Leaf Wax Lipid Extraction for Archaeological Applications
Robert Patalano, Robert Patalano, Jana Zech, Jana Zech, Patrick Roberts, Patrick Roberts
Abstract
Plant wax lipid molecules, chiefly normal (n -) alkanes and n -alkanoic acids, are frequently used as proxies for understanding paleoenvironmental and paleoclimatic change. These are regularly analyzed from marine and lake sediments and even more frequently in archaeological contexts, enabling the reconstruction of past environments in direct association with records of past human behavior. Carbon and hydrogen isotope measurements of these compounds are used to trace plant type and water-use efficiency, relative paleotemperature, precipitation, evapotranspiration of leaf and soil moisture, and other physiological and ecological parameters. Plant wax lipids have great potential for answering questions related to human-environment interactions, being for the most part chemically inert and easily recoverable in terrestrial sediments, including those dating back millions of years. The growing use of this technique, and comparison of such data with other paleoenvironmental proxies such as pollen and phytolith analysis and soil carbonate and tooth enamel isotope records, make it essential to establish consistent, best-practice protocols for extracting n -alkanes and n -alkanoic acids from archaeological sediments to provide comparable information for interpreting past climatic, ecosystem, and hydrological changes and their interaction with human societies. © 2020 The Authors.
Basic Protocol 1 : Total lipid extraction
Support Protocol 1 : Weighing the total lipid extract
Support Protocol 2 : Cleaning the PSE extraction cells
Alternate Protocol 1 : Soxhlet total lipid extraction
Alternate Protocol 2 : Ultrasonic total lipid extraction
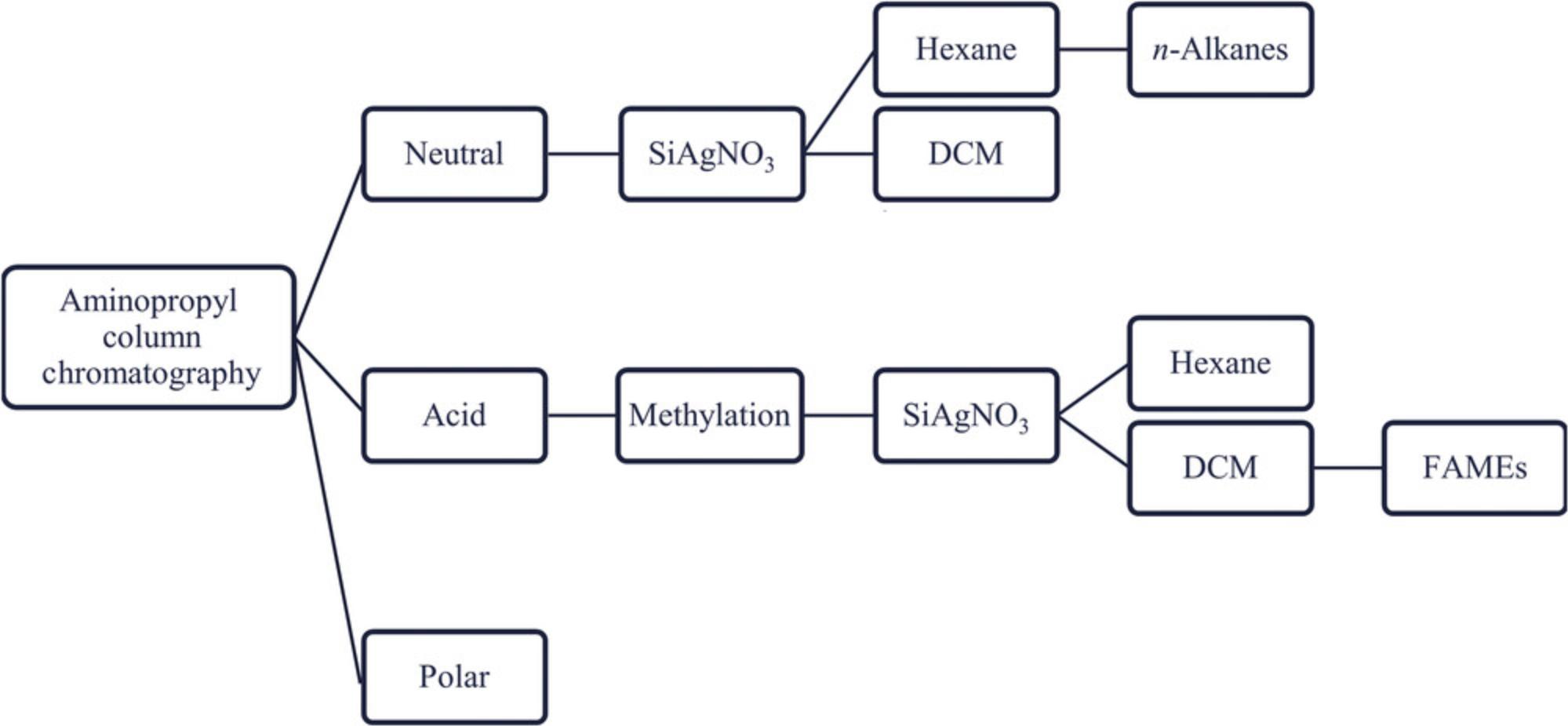
Basic Protocol 2 : Separation of lipids by aminopropyl column chromatography
Basic Protocol 3 : Separation of lipids by silver-nitrate-infused silica gel column chromatography
Support Protocol 3 : Preparation of silica gel infused with 10% silver nitrate
Basic Protocol 4 : Methylation of n -alkanoic acids
Basic Protocol 5 : Gas chromatography mass spectrometry (GC-MS)
Basic Protocol 6 : Gas chromatography isotope ratio mass spectrometry (GC-IRMS)
INTRODUCTION
The external surfaces of vascular plant leaves are coated with protective waxes that contain a wide range of organic compounds. These waxes preserve the water balance of the plant and minimize damage to leaf cells from fungal and insect attack, wind abrasion, and excessive ultraviolet radiation. The lipid component of the leaf wax is, for the most part, chemically inert and resistant to biodegradation in sediments over geologic time, making these lipids an excellent proxy measure for paleoclimatic and paleoenvironmental conditions (Eglinton & Eglinton, 2008). Palaeoclimatological research extending back to the Miocene (Yang & Huang, 2003), and even the early Eocene (Pagani et al., 2006), highlights the long-term molecular fingerprinting capability of plant biomarkers. Plant wax lipids pass into the environment as leaf debris and can be transported long distances by wind and water before the intact molecules are deposited. Traditionally, geographers and earth scientists have sought to recover plant waxes from terrestrial, ocean, and lake sediments in order to reconstruct biotic and abiotic factors of past ecosystems, using solvent extraction of ancient sediments or rocks followed by qualitative and quantitative profiling through a combination of chromatographic separation and mass spectrometry techniques. For example, the characterization of biomarker chemical structures to assess biosynthesis is now complemented by isotopic analyses of individual compounds to study ancient biodiversity or climatic conditions (Simoneit, 2004). There are growing attempts to extract the same compounds from archaeological sites, providing a record of past environments that can be directly compared to sequences of past human technology, culture, and settlement.
