Immunohistochemistry of Human Brain Striatum: ciliation of cholinergic neurons and astrocytes
Suzanne R Pfeffer, Ebsy Jaimon, Shahzad S. Khan, Yu-En Lin
Abstract
We study cholinergic neurons and astrocytes of the dorsal striatum that are important components of the nigrostriatal circuit. We have shown previously that these cell types lose primary cilia in 4 mouse models of LRRK2 Parkinson’s disease (Dhekne et al., 2019; Khan et al., 2021). Here we present a method to identify the same brain region in human postmortem tissue to evaluate ciliary status. The striatum is identified morphologically and also by immunofluorescence microscopy using anti-DARPP-32 antibodies (Ouimet & Greengard, 1990; Arlotta et al., 2008). Cholinergic cell bodies stain brightly with anti-choline acetyltransferase (ChAT) antibodies and these cells are also identifiable using antibodies that recognize the choline transporter, CHT. Auto-fluorescence is decreased using Sudan Black B.
We are grateful to our colleagues at the Banner Institute at the University of Arizona (Drs. Geidy E. Serrano and Ileana Lorenzini) for providing tissue and guidance (Beach et al., 2015).
References
Arlotta P, Molyneaux BJ, Jabaudon D, Yoshida Y, Macklis JD. Ctip2 controls the differentiation of medium spiny neurons and the establishment of the cellular architecture of the striatum. J Neurosci. 2008 Jan 16;28(3):622-32.
doi: 10.1523/JNEUROSCI.2986-07.2008.
Beach, T. G., Adler, C. H., Sue, L. I., Serrano, G., Shill, H. A., Walker, D. G., Lue, L., Roher, A. E., Dugger, B. N., Maarouf, C., Birdsill, A. C., Intorcia, A., Saxon-Labelle, M., Pullen, J., Scroggins, A., Filon, J., Scott, S., Hoffman, B., Garcia, A., Caviness, J. N., … Sabbagh, M. N. (2015). Arizona Study of Aging and Neurodegenerative Disorders and Brain and Body Donation Program. Neuropathology : official journal of the Japanese Society of Neuropathology, 35(4), 354–389. https://doi.org/10.1111/neup.12189
