Isolation of Schistosoma mansoni eggs, miracidia, and sporocysts for in vitro cultivation
Sarah K Buddenborg, Matt Berriman, Geetha Sankaranarayanan, Magda E Lotkowska, Catherine McCarthy, Lisa Seymour, Gabriel Rinaldi
Abstract
The purpose of this procedure is to isolate eggs from livers collected from mice infected with Schistosoma mansoni . This protocol ensures a sterile prep of eggs to be used for culture of eggs and/or collection and culture of sporocysts, and snail infection.
Steps
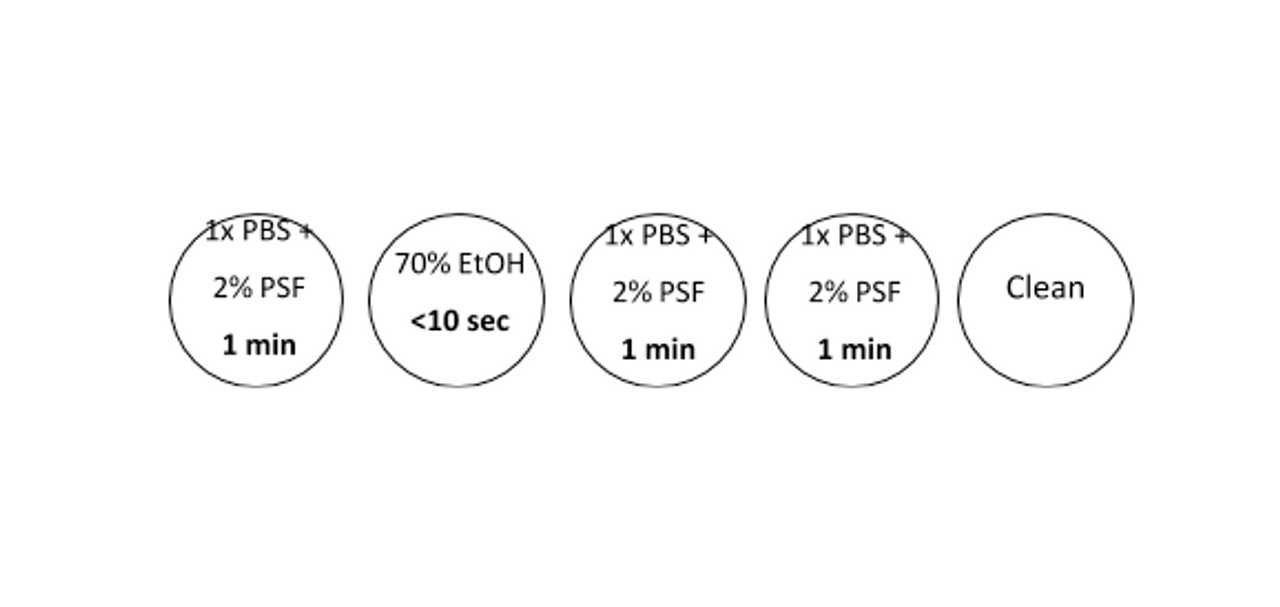
Liver washing
Decant the livers into the first petri dish using ethanol-cleaned tweezers to submerge and continuously move them for 1 minute to ensure complete saturation of the tissue
Repeat step 3 for each petri dish with the exception of the 70% ethanol dish which should be submerged and rinsed for less than 10 seconds
Liver dissociation
Place all livers into a clean petri dish and using a sterile scalpel finely mince them
Transfer the minced livers to a 50ml falcon tube, re-suspend in 40ml of 1x DPBS + 2% anti-anti and label with necessary identifying information
Weigh out 0.05g of collagenase into a labelled 15ml falcon tube and add 10ml of dH20. Mix well (The amount of collagenase to prepare depends on the number of livers to be processed – collagenase is always prepared fresh)
Add 5ml of 0.5% collagenase solution to the liver suspension and mix well
- Optional: Add to the mix 500 ul of polymixin B (100K Units) (Sigma-Aldrich, P4932-1MU), a gram negative bactericidal antibiotic that reduce LPS contamination in the egg prep, in particular when SEA will be prepared from eggs and immunological studies or co-culture with cells, will be performed
Wrap securely in parafilm and secure the tube horizontally in a 37°C rocker overnight
Egg isolation
The following day, centrifuge the liver suspension tube at 400g for 5 min (acceleration and deceleration 9)
Aspirate the supernatant and re-suspend in 50ml of 1x DPBS + 2% anti-anti.