The two main plant wax biomolecules used as paleoenvironmental proxies are normal (n -) alkanes and n -alkanoic acids. Through their carbon (δ13C) and hydrogen (δD) isotope ratios, these water-insoluble compounds can be used to research the relationship between hydrological patterns (e.g., rainfall amount, aridity vs. humidity) and plant ecology (e.g., C3 vs. C4 ecosystems, canopy structure) and, when applied in archaeological situations, to answer questions about cultural adaptive responses to ecological changes, site-use patterns across diverse plant landscapes, and the human selectivity and usage of specific plants within a wider ecological biome. The rising use of this technique in archaeological contexts and in the study of past human-environment interactions necessitates the development and presentation of appropriate standardized protocols to an archaeological audience. Nevertheless, published protocols remain variable, are rarely compared or presented in detail, and are generally lacking in the archaeological literature.
This protocol paper attempts to amend these problems by presenting a standardized geochemical laboratory workflow that is applicable in archaeological research settings. The workflow for extracting and analyzing n -alkanes and n -alkanoic acids is divided into six parts, as shown in Figure 1: (i) the solvent extraction of total lipids from soils and sediments (Basic Protocol 1); (ii) the column chromatography separation of the total lipid extract to obtain distinct compound fractions (Basic Protocol 2); (iii) the further separation of distinct fractions to isolate n -alkanes or n -alkanoic acids (Basic Protocol 3); (iv) the methylation of the n -alkanoic acid fraction (Basic Protocol 4); (v) the identification and quantification of component compounds using gas chromatography mass spectrometry (Basic Protocol 5); and (vi) carbon (δ13C) and hydrogen (δD) isotope analyses using isotope ratio mass spectrometry (Basic Protocol 6).
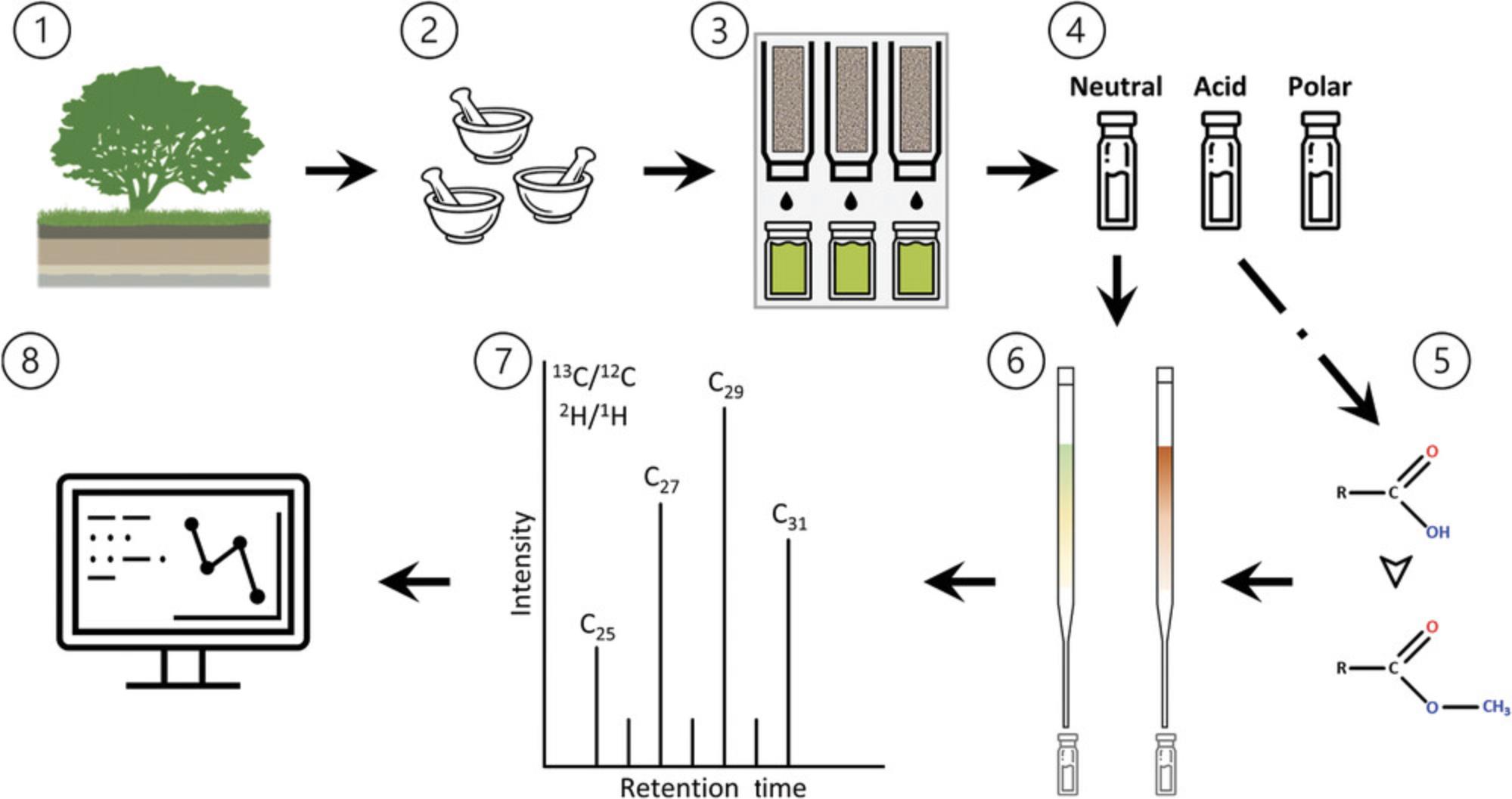
Basic Protocol 1: TOTAL LIPID EXTRACTION
Total lipid extraction (TLE) is a technique based on the like-dissolves-like principle of solvent separation, whereby solvents readily dissolve analytes of approximately the same polarity. Typically, an azeotrope of solvents is refluxed through a ground plant, rock, or unconsolidated soil or sediment sample to isolate leaf waxes. There are multiple ways to extract lipids, of which the most common are the use of a Soxhlet apparatus, accelerated or pressurized solvent extraction (ASE or PSE), and ultrasonic extraction. Although Basic Protocol 1 will focus on PSE, the different techniques share inherent principles in that the goal of each is to ensure high analyte recovery while reducing time and solvent consumption and avoiding carryover or cross-contamination between samples. Each extraction technique requires samples to be homogenous and dry and equipment to be precleaned, so sample pretreatment and cleaning recommendations are included in each protocol. See Alternate Protocols 1 (Soxhlet extraction) and 2 (ultrasonic extraction) for the extraction of total lipids when PSE is not available.