Dhekne, H. S., Yanatori, I., Gomez, R. C., Tonelli, F., Diez, F., Schüle, B., Steger, M., Alessi, D. R., & Pfeffer, S. R. (2018). A pathway for Parkinson's Disease LRRK2 kinase to block primary cilia and Sonic hedgehog signaling in the brain. eLife, 7, e40202. https://doi.org/10.7554/eLife.40202
Khan, S. S., Sobu, Y., Dhekne, H. S., Tonelli, F., Berndsen, K., Alessi, D. R., & Pfeffer, S. R. (2021). Pathogenic LRRK2 control of primary cilia and Hedgehog signaling in neurons and astrocytes of mouse brain. eLife, 10, e67900. https://doi.org/10.7554/eLife.67900
Format:
Ouimet CC, Greengard P. Distribution of DARPP-32 in the basal ganglia: an electron microscopic study. J Neurocytol. 1990 Feb;19(1):39-52.
doi: 10.1007/BF01188438.
Steps
Identification of the Striatum
Use paintbrushes to retrieve a tissue section from a 15 mL falcon tube containing brain tissue sections.
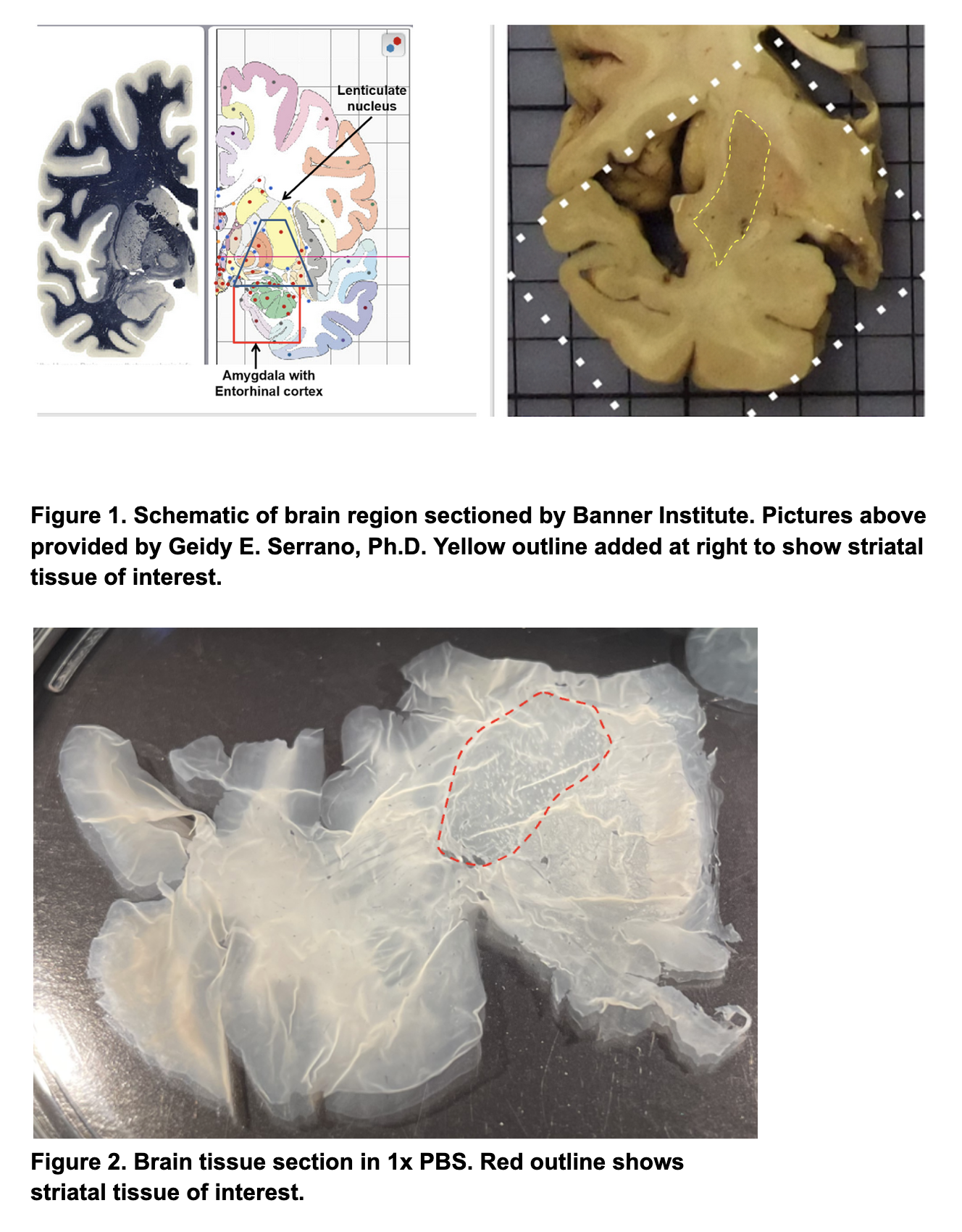
Use a dissecting microscope to identify pieces of tissue sections that contain striatum (See Figures 1 and 2).
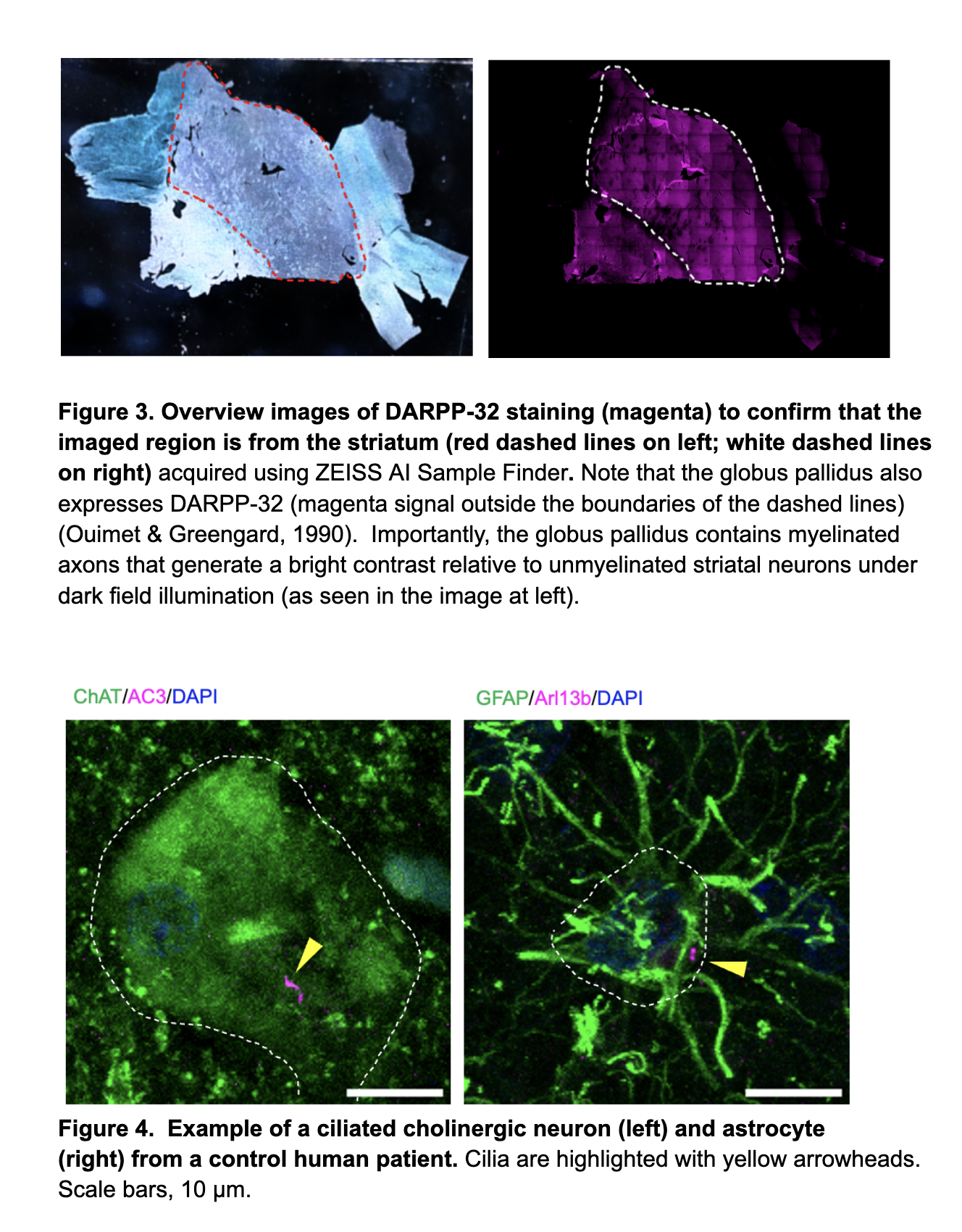
Free floating tissue should be submerged in 1x PBS in 10 cm tissue culture dishes and handled primarily with paintbrushes. Allow tissue to unfold in the PBS and identify the areas of interest (i.e. striatum) using visual guides in Figures 1-2. Note that not all sections are “whole pieces” and that some tissues may be torn and difficult to handle even with paintbrushes.
