Introductory Hydra Activities
Callen Hyland
Abstract
This is a series of introductory lab activities for BIOL309-03: Research Methods. The purpose is to master vocabulary related to Hydra anatomy, become familiar with compound microscopes and dissecting microscopes, and practice microdissections and micropipette use. The second section sets up an experiment that will be documented in a second class section to provide quantitative data for a statistics activity.
Before start
Please read through the entire protocol before beginning.
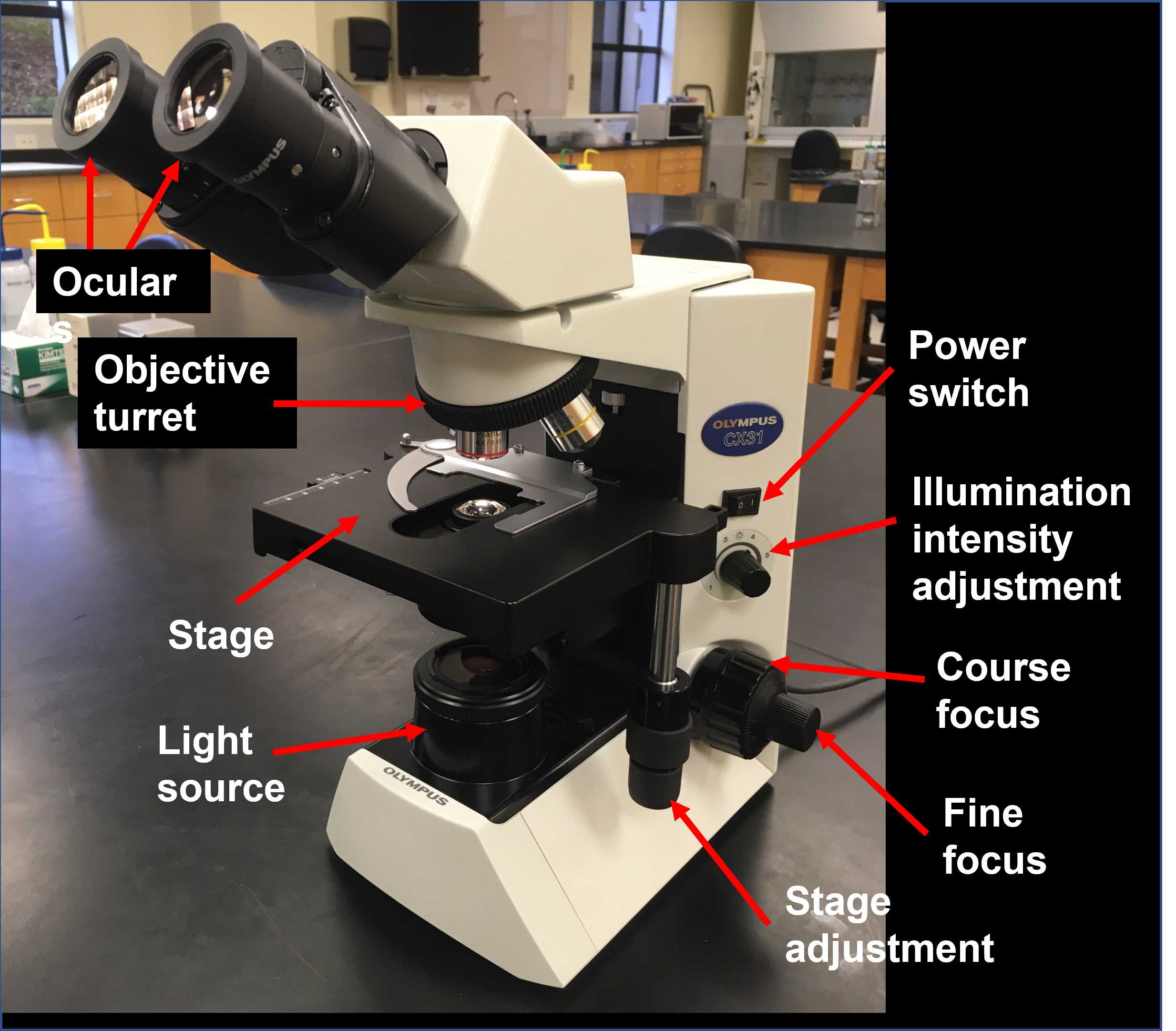
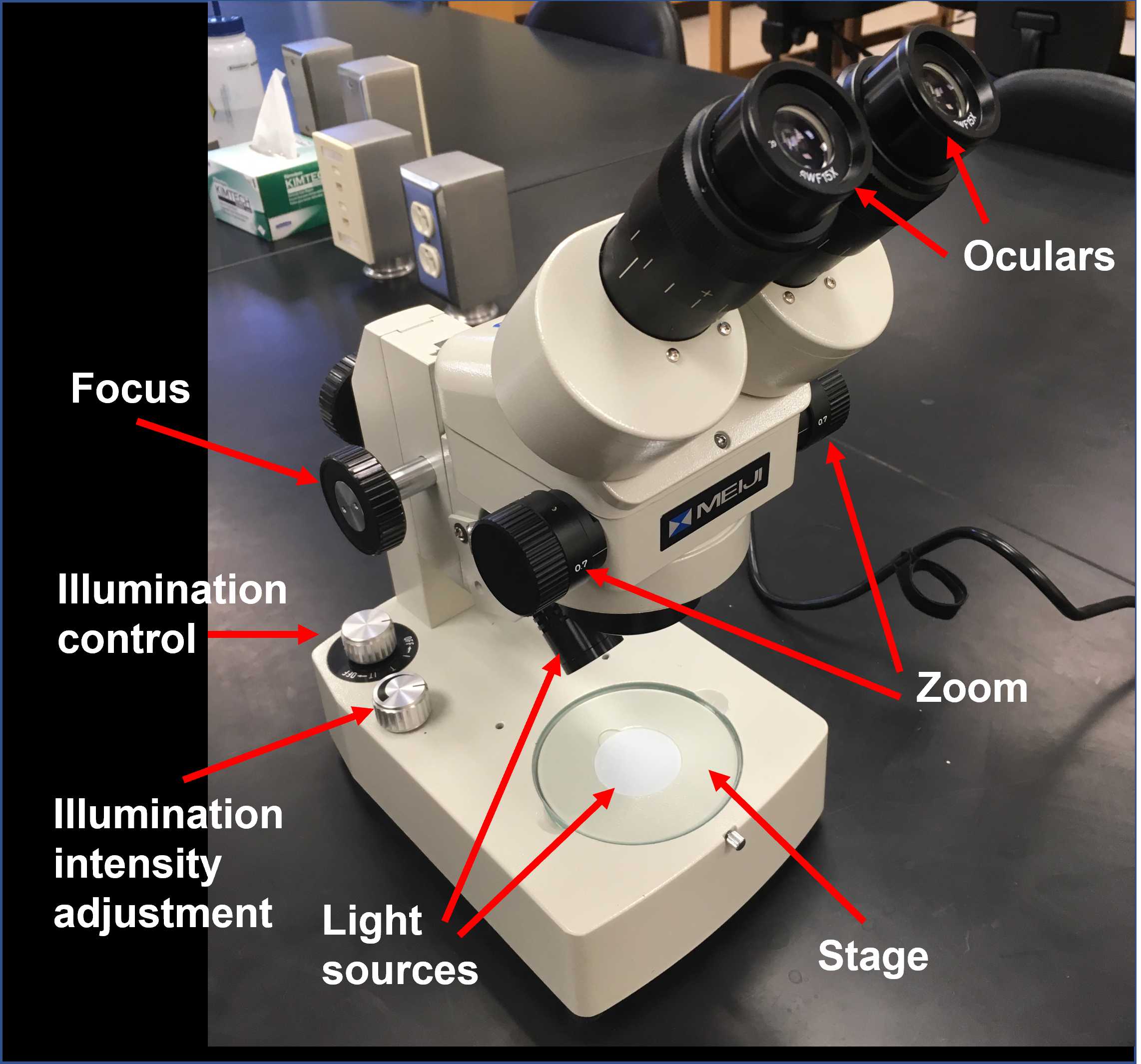
Familiarize yourself with the components of the microscope and its basic operation and review the basic anatomy of Hydra.
Steps
Observation Activities
In this activity you will become familiar with the anatomy of Hydra including areas of the body, cell layers, and nematocysts, while practicing the new vocabulary we learned in lecture.