Repeat steps 9-10 three more times
After the final aspiration, re-suspend the pellet in 25ml of 1x DPBS + 2% anti-anti
Using a 50ml stripette, pass the suspension through the 250uM sieve into a 1L sterile beaker
Pass this filtrate through the 150uM sieve into a second 1L sterile beaker
Wash the beaker with 5ml of 1x DPBS + 2% anti-anti to collect any remaining eggs and add this to the filtrate by passing through the 150uM sieve
Decant the filtrate into a 50ml falcon tube and centrifuge (400g, 5 minutes, acceleration and deceleration 9)
Aspirate the supernatant and resuspend in 10ml 1x DPBS + 2% anti-anti
Prepare a Percoll gradient (one percoll gradient per 5 livers):
Prepare a 0.25M sucrose solution (4.27g sucrose + 50ml diH2O) and filter the solution through 0.22um syringe filter
In 50ml falcon tube mix 8ml Percoll and 32ml of the 0.25M sucrose solution. Invert 5 times
Very carefully pipette the resuspended eggs onto the surface of the Percoll gradient around the circumference to create a defined layer
Centrifuge the gradient at 800g for 10 minutes (acceleration 2 and deceleration 1)
Aspirate the supernatant and re-suspend in 10ml of 1x DPBS + 2% anti-anti. Transfer to a 15ml falcon tube
Centrifuge at 400g for 5 min (acceleration and deceleration 9)
Repeat steps 21-22 two more times
IMPORTANT. Check the eggs under microscope, if the prep is still ‘dirty’ or ‘contaminated with liver debris’ proceed with a second percoll gradient
Resuspend the pellet in 10ml 1x DPBS + 2% anti-anti and count 12 aliquots of 5µl to estimate total number of collected eggs
Eggs in culture
Centrifuge eggs in falcon tube at 400g for 5 mins (acceleration and deceleration 9)
Resuspend eggs in adult media (DMEM + 10% FBS + 2% anti-anti) and transfer to 6 well plates (5-6ml of media per well)
Keep eggs in culture at 37°C and 5% CO2
- Eggs can be kept in culture for up to ~10 days and retain the ‘hatchability’ however, the hatching rate will drop over time
Hatching eggs and collecting miracidia
Centrifuge eggs in falcon tube at 400g for 5 mins (acceleration and deceleration 9)
In the culture hood, aspirate supernatant and re-suspend in 6ml diH2O
Aliquot 1ml each in a 24 well plate
- It is important to use 24 well plate given the miracidia get more diluted in 12 or 6 well plate and more egg shells are picked up when collecting the miracidia)
Rinse the original falcon tube with 6 ml of water and distribute 1 ml to each well containing 1ml of eggs (i.e. the eggs will be in 2ml of water)
Place under light for hatching
At 30-40 min intervals over ~3 hrs, gently remove the top 1ml of water containing the miracidia into a 50ml falcon tube and top up the wells with 1ml of diH2O
Count miracidia and proceed with snail infections dx.doi.org/10.17504/protocols.io.36wgqjkkxvk5/v1 or sporocyst transformation
Sporocyst transformation
Place the tube containing miracidia on ice for ~20 min
Centrifuge at 800g for 15min (acceleration and deceleration 9)
Quickly aspirate the water and resuspend the pellet of miracidia in complete sporocyst media
- Sporocysts can be kept in culture at 28°C with malaria gas (90-92% N, 5% CO2, 3-5% O2) in a sealed container changing the media once to twice a week
- IMPORTANT. To avoid contamination always replace the media with fresh complete media the day after transformation