room temperature
1× (PBS) : Dilute 10× PBS stock solution 1:9 (100mL of 10× PBS into 900mL of Milli-Q water) and then sterilize by autoclaving for 0h 20m 0s at121°C with1 Bar. Store at 121Room temperature.
Saline solution: 0.9 NaCl. Dissolve 9g of NaCl in 1L of Milli-Q water. Pass through 0.45-µm filters and sterilize by autoclaving for 0h 20m 0s at 121°C with 1 Bar. Store at 121Room temperature .
Fixing solution : 30% (w/v) sucrose in PBS–4% (w/v) paraformaldehyde. In the first place, a solution of PBS–4% (w/v) paraformaldehyde was prepared as follows: for 1L, add 40g of paraformaldehyde powder to 800mL of 1× PBS. Heat to 60°Cin a fume hood (no dot boil). If it does not dissolve, raise the pH adding 5N NaOH drop by drop until a clear solution is formed. Cool the solution, adjust the pH to 8.0, and adjust the volume to 1L with 1× PBS. Pass thought 0.45-µm filter to remove particles. Aliquot in small volume and store at 4°Cfor use in 1 to 2 weeks or store at -20°C for up to 1 year. To prepare 100mL of 30% (w/v) sucrose in PBS–4% (w/v) paraformaldehyde, 30g of sucrose were dissolved in PBS–4% (w/v) paraformaldehyde with a final volume of 100mL.
Permeabilizing solution: a PBS–0.2% (v/v) Triton X-100 solution was prepared as follows: a stock solution of 10% (v/v) Triton X-100 was prepared in PBS. To prepare 10mL of the stock solution, dilute 1Molarity (M) of Triton X-100 in 9mL of PBS with gentle shaking. PBS–0.2% (v/v) Triton X-100 solution was made by dilution 1:49 of the stock with sterilized 1× PBS. The stock solution was stored at 4°C .
Blocking solution: PBS–3% (w/v) BSA solution. To prepare 10mL of the solution, 0.3gof BSA was dissolved in 8mL of 1× PBS, then add PBS to 10mL and sterilize by filtering with 0.2-µm syringe filter. The solution was stored at 4°C.
Surgery
Day 1 All surgical procedures were performed under clean but non-sterile conditions. The surgical scissors and forceps were autoclaved before usage except the Forceps Dumont Nº5, that are not autoclavable, so they were washed with soap, 0.5% (v/v) bleach, and 70% (v/v) ethanol.All the required materials such as isoflurane, povidone-iodine, 70% (v/v) ethanol, ophthalmic ointment, heat-pad, a stainless-steel surgical tray attached to an isoflurane mask, syringe with saline solution, syringes with C. difficile spores, silk braided silicon suture, autoclaved towel paper, masking tape, and the surgical material as forceps, scissors, were organized in a manner that they are easily accessible to the hand ( Fig 1 ).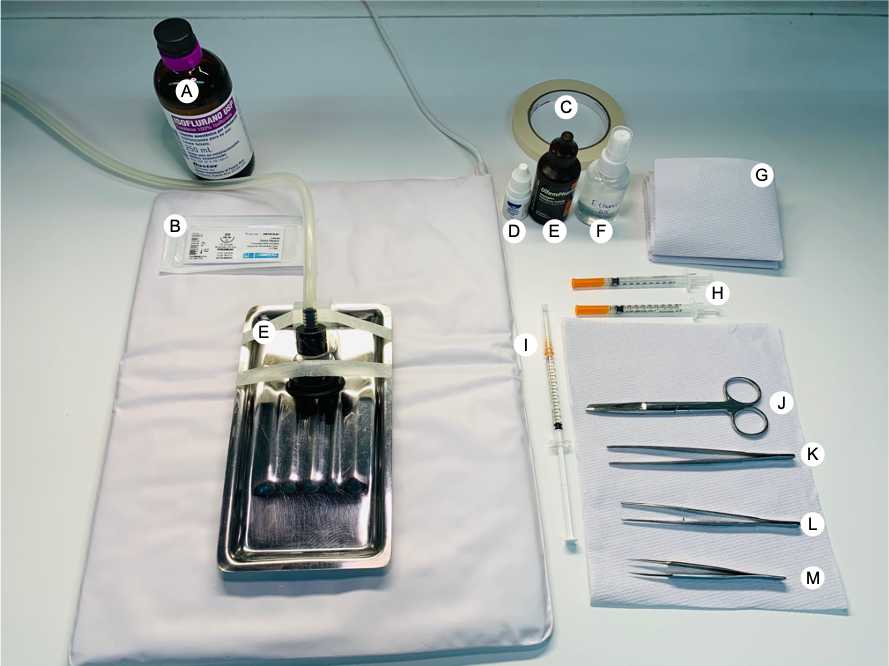
Male or female, 18–25g mice C57BL/6 of 8–12 weeks were fasted overnight (15h 0m 0s) before the surgery with free access to water.