Learning objectives:
- Use a compound microscope to examine prepared slides
- Master vocabulary related to Hydra anatomy
- Correctly identify body parts and cell layers in fixed Hydra specimens
- Sketch biological structures under a microscope
- Identify similarities and differences in animal anatomy
Materials:
- Compound microscope
- Microscope slides with Hydra whole mount, cross section, and nematocysts
- Selection of preserved Cnidarian specimens
- Lab notebook, pen, and pencil (optional)
Note
In this activity, you will be sketching biological structures that you observe under the microscope. Your sketches don't have to be "good", they just have to show the general shapes and relative positions of the structures you are asked to examine. You probably know not to write in pencil in your lab notebook, but you can make an exception here. It may be convenient to sketch the structures first with pencil then copy over the final lines in pen.
Basic Hydra anatomy
Examine the whole mount Hydra slides with the 4X objective. Adjust the distance between the oculars and focus on the specimen if necessary. Sketch the whole Hydra in your lab notebook and label the following body parts:
- Tentacles
- Hypostome
- Head
- Body column
- Peduncle
- Basal disk
- Bud
- Budding zone
- Label the hypostome and tentacles on the bud
Body wall structure
Examine the Hydra cross section (transverse section) with the 40X objective. The cross section should already be centered in the field, so don't move the stage. Adjust the distance between the oculars and focus on the specimen if necessary (use only the fine focus adjustment). In your lab notebook, sketch just a section of the body wall and label:
- Endodermal cells
- Ectodermal cells
- Mesoglea
Hydra nematocysts
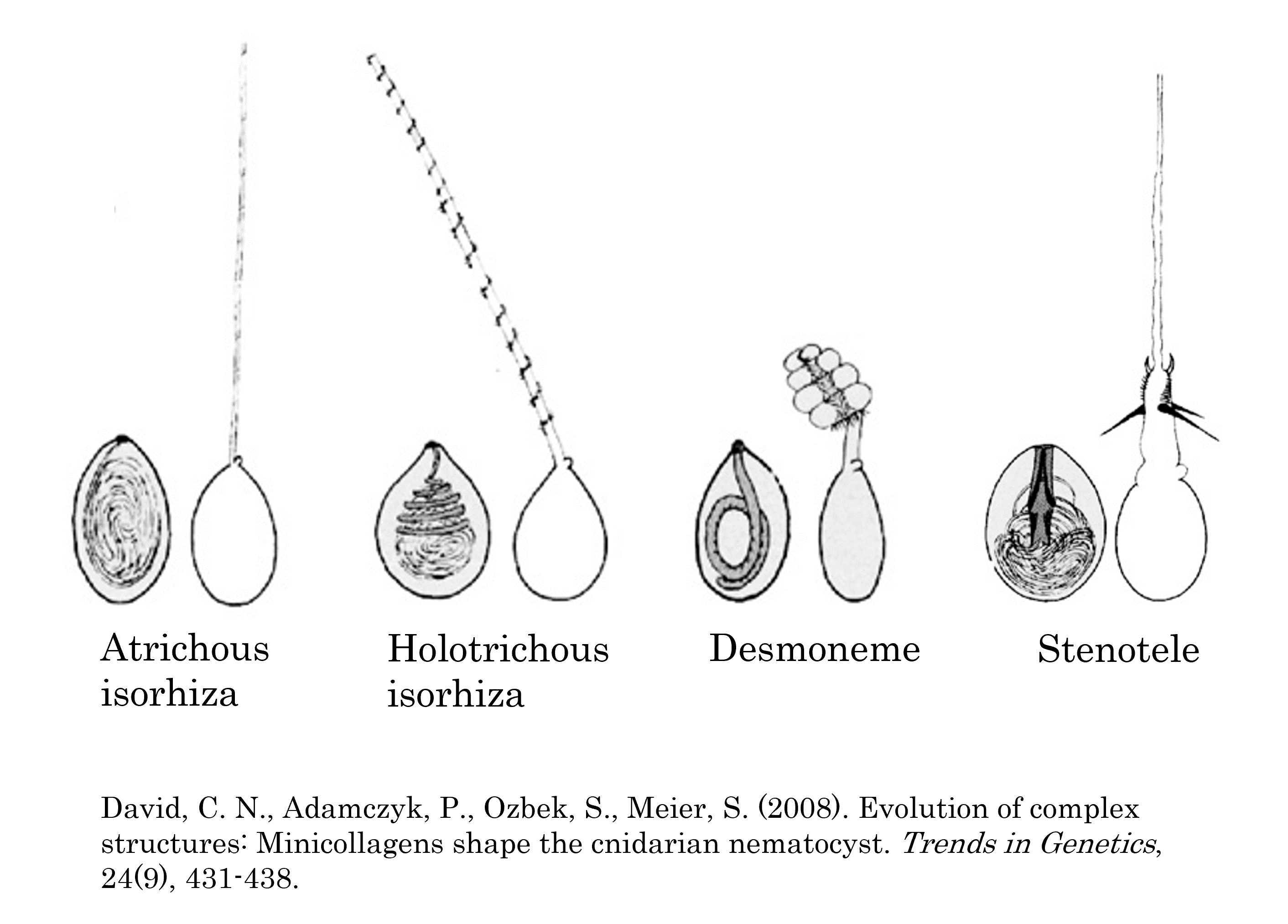
Examine the smear of Hydra nematocysts with the 40X objective. Adjust the distance between the oculars and focus on the specimen if necessary. Feel free to move the stage around to view different parts of the smear. Try to find at least two of the four different types of nematocysts (Figure 1) and sketch them in your lab notebook.