A PSE apparatus is an automated system for extracting organic compounds from a variety of solid and semisolid samples. Although these are still relatively rare pieces of equipment in many labs, they are quickly being adopted because of their efficiency in extracting compounds over short time periods. PSE uses elevated temperature and pressure in combination with organic solvents to increase the efficiency of the extraction process. During the procedure, an extraction cell containing the sample is filled with solvent and then heated and pressurized to maintain the solvent in a liquid state for a predetermined amount of time. After this hold time, the extract is discharged from the cell into a collection vessel , and then the process is repeated. PSE has major benefits over other methods in terms of speed and overall compound recoverability. First, higher temperatures increase the extraction speed and the solubility of lipid analytes. Elevated pressure allows temperatures above the solvent boiling points to be used, which is essential given that the boiling points of organic solvents and target lipid compounds are often low (see solvents listed in Materials). Operating at elevated pressure also expedites and enhances the overall extraction, as pressurized solvent is forced into and through the pore spaces of the sediments, improving biomarker recovery.
The type of solvent used has a major impact on the extraction, and it is best to choose solvents with polarities similar to those of the target lipids; in this instance, the target analytes are nonpolar (n -alkanes) and slightly polar (n -alkanoic acids as fatty acid methyl esters). Mixtures of solvents with different polarities often provide better recoveries. A temperature of 100°C, a pressure of 1500 psi (103 bar), and a 9:1 volume/volume mixture of dichloromethane and methanol have been shown to be good starting points for a wide range of extraction applications.
Materials
-
Compressed nitrogen gas (N2), 99.99% purity
-
Dichloromethane (DCM), HPLC grade, 39.7°C boiling point, 3.1 polarity index (Millipore Sigma cat. no. DX0831)
-
Methanol (MeOH), HPLC grade, 64.7°C boiling point, 5.1 polarity index (Millipore Sigma cat. no. MX0488)
-
5-20 g dry, homogenized archaeological sediment or paleosol sample
-
Quartz Ottawa sand, 0.3-0.9 mm (Büchi part no. 037689)
-
Pressurized speed extractor: Büchi Speed Extractor E-916 (shipped with all parts needed for initial installation and use, but additional items are likely to be needed and are listed below)
-
Stainless steel funnel (Büchi part no. 053396)
-
20- and 40-ml ml stainless steel E-916 extraction cells (Büchi part nos. 051236 and 051235)
-
Cellulose top and bottom filters for extraction cell (Büchi part nos. 049572 and 049569)
-
150-ml wide-necked collection vessel with 1 ml residual volume (Büchi part no. 11056498)
-
Portable cell rack (Büchi part no. 053690)
-
1-liter solvent bottle (Büchi part no. 053203; or Kimble GL 45 Media Bottle, Kimble cat. no. 14395-1000)
-
Porcelain evaporating dishes
-
Mortar and pestle (CoorsTek™ Porcelain recommended)
-
Analytical balance
-
Polystyrene balance weigh boats
-
4- and 60-ml borosilicate glass screw-thread vials with PTFE-lined caps (glass vials oven cleaned for 8 hr at 500°C)
-
Kimwipes (Kimberly-Clark Professional™ Kimtech Science™, mfg. no. 34120)
-
Stainless steel forceps (precleaned with DCM)
-
14.6-cm (5 3/4-inch) borosilicate glass Pasteur pipet
-
Rotary or vacuum evaporator (e.g., Syncore® Analyst Vacuum Evaporator)
-
Nitrogen evaporator (e.g., Thermo Scientific Reacti-Vap Nitrogen Evaporator)
Setup of Büchi SpeedExtractor E-916 Pressurized Speed Extractor (PSE)
1.Connect the nitrogen gas cylinder to the PSE, and set the pressure range between 6 and 10 bar (90 and 150 psi).