Depth anesthesia was induced in ~0h 3m 0s with 4% (v/v)
Equipment
| Value | Label |
|---|---|
| Small animal Anesthesia Machine | NAME |
| RWD | BRAND |
| RWD-R510IP | SKU |
| https://www.rwdstco.com | LINK |
Take out the mouse from the isoflurane induction chamber, put the mouse prone into the surgery tray, and put the snout (nose and mouth) into the isoflurane mask. The surgery bed is over a heating pad to avoid hypothermia during the procedure. Reduce the isoflurane to 2% (v/v).
Add ophthalmic drops in the eyes to avoid corneal drying.
Turn the mouse to supine position.
Check anesthetic depth by non-response to hind limb toe pinch.
Using small pieces of masking tape, fix the limbs of the mice to the surgery bed.
Dampen the hair of the abdominal area with 70% (v/v) ethanol either by spraying or by dipping. Clean with a paper towel. Let it dry.
With a disposable razor, shave the abdominal zone.
Clean the shaved abdominal zone with povidone iodine, and clean it with towel paper ( Fig 2A ).

For steps 8–17, see S1 Video in the next link:
https://www.dropbox.com/s/bnnu71l2rewa220/S1_Video.mp4?dl=0
S1 Video. Mouse preparation for surgery (steps 8–17). This video shows how to anesthetize the mouse, apply ophthalmic solution, disinfect, and shave the abdomen.
Using forceps and a surgical scissor sharp/blunt, perform an incision of ~2 cm in the midline of the abdominal skin ( Fig 2B ).
The skin is separated from the peritoneum using anatomical forceps.
Identify the linea alba, a semitransparent longitudinally white line in the peritoneum ( Fig 2C ).
Open the abdominal cavity, incise the abdominal musculature on the linea alba. For this, gently grab the musculature with anatomical forceps and retracting up. The abdominal organs are not adjacent to the muscles. Using a scissor or a scalpel makes a small opening into the peritoneal cavity in the linea alba. Extend the incision in the midline until it reaches the size of the skin cut.
For steps 19–22, see S2 Video in the next link:
https://www.dropbox.com/s/2ynycw9f8vwz1ot/S2_Video.mp4?dl=0
S2 Video. Midline laparotomy (steps 19–22). This video shown how to open the abdomen skin, identify the linea alba and open the peritoneal cavity.
Using forceps, gently move the intestines to identify the cecum, a large J-shaped blind sac curved. Extract the cecum through the incision and identify the ileum and colon ( Fig 3A ). The ileum is the distal last part of the small intestine that is attached to the cavity by mesentery tissue that derives blood supply from the mesenteric artery. The proximal colon is the first part of the colon that begins in the cecum.
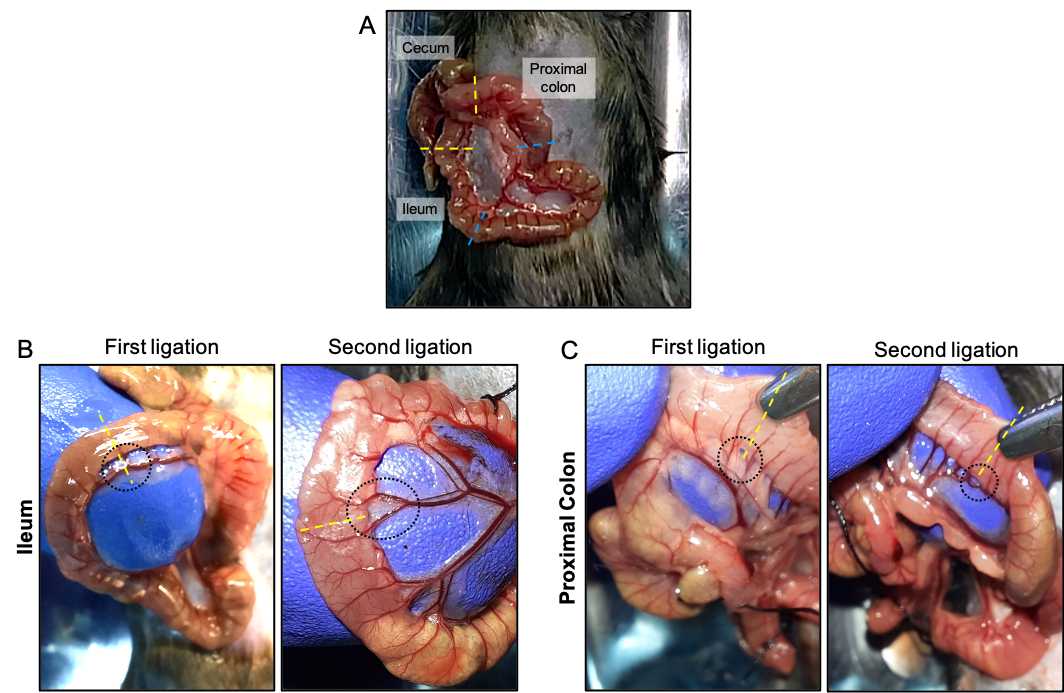
When the ileum or colon has fecal material, it can be removed, pressing gently with a blunt tip of the forceps against your fingers and move it in the direction of the flow of the fecal material or to the cecum.