Comparing Hydra to other Cnidarians
Examine the preserved specimens. In your lab notebook, make a list of the features that all of these animals have in common. What do they have in common with Hydra? What features make them appear different from each other and from Hydra?
Regeneration Experiment
Anyone who examines a large number of Hydra in a dish can clearly see that they do not all have the same number of tentacles. You may wonder, does each animal have a fixed number of tentacles? If we were to hypothesize that each animal has a fixed number of tentacles, it might follow that we cut a Hydra in half and allowed the foot-end to regenerate a head, this head would have the same number of tentacles as the head that was removed. We will keep the head-end of the Hydra to test whether the number of tentacles on an intact Hydra head spontaneously changes in the course of a week.
Learning objectives:
- Use a dissecting microscope to view live animals
- Become proficient with using an adjustable volume micropipette
- Practice moving Hydra with a Pasteur pipette
- Apply micro-dissection skills to create transverse sections of Hydra
Materials:
- Common dish with Hydra
- Dissecting microscope
- scalpel
- 60 mm Petri dish
- Hydra medium
- 100-1000 µL micropipette and tips
- 24-well plate
- Glass Pasteur pipette and bulb
- Waste container
- Permanent marker
- Labeling tape
Check your workstation to make sure you have all materials listed above.
Fill your Petri dish halfway with Hydra medium.
Use your glass Pasteur pipette to transfer six adult asexual Hydra without buds, one by one, from the common dish to your Petri dish.
Set your 100-1000 µL adjustable volume micropipette to 1000 µL. Get a new tip.
Fill 18 wells of your 24-well plate with 1 mL (1000 µL) of Hydra medium using your micropipette.
Dispose of used tips in the waste container.
Put a piece of labeling tape on the lid of your 24-well plate.
Use a permanent marker to label it with your name and date.
Place your Petri dish on the stage of your dissecting microscope and turn the light control knob to the "I" (incident light) setting.
Adjust the zoom to the lowest setting.
Adjust the distance between the oculars until you see a single field.
Adjust the focus knob to get a crisp image of one of your Hydra.
You'll have to adjust the zoom for your own preference when doing the dissection.
Count the number of tentacles that the Hydra has and record it in your lab notebook.
Since you are going to dissect six Hydra you may want to set up a table to record the following information for each Hydra:
- Initial number of tentacles
- Well containing head-end
- Number of tentacles on head-end after a week
- Well containing foot-end
- Number of tentacles on foot-end after a week
Use your scalpel to cut the Hydra transversely (horizontally) in two. Try to cut it right above the budding zone. In some adult Hydra, you can see a visible "bulge" near the budding zone. If this is not visible, just try to cut it into two equal parts.
What happened to your Hydra right after you cut it?
Record any behaviors you observed in your lab notebook.
You should not have a head-end and a foot-end. Transfer both into separate wells of your 24-well plate.
Record which well the head and foot ends were placed in.
Repeat steps 11 through 15 for five more Hydra.
When you are done, place the lid on your 24-well plate and give it to your instructor.