2.Fill a 1-liter solvent bottle with DCM and another 1-liter solvent bottle with MeOH. Connect the bottles on the right side of the PSE.
3.Turn on the PSE.
4.After initialization, use the PSE LCD control panel to open the MENU >> SOLVENT LIST and make sure DCM and MeOH are recorded on the solvent list.
5.Open MENU >> FLUSH and make sure DCM and MeOH are listed under Solvent.
If this is the first time you are using the PSE, or after it has been shut down for an extended period, also perform the following steps.
- a.Place empty, clean extraction cells in the heat block.
- b.Flush with solvent and gas for 120 s.
- c.Set Flow Rate to 50 ml/min.
- c.Set the solvent ratio to 90% DCM and 10% MeOH.
- d.Flush the solvent into the collection vessels. Ideally, have a set of “waste” vessels that can be used when flushing the PSE with solvent.
Creation of a new method
6.Use the PSE LCD control panel and select EXTRACTION >> EDIT METHOD to create a new method.
7.Set TEMPERATURE to 100°C and PRESSURE to 103 bar using the selection knob.
8.Specify a 150-ml vial volume using the selection knob.
9.Specify cell volume using the selection knob (as 20 or 40 ml depending on that being used).
10.Go to the SOLVENT submenu and choose DCM (as Type 1) and MeOH (as Type 2) from the solvent list by pressing SELECT. Set the solvent ratio to 90% DCM and 10% MeOH.
11.Use the selection knob to specify three cycles under NUMBER OF CYCLES.
12.Define the parameters of the cycles under the CYCLES submenu:
-
Set the HOLD and DISCHARGE times to 10 min and 2 min, respectively.
-
Select the solvent for each cycle, if not already set, to 90% DCM and 10% MeOH (from step 5).
13.Set FLUSH WITH SOLVENT and FLUSH WITH GAS to 2 min each.
14.To save the new method, return to the EXTRACTION menu and select SAVE METHOD, select an empty position, name the new method, and press OK.
Sample preparation
15.Homogenize samples using mortar and pestle (precleaned with DCM and MeOH) so that each is uniform in particle size.
16.Transfer samples to individual, precleaned, labeled 60-ml glass vials or porcelain evaporation dishes, and oven dry or lyophilize. Residual water can reduce extraction efficiency, so be sure that samples are completely dry before extracting.
17.After drying, weigh out sample amounts for extraction using an analytical balance and weigh boats.
18.Mix the weighed sample with Ottawa sand.
19.Gently pour the sample-sand mixture into a cleaned PSE extraction cell using the precleaned (DCM and MeOH) stainless steel funnel that is provided with the PSE. Do not fill the cell completely, but retain a void ∼0.5-1 cm in height from the top of the inner shoulder of the cell to prevent sample clogging.
20.Use DCM-rinsed forceps and Büchi-provided plunger to place a cellulose top filter into the open-ended PSE extraction cell so that it rests on the inner shoulder.
21.Label the 150-ml wide-necked collection vessel with permanent marker; include initials, date, sample name, and project name.
Extraction process
22.Make sure the solvent reservoirs with DCM and MeOH are filled and connected to the corresponding ports on the right side of the PSE.
23.Select EXTRACTION >> OPEN METHOD, and select the method created in steps 6-14.
24.Select EXTRACTION >> PREHEAT. Wait for the oven to reach 100°C.
25.Place the filled extraction cells in the heating block and the corresponding labeled collection vessels in the vessel tray, so that Sample X goes in heating block position 1 and Collection Vessel X goes in vessel tray position 1.
26.Push the heating block with samples into the PSE, close the glass screen, and mount the vessel tray into the mounting fixture.
27.Activate the extraction positions by using the LCD control panel and select EXTRACTION >> OCCUPIED POSITIONS >> OCCUPY ALL >> YES.
28.Push START to extract the samples.
Post-extraction procedures
29.CAUTION : Be careful handling the cells after extraction, as they will be extremely hot and can cause burns.
30.Remove the heating block, and take out the extraction cells using the extraction cell gripper (provided by Büchi).
31.Once the cell has cooled, remove the top cellulose filter and pour the sample-sand mixture into a labeled sample bag.
32.Disassemble the PSE cell by removing the bottom plug screw, metal frit, and bottom cellulose filter.
33.Remove the collection vessels from the tray rack, and evaporate the solvent using rotary or vacuum evaporation until only ∼1 ml of solvent remains.
34.Transfer the ∼1 ml of solvent to an oven-cleaned 4-ml vial labeled with the sample name, date, and “TLE.”