In the ileum, identify regions to be ligated where blood vessels are finely separated from the ileum's external wall ( Fig 3B ). Once identified, pass the fine tip of the Dumont Nº5 Forceps between the outer wall of the ileum and the blood vessels having care of not damage or puncture the blood vessels. With the tip of the Dumont Nº5 Forceps, on the other side of the hole formed between the external wall of the ileum and the blood vessels, grasp the thread of the surgical suture, and gently pass to the other side of the hole.
Perform a firm but gentle double "simple knot," performing a blind knot so as not to cut the tissue and having special care of not ligate neither interfering with the blood flow.Note: When necessary, hydrate the intestines with drops of sterile saline solution.
A second ligation is then performed at ~1.5 cm of distance from the first ligation using the same strategy described above ( Fig 3B ). However, in this case, perform a simple knot without closing.
At 0.5 cm –1 cm out of the second ligation, insert the needle of a tuberculin syringes with 5×108 C. difficile spores in 100µLof saline in the direction of the ligation and cross the ligation by inside the intestine the and close the knot with the syringe needle inside. C. difficile spores strain R20291 (CM210) were purified as was described previously (Calderón-Romero et. al. 2018)
Release the C. difficile spores inside the loop, keeping the pressure in the knot. This is performed to avoid inoculum loss and splashing that occurs when the ligated loop is injected directly.
Remove the syringe and close the ligation with a simple double knot.
For steps 24–31, see S3 Video in the next link:
https://www.dropbox.com/s/8ckq11o8enpc8s4/S3_Video.mp4?dl=0
S3 Video. Procedure to ligate loops (steps 24–31). This video shows how to identify the ileum and the proximal colon, remove fecal material from the section to be ligated, identify the sites to be ligated. Also, shown how to perform the ligations without interruption of the blood vessels and injection of C. difficile spores on ileum and colon.
To perform the colonic loop, identify the regions to be ligated ( Fig 3C ) and repeat the points 24–31 in the proximal colon.
Carefully using anatomical forceps return intestines to the abdominal cavity.
For steps 33 and 34, see S3 Video .
Close the peritoneum of the abdominal wall by a continuous or interrupted suture using silk suture.
Close the skin of the abdominal wall by continuous or interrupted suture using silk suture.
Remove mice from the isoflurane mask and from the heating pad and allow the mouse to recover from the anesthesia under a heat lamp. The awareness recovery usually takes 0h 1m 0s–0h 2m 0s.
For steps 37–38, see S4 Video in the next link:
https://www.dropbox.com/s/5kaqmdjw1tu2rlq/S4_Video.mp4?dl=0
S4 Video. Midline laparotomy closure with suture (steps 37–38). This video shows how to suture the abdominal wall and the abdominal skin with silk suture by continuous suture technique to close the incision and let mice recover from the procedure.
Apply postoperative analgesia when required according to the animal care protocol at your institution.
Usually, the complete procedure for one mouse and one loop takes ~0h 15m 0s: and with 2 loops ~0h 10m 0s. Animals were kept in the cage for 5h with free access to water and close to a heat lamp. Animals were monitored every 0h 30m 0s.
Necropsy and tissue collecting
Depth anesthesia is induced by isoflurane inhalation, as is indicated above in step 9.
Check anesthetic depth by non-response to hind limb toe pinch.
Perform cervical dislocation by separating the vertebrae in the cervical area with a firm pinch to the neck using a rigid metallic tool and firmly pull the mouse from the tail. The separation of the skull and brain from the spinal cord is caused by anterior pressure applied in the skull base.
Using forceps, gently grab the skin and retract it up, so the abdominal organs and the ligated loops are not adjacent to the muscles.