Add 1mL of 10 mM Sodium Citrate Buffer pH 6.0 into a clean 1.5mL Eppendorf tube for each dissected tissue piece and preheat these tubes on a 95° C heat block for > 5 < 15 minutes.
Antigen Retrieval
- Transfer dissected tissues to the preheated 1.5 mL tubes containing Sodium Citrate Buffer and allow tissues to incubate for 15 minutes.
- Transfer dissected tissue into a 24-well dish containing 0.75-1mL PBS. Incubate on a Nutator (90-120 rpm) for 5 minutes. Repeat PBS rinse and incubation two more times.
Permeabilization
- Permeabilize with PBS containing 0.1% Triton for 1 hour at room temperature on a Nutator.
- Wash tissue (3x) with PBS to remove excess Triton (5 minutes each).
Block
Block tissue for 2 hours @ room temperature using PBS containing 2% FBS and 1% BSA.
Primary Antibody Incubation
- Incubate tissue overnight at 4°C on a Nutator with mouse-anti-human AC3 to stain primary cilia (E-8 #sc-518057) (1:100), goat-anti-human Choline acetyltransferase (ChAT) (Millipore #AB144P) (1:200) or rabbit-anti-human choline transporter (Millipore #ABN458) (1:500) to identify cholinergic neurons, and rabbit-anti-human DARPP-32 (CST #2306S) (1:400) in antibody diluent (PBS containing 1% BSA and 1% DMSO) to identify the correct brain region. For astrocyte detection, use chicken-anti-human-GFAP (EnCor Biotechnology Inc. #CPCA-GFAP) (1:2000) and label primary cilia in astrocytes using mouse-anti-human Arl13b (NeuroMab #N295B/66) (1:500).
- Wash 3x with 1% BSA containing 1% DMSO to remove primary antibody solution (5 minutes each) with rotation on a Nutator.
Secondary Antibody Incubation
- Incubate tissue for 2 hr at room temperature in donkey-anti-rabbit Alexa Fluor 568 (1:2000), donkey-anti-goat Alexa Fluor 488 (1:2000), donkey-anti-mouse Alexa Fluor 647 (1:2000), and DAPI (1:500) diluted in antibody diluent (PBS containing 1% BSA and 1% DMSO). IMPORTANT: Protect samples from light
- Rinse samples (3x) with PBS (5 minutes each) with rotation on a Nutator.
Reduction of Auto-fluorescence
Reduce autofluorescence using freshly prepared 0.1% Sudan Black B in 70% ethanol for 20 minutes.
To prepare Sudan Black B, add dry powder (0.5 g) to 50 mL of 70% ethanol. If Sudan Black B does not readily dissolve you can warm the mixture to 37° C. Most of the Sudan Black B should go into solution. If you observe small impurities that do not dissolve, filter the solution using a 40 µm strainer into a clean 50 mL falcon tube.
- Rinse (3x) with PBS containing 0.3% Triton for 5 minutes each with rotation.
- Remove excess detergent by transferring the tissue to a well containing ddH2O.
Mounting Tissues onto Slides
- Add ddH2O to a microscope slide until it forms a droplet large enough to encase the dissected tissue. Then, transfer the tissue onto the microscope slide and unfold the tissue using tissue paintbrushes.
- Once the tissue has been unfolded and is flat, remove excess water using a disposal dropper. Add a few drops of Fluoromount G to the center of the tissue section using a clean dropper and slowly lower an appropriate glass slide onto the tissue. Aspirate any excess mountant along the corners of the glass slide.
- Image the slides the next day using a 63X/1.4 oil immersion objective. Acquire images with 0.5 µm Z steps for astrocytes and 0.75 µm Z steps for cholinergic neurons.