35.Add 1-2 ml DCM to the collection vessels, rinse the insides thoroughly, and transfer the solvent to the 4-ml vial; repeat this step three times.
36.Evaporate the remaining solvent from the 4-ml vial using nitrogen gas.
37.Store the TLE in a refrigerator.
Support Protocol 1: WEIGHING THE TOTAL LIPID EXTRACT
A simple method for calculating the total amount of lipid extracted from a sample is to weigh the 4-ml vials before and after adding the TLE. This provides a general understanding of the lipid content of a soil, sediment, or paleosol, which can then be compared across all samples analyzed. It also indicates whether the total amount of sample needed for extraction must be increased and whether the extraction protocol is working efficiently. If the TLE weight is >35 mg, split into two aliquots (store one, use another for analysis) to avoid overloading the column during chromatography.
Materials
-
Total lipid extract (TLE) from Basic Protocol 1
-
Compressed nitrogen gas (N2), 99.99% purity
-
4-ml borosilicate glass screw-thread vials with PTFE-lined caps
-
Analytical balance
-
14.6-cm (5 3/4-inch) borosilicate glass Pasteur pipet
-
Nitrogen evaporator (e.g., Thermo Scientific Reacti-Vap Nitrogen Evaporator)
1.Label 4-ml vials with the sample name, date, and “TLE.”
2.With the caps on, record the weight of the labeled 4-ml vials.
3.Transfer the TLE to the 4-ml vials (see Basic Protocol 1, steps 29-37).
4.Evaporate the solvent from the 4-ml vials using nitrogen gas.
5.Reweigh and record the weight of the 4-ml vials now containing the TLE.
6.Subtract the weights of each sample from step 2 (empty vial) from that of step 5 (vial with TLE).
7.To compare TLE across samples, divide the weight of the TLE (likely in mg) from the weight of the sediment (likely in g) used in the extraction.
Support Protocol 2: CLEANING THE PSE EXTRACTION CELLS
The best method for cleaning the stainless-steel extraction cells is through the PSE. After they are washed with mildly alkaline liquid detergent and thoroughly rinsed with water to remove all soap traces, empty cells can be added to the PSE and cleaned with the same solvents used during extractions. Although this method uses solvent that must be discarded, it is quick and efficient and the most reliable way to avoid contamination.
Materials
-
Mild-alkalinity liquid detergent: e.g., Alconox® LIQUINOX
-
Dichloromethane (DCM), HPLC grade, 39.7°C boiling point, 3.1 polarity index (Millipore Sigma cat. no. DX0831)
-
Methanol (MeOH), HPLC grade, 64.7°C boiling point, 5.1 polarity index (Millipore Sigma cat. no. MX0488)
-
Büchi Speed Extractor E-916 Pressurized Speed Extractor (shipped with all parts needed for initial installation and use, but additional items are likely to be needed and are listed below)
-
Torx TX20 screwdriver
-
Laboratory glassware washer (MÍele PG 8583, WW AD LD OIL)
-
150-ml wide-necked collection vessel with 1-ml residual volume (Büchi part no. 11056498)
-
1-liter solvent bottle (Büchi part no. 053203; or Kimble GL 45 Media Bottle, Kimble cat. no. 14395-1000)
-
Extraction cell gripper (Büchi part no. 053030)
-
20-ml stainless steel E-916 extraction cell (Büchi part no. 051236+)
-
Cellulose bottom filter for extraction cell (Büchi part no. 049569)
-
Portable cell rack, Büchi part no. 053690
-
Combusted (i.e., oven cleaned for 8 hr at 500°C) aluminum foil
1.Disassemble the PSE cell by removing the bottom plug screw, metal frit, and bottom cellulose filter. Discard the cellulose filter.
2.Wash the disassembled cell in a laboratory glassware washer using a mildly alkaline liquid detergent.
3.Reassemble the extraction cells by inserting first a new cellulose filter, then the metal frit, and finally the plug screw. Hand tighten with the Torx screwdriver.
4.Create a new PSE method, set TEMPERATURE to 120°C, and specify 2 cycles under NUMBER OF CYCLES.
5.Save the method as “Cleaning.”
6.Make sure the solvent reservoirs with DCM and MeOH are filled and connected to the corresponding ports on the right side of the PSE.
7.Activate the extraction positions by using the LCD control panel and select EXTRACTION >> OCCUPIED POSITIONS >> OCCUPY ALL >> YES.
8.Select EXTRACTION >> OPEN METHOD and select “Cleaning.”
9.Select EXTRACTION >> PREHEAT.
10.Once the temperature reaches 120°C, push START to clean the cells.
11.After cleaning, remove the heating block and take out the extraction cells using the extraction cell gripper (provided by Büchi).
12.Place the hot extraction cells into the portable cell rack and let cool.
13.Once the cell has cooled, store in oven-cleaned aluminum foil in a clean, dry space if not extracting samples immediately.
Alternate Protocol 1: SOXHLET TOTAL LIPID EXTRACTION
Soxhlet is designed to extract organic materials from crushed rocks, sediments, and recent biological materials by distillation and the continuous recycling of solvent. This method is the most commonly used extraction technique, even though it is gradually being replaced by automated solvent extraction (Richter, Jones, Ezell, & Porter, 1996). This is the longest of the techniques described here and can take up to 72 hr to finish extracting total lipids. The advantages are that it uses small amounts of solvent and is relatively maintenance free except for cleaning the Soxhlet after the extraction. During the procedure, organic solvent is evaporated and refluxed into an Allihn (bulb) condenser where cool temperatures condense the vapor back into liquid solvent, causing it to drip into the extraction chamber containing the sample. The condensed solvent fills the extraction chamber while bonding to lipids and siphons the extracted organics into the boiling flask. The evaporation-condensation process is repeated while the TLE accumulates in the flask.