Using scissors, open the abdominal cavity, cutting the skin and peritoneum. Extend the incision to visualize the intestines and the loops.
Remove the ligated ileum and colonic loop by cutting at ~0.5 mm from the outside of ligatures and put the intestinal loops in a petri dish.
For steps 46–47, see S5 Video in the next link:
https://www.dropbox.com/s/1wl5v3u3qadfwiv/S5_Video.mp4?dl=0
S5 Video. Extraction of the ligated loop (steps 33–34). This video shows how to extract the ligated loop in a euthanized mouse.
Fixing the tissues
In a biosafety cabinet, put drops of 1mL of PBS over a petri dish.
To fix the tissues, prepare a "fixation chamber": we used a petri dish, but you can use any other tupperware with lid that you have available. Put inside a filter paper (extra thick blot paper). If the filter paper is larger than the used container, cut the filter paper with scissors o fit it inside the container.
Imbibe filter paper with the solution of 30% (w/v) sucrose in PBS–4% (w/v) paraformaldehyde enough to wet the entire filter paper without adding an excess of solution. Remove the excess of fixing solution from one edge using a 100µL–1000µL micropipette.
To remove the ligatures, cut the ligated loop as close as possible from the ligation. A liquid with gelatinous consistency comes out of it.
Put the scissor tip inside the lumen of the intestine and perform a longitudinal cut in the tissue to extend it.
Grasp the tissue from one end with the forceps and wash the tissue by immersion in the PBS drops for ~20 immersions and repeat in 2–3 different drops of fresh PBS as is necessary for each tissue.
With anatomical forceps, grasp the opened intestinal tissues from a corner with the muscular layer downwards and the luminal side upwards. This can be identified because when the longitudinally cut tissue is grasped with the forceps from one end, it tends to recover its uncut shape, where the luminal side is inwards and the muscular side is outside. Using 2 pairs of forceps, stretch the tissues on the filter paper with the fixing solution.
Using a 100µL–1000µLmicropipette, add fixing solution directly over the tissues. Repeat each ~0h 5m 0s.
Let samples fixing for at least 0h 15m 0s.
Using forceps, transfers each tissue to one independent 1.5mL microcentrifuge tube containing 1mL of 30% (w/v) sucrose in PBS–4% (w/v) paraformaldehyde solution, having care that the tissue is completely submerged in the fixing solution and there are no air bubbles in the tissue. Is common that some tissues should be folded in half so that it remains immersed in the solution. If the intestine has adipose tissue attached, it will tend to float. Therefore, it is recommended to remove the adipose tissue using scissors and forceps. Incubate the intestines in fixing solution 0h 15m 0s at 4°C.
For steps 50–58, see S6 Video in the next link:
https://www.dropbox.com/s/w1xacz5hqxc0m4z/S6_Video.mp4?dl=0
S6 Video. Washing and fixing of extracted tissues (steps 50–58). This video shows how to open and wash the infected ligated loops and the procedure of fixing with 30% sucrose in PBS–4% paraformaldehyde.
Immunofluorescence
Day 2
Using forceps, transfer the tissues to a new 1.5mL microcentrifuge tube containing 1mL of PBS. Perform this step carefully to avoid paraformaldehyde splashing. Incubate for ~0h 5m 0s a Room temperature .
Wash the tissues. Using a 100µL–1000µL micropipette, remove the PBS and discard it to an autoclavable glass bottle, from now on, waste bottle. Add 1.0mL of PBS to the edges of the tube. And repeat one more time.
Using forceps put the tissues over a clean and sterile open petri dish, and using surgical scissors, cut a section of the tissues of ~5 mm × 5 mm .
Using forceps, transfer the tissues to a 0.5mL microcentrifuge tube containing 150µL permeabilizing solution; PBS–0.2% (w/v) Triton X-100 and incubated for 2h 0m 0s at 4Room temperature
Using a 20µL–200µL micropipette, remove the permeabilizing solution as much as possible from the walls of the tube and discard it in the waste bottle. Add 200µL of PBS to wash the samples and incubate for ~0h 3m 0s at 4Room temperature in an orbital shaker at 60rpm. Repeat 2 more times.
In the same tubes, incubate the tissues with 150µL of blocking solution; PBS–3% (w/v) BSA for 3h 0m 0s at 4Room temperature in an orbital shaker at 60rpm.
Using a 20µL–200µL micropipette, remove the blocking solution and discard it in the waste bottle.
Add 70µL–90µL of 1:1,000 chicken primary antibody anti- C. difficile spore IgY batch 7246 (AvesLab, USA) and 1:150 phalloidin Alexa-Fluor 568 (A12380 Invitrogen, USA); in PBS–3% BSA overnight at 4°C. This antibody does not immunoreacted with epitopes of vegetative cells neither with murine microbiota [see references]. After adding the antibody solution, check that there are no bubbles in the tube and that the tissue is completely submerged.
Day 3
Wash the tissues. Using a 20µL–200µL micropipette, remove the primary antibody solution as much as possible from the walls of the tube and discard it in the waste bottle. Add 200µL of PBS to wash the samples and incubate for ~0h 3m 0s at 4Room temperature in an orbital shaker at 60rpm. Repeat 2 more times.
In the same tube, incubate the tissue with 70µL–90µL of 1:350 secondary antibodies goat anti-chicken IgY Alexa Fluor-647 (ab150175, Abcam, USA) and 4.5µg/µL of Hoechst 33342 and incubate for 3h 0m 0sat 4Room temperature in an orbital shaker at 60rpm.
Wash the tissues 3 times as was described in step 68.
Tissue Mounting
At this point is difficult to identify the luminal side and the muscular side of the tissues at the naked eye. However, sample mounting is essential to identify the tissue orientation. For this:
Using forceps, place the samples in a clean glass slide.
First, using a light -upright or -inverted microscope with 20× or 40× magnification coupled to epifluorescence with a blue filter to visualize Hoechst 33342 staining, orientate the tissues to put the liminal side up as follow.
In the case of the ileum, villi can be visualized, and in the case of the colon, crypts are easy to identify. In both cases, on the other side, the muscular layer is seen.
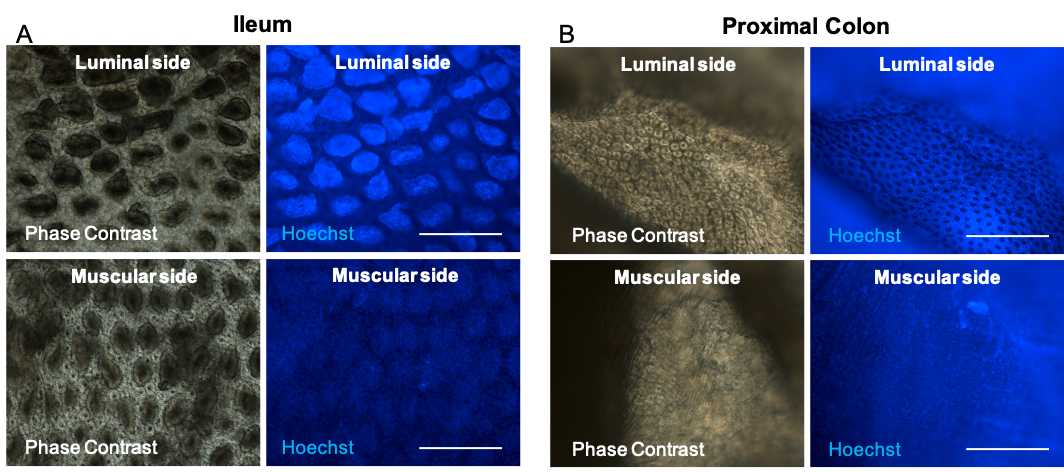
Clean the coverslips and slides with 70% (v/v) ethanol and towel paper.
Using towel paper removes the excess PBS from the edges of the tissues.
In a new clean glass slide, using a 2µL–20µL micropipette, put a drop of 5µL of fluorescent mounting medium for each tissue to be mounted.
Using forceps put the tissues over the drops of the mounting medium of the clean slide.
Using a 2µL–20µL micropipette, put 15µL of fluorescent mounting medium over the tissues having care of not to damage the tissue with the tip of the micropipette.
Put Scotch transparent tape (3M, USA) on the upper and lower edges of the coverslip, leaving half of the Scotch transparent tape on the coverslips and the other half free.
Put a coverslip over the samples, not allowing air bubbles to remain in the tissue.
With your fingers, fold the piece of Scotch transparent tape under the slide firmly.
Seal the remaining edges with Scotch transparent tape.
Store the samples at 4°C 3h 0m 0s, and then the samples are ready to visualize under confocal microscopy.
For steps 74–84, see S7 Video in the next link:
https://www.dropbox.com/s/to07xfy6fyi4xtd/S7_Video.mp4?dl=0
S7 Video. Mounting of immunostained tissues for confocal microscopy (steps 74–84). This video shows how to orientate the tissues to put the luminal side up of the ileum and the colon, the mounting using mounting medium, and sealing it with Scotch transparent tape.