Materials
-
Dichloromethane (DCM), HPLC grade, 39.7°C boiling point, 3.1 polarity index (Millipore Sigma cat. no. DX0831-1)
-
Boiling chip granules (Millipore Sigma, VWR® cat. no. CA1.07912.0250)
-
Copper, granular, 10-40 mesh (Sigma Aldrich cat. no 311405-100G)
-
Glass wool
-
Methanol (MeOH), HPLC grade, 64.7°C boiling point, 5.1 polarity index (Millipore Sigma cat. no. MX0488-1)
-
Compressed nitrogen gas (N2), 99.99% purity
-
5-30 g dry, homogenized archaeological sediment or paleosol sample
-
DWK Life Sciences Kimble™ KIMAX™ Soxhlet Apparatus with Allihn condenser, DWK mfr. no. 2400540
-
250-ml flat-bottom boiling flasks (VWR® cat. no. CORN4320-250)
-
GE Whatman High Performance Cellulose Extraction Thimble, double thickness (2 mm wall), VWR® cat. no. 27732-145
-
Fume hood with running water
-
Hot plates
-
Mortar and pestle (CoorsTek™ Porcelain recommended)
-
4- and 20-ml borosilicate glass screw-thread vials with PTFE-lined caps (vials combusted for 8 hr at 500°C)
-
60-ml borosilicate glass screw-thread vials with PTFE-lined caps, or porcelain evaporation dishes, precleaned by combustion (vials) or with DCM and MeOH (evaporating dishes)
-
Analytical balance
-
Combusted (i.e., oven cleaned for 8 hr at 500°C) aluminum foil
-
Porcelain evaporation dishes, optional
-
Kimwipes™ (Kimberly-Clark Professional™ Kimtech Science™ , mfg. no. 34120)
-
14.6-cm (5 3/4-inch) borosilicate glass Pasteur pipet
-
Nitrogen evaporator (e.g., Thermo Scientific Reacti-Vap Nitrogen Evaporator)
Precleaning of Soxhlet apparatus and consumables
1.Oven clean the Soxhlet extraction chamber and flat-bottom boiling flasks for 8 hr at 500°C.
2.When those have cooled, pour 200 ml DCM into the 250-ml flat-bottom boiling flask. Add 3-5 anti-bumping granules and some copper granules.
3.Connect the bottom of the Soxhlet apparatus to the 250-ml flask.
4.Fill an extraction thimble with glass wool, and gently place the extraction thimble into the Soxhlet apparatus.
5.Pour 50-75 ml DCM solution into the extraction thimble containing the glass wool.
6.Connect the top of the Soxhlet apparatus to the Allihn condenser.
7.Connect the Allihn condenser to the faucet (or water recycler) and turn on the water flow.
8.Turn on the hot plate and begin heating the 250-ml flat-bottom flask to ∼40°C.
9.Check to make sure the solvent is evaporating, travelling to the Allihn condenser, and condensing into the extraction chamber.
10.Let the cleaning conduct for the next 24 hr, checking it periodically to make sure that solvent is not being lost.
11.When cleaning is complete, turn off the hot plate and let cool. Siphon any remaining solvent in the extraction chamber into the flat-bottom flask. Remove the flask from the Soxhlet apparatus and the Soxhlet apparatus from the Allihn condenser.
12.Discard the DCM from the flat-bottom flask.
13.Remove the extraction thimble from the Soxhlet and the glass wool from the thimble, and let both air dry. Store in oven-cleaned aluminum foil until use.
14.Oven clean the flat-bottom boiling flasks again for 8 hr at 500°C.
Sample preparation
15.Homogenize samples using mortar and pestle (precleaned with DCM and MeOH) so that each is uniform in particle size.
16.Transfer samples to individual precleaned, labeled 60-ml glass vials or porcelain evaporation dishes, and oven dry or lyophilize.
17.After drying, weigh out sample amounts for extraction using an analytical balance and precleaned extraction thimbles.
18.Place a precleaned extraction thimble in an oven-cleaned glass beaker on top of the analytical balance. Tare the balance so that it reads “0.”
19.Add the sample to the precleaned extraction thimble. Record the weight of the sample.
20.Place precleaned cotton wool on top of the sample inside the extraction thimble to cover it to prevent spillage.
21.It is best not to label the extraction thimbles, so cover the top of the thimble with oven-cleaned aluminum foil and write the sample name on the foil.
Soxhlet extraction
22.Pour 200 ml 9:1 (v/v) DCM/MeOH into the 250-ml flat-bottom boiling flask. Add 3-5 anti-bumping granules and some copper granules.
23.Connect the bottom of the Soxhlet apparatus to the 250-ml flask.
24.Remove the aluminum foil and gently place the extraction thimble with sample into the Soxhlet apparatus.
25.Pour 50-75 ml 9:1 DCM/MeOH solution into the extraction thimble containing the sample.
26.Connect the top of the Soxhlet apparatus to the Allihn condenser.
27.Connect the Allihn condenser to the faucet (or water recycler) and turn on the water flow.
28.Turn on the hot plate and begin heating the 250-ml flat-bottom flask to ∼40°C.
29.Check to make sure the solvent is evaporating, travelling to the Allihn condenser, and condensing into the extraction chamber.
30.Let the extraction conduct for the next 72 hr, checking it periodically to make sure solvent is not being lost.
31.When extraction is complete, turn off the hot plate and let cool. Siphon any remaining solvent in the extraction chamber into the flat-bottom flask. Remove the flask from the Soxhlet apparatus and the Soxhlet apparatus from the Allihn condenser.
32.Concentrate the sample in the flask by evaporation (ideally rotary evaporation) and pipet transfer the concentrated solvent to labeled, oven-cleaned glass vials.
33.Dry the transferred sample in the vials by evaporation using nitrogen gas. What remains is TLE.
Alternate Protocol 2: ULTRASONIC TOTAL LIPID EXTRACTION
Ultrasonic extraction is typically used when only a small amount of sample (≤10 g) is available or where it is desirable to quickly test the organic content in a sediment. Depending on the number of samples being extracted, however, it can be a very time-consuming process. It involves agitating the sample using temperature and high-frequency vibrations in dichloromethane and methanol to isolate organic compounds from a soil or sediment sample. In this technique, cavitation bubbles generated by high-frequency sound remove total lipids bound in a sedimentary matrix. Samples are then centrifuged and the organic solvent (with TLE) is filtered into glass vials and evaporated. The major benefit of ultrasonic extraction is that an ultrasonic cleaner is a relatively inexpensive instrument and can be purchased for about the same cost as one Soxhlet apparatus (∼$600 USD for a 3-liter tank). This may be the most practical extraction option for laboratories just beginning leaf wax analysis.
Materials
-
10 g (or less) dry, homogenized archaeological sediment or paleosol sample
-
Methanol (MeOH), HPLC grade, 64.7°C boiling point, 5.1 polarity index (Millipore Sigma cat. no. MX0488-1)
-
Dichloromethane (DCM), HPLC grade, 39.7°C boiling point, 3.1 polarity index (Millipore Sigma cat. no. DX0831-1)
-
Mortar and pestle (CoorsTek™ Porcelain recommended)
-
60-ml borosilicate glass screw-thread vials with PTFE-lined caps, or porcelain evaporation dishes (precleaned by combusion for 8 hr at 500°C)
-
Kimwipes™ (Kimberly-Clark Professional™ Kimtech Science™, mfg. no. 34120)
-
Analytical balance
-
40-ml (or other appropriate size depending on sample) borosilicate glass screw-thread vials with PTFE-lined cap, precleaned
-
Vortex mixer
-
Benchtop centrifuge
-
14.6-cm (5 3/4-inch) borosilicate glass Pasteur pipet
-
Borosilicate glass Pasteur pipet with glass wool filter
-
Compressed nitrogen gas (N2), 99.99% purity
-
Nitrogen evaporator (e.g., Thermo Scientific Reacti-Vap Nitrogen Evaporator)
-
Ultrasonic cleaner with heater (e.g., VWR® cat. no. 97043)
Sample preparation
1.Homogenize samples using mortar and pestle (precleaned with DCM and MeOH) so that each is uniform in particle size.
2.Transfer samples to individual precleaned, labeled 60-ml glass vials or precleaned porcelain evaporation dishes, and oven dry or lyophilize.
3.After drying, weigh 10 g of the sample for extraction using an analytical balance into a precleaned, labeled glass vial (e.g., 40 ml).
Ultrasonic extraction
4.Add 20 ml MeOH to the sample vials.
5.Vortex so that the sample and solvent are well mixed.
6.Ultrasonicate the samples 15 min at 40°C using the ultrasonic cleaner.
7.After sonication, centrifuge the vials 5 min at 4000 rpm, room temperature.
8.Transfer the TLE through oven-cleaned glass pipets containing glass wool filters into a labeled 40-ml vial.
9.Add 20 ml 2:1 (v/v) MeOH/DCM to the sample vials.
10.Repeat steps 2-4.
11.Transfer the TLE through the pipets with filters to the labeled 40-ml vial from step 5.
12.Add 20 ml 2:1 (v/v) DCM/MeOH to the sample vials.
13.Repeat steps 2-4.
14.Transfer the TLE through the pipets with filters to the second labeled 40-ml vial.
15.Add 20 ml DCM the sample vials.
16.Repeat steps 2-4.
17.Transfer the TLE through the pipets with filters to the second labeled 40-ml vial from step 11.
18.Completely evaporate the first 40-ml vials (containing the MeOH and MeOH/DCM TLE) using N2 gas.
19.Evaporate half the contents of the second 40-ml vial (containing the DCM/MeOH and DCM TLE) using N2 gas.
20.Transfer the remaining solvent in the second 40-ml vial to the first 40-ml vial. Discard the empty second 40-ml vial.
21.Add 5 ml pure DCM to the second 40-ml vial, rinse the inner walls, and transfer to the first 40-ml vial.
22.Completely evaporate the solvent from the first 40-ml vial using nitrogen gas. What remains is the total lipid extract (TLE).
Basic Protocol 2: SEPARATION OF LIPIDS BY AMINOPROPYL COLUMN CHROMATOGRAPHY
Column chromatography is an analytical technique used to separate organic compounds into individual components, so that these components can be analyzed by gas chromatography and mass spectrometry. Like total lipid extraction, column separation is based on the principle that like dissolves like, whereby solvents readily elute analytes of approximately the same polarity. Generally, the targeted analyte is dissolved in various organic solvents and loaded onto the column, and due to solvent polarity, the different compounds of the analyte either adhere to or elute through the column. Two different stationary-phase separation techniques are outlined in the following protocols: (1) aminopropyl column chromatography (this protocol) and (2) silver nitrate (SiAgNO3)-infused silica gel column chromatography (Basic Protocol 3); and (3) silica gel column chromatography.
Aminopropyl (NH2(CH2)3) column chromatography is a silica-based stationary-phase extraction technique in which the silica gel is functionalized with amino groups. Silica is a polar adsorbent, so nonpolar compounds (e.g., n -alkanes) elute before more polar compounds. This is a normal-phase ion-exchange chromatographic type that uses stationary-phase columns to retain polar analytes, and nonpolar mobile-phase solvents to separate neutral lipids from fatty acids and phospholipids out of the TLE. Neutral lipids, or those soluble only in solvents of very low polarity, are eluted with 2:1 DCM/isopropanol, fatty acids with 4% glacial acetic acid in diethyl ether, and polar phospholipids with MeOH. The ability of aminopropyl columns to retain acids when total extracts are eluted with DCM/isopropanol, and then release the acids when eluted with acetic acid in ether, make this the ideal technique for quickly separating carboxylic acids from the neutral lipid fractions before further separation into n -alkanes and n -alkanoic acids.
Materials
-
Dichloromethane (DCM), HPLC grade, 39.7°C boiling point, 3.1 polarity index (Millipore Sigma cat. no. DX0831-1)
-
99.9% isopropanol, HPLC grade, 82°C boiling point, 3.9 polarity index (Millipore Sigma cat. no. 34863)
-
Extraction cells containing total lipid extract (TLE) from Basic Protocol 1
-
Glacial acetic acid, HPLC grade, 117.9°C boiling point (Millipore Sigma cat. no. 338826)
-
≥99.9% diethyl ether, HPLC grade, 34.5°C boiling point, 2.8 polarity index (Millipore Sigma cat. no. 309966)
-
Methanol (MeOH), HPLC grade, 64.7°C boiling point, 5.1 polarity index (Millipore Sigma cat. no. MX0488-1)
-
Compressed nitrogen gas (N2), 99.99% purity
-
4-ml borosilicate glass screw-thread vials with PTFE-lined caps
-
DWK Life Sciences Kimble™ Kontes™ Extraction Manifold (DWK Life Sciences cat. no. 971000-6047; optional)
-
14.6-cm (5 3/4-inch) borosilicate glass Pasteur pipets
-
Pipettor (e.g., Eppendorf Research® Plus G Variable Volume Mechanical Pipette)
-
Agilent Bond Elut NH2 cartridge, 500 mg, 6 ml, 40 μm (Agilent part no. 12256045)
-
Vacuum manifold, Millipore Sigma Visiprep Manifold, cat no. 57030-U (optional)
1.For each sample, label three 4-ml vials with the sample name, date, and solvent fraction.
2.Label an additional set of vials “Waste.”
3.Place the aminopropyl columns into the extraction manifold with the waste vials set underneath.
4.Add 3 ml 2:1 (v/v) DCM/isopropanol to the aminopropyl columns and collect in the waste vials. Empty waste vials and repeat.
5.Add 1 ml 2:1 (v/v) DCM/isopropanol to the TLE vials with a pipettor.
6.Use a separate pipet (for each sample) to rinse the inside of the TLE vials three times.
7.Add the 1 ml DCM/isopropanol (now containing the neutral compounds) to the top of the aminopropyl column.
8.Replace the “Waste” vials with the “Neutral” vials.
9.Add another 1 ml DCM/isopropanol to the TLE vials, and rinse the inside of the TLE vial six times.
10.Add the 1 ml DCM/isopropanol to the top of the aminopropyl column.
11.Add another 1 ml DCM/isopropanol to the TLE vial, and thoroughly rinse the inside of the TLE vial 10 times.
12.Add the 1 ml DCM/isopropanol to the top of the aminopropyl column.
13.Add 1 ml of DCM/isopropanol to the top of the aminopropyl column to elute any remaining neutral compounds on the column.
14.Add 1 ml 4% acetic acid in diethyl ether (4:96 [v/v]; AADE) to the TLE vials with a pipettor.
15.Use a separate pipet (can be the same pipet from step 6) to rinse the inside of the TLE vials three times.
16.Add the 1 ml AADE (now containing the acid compounds) to the top of the Aminopropyl column.
17.Replace the “Neutral” vials with the “Acid” vials.
18.Repeat steps 9-13 with 4% AADE as the solvent.
19.Add 1 ml MeOH to the TLE vials with a pipettor.
20.Use a separate pipet (can be the same as that from step 6) to rinse the inside of the TLE vials three times.
21.Add the 1 ml MeOH (now containing the polar compounds) to the top of the aminopropyl column.
22.Replace the “Acid” vials with the “Polar” vials.
23.Repeat steps 9-13 with MeOH as the solvent.
24.Evaporate all fractions to dryness with N2, cap the vials with PTFE-lined caps, and store in a refrigerator.
Basic Protocol 3: SEPARATION OF LIPIDS BY SILVER-NITRATE-INFUSED SILICA GEL COLUMN CHROMATOGRAPHY
SiAgNO3 is chromatographic technique designed to separate organic compounds according to polarity. It is based on the ability of silver ions (Ag+) to interact with unsaturated compounds (e.g., alkenes; McWilliams & Angelici, 2016; Nikolova-Damyanova, 2009) so that straight-chain hydrocarbons (n -alkanes and n -alkanoic acids) can be isolated from other neutral or acidic compounds. Silver ions form weak, reversible electron donor–acceptor complexes with multiple-bonded compounds to separate lipids based on their degree of unsaturation and resolve complex lipid mixtures into simpler molecular species (Nikolova-Damyanova, 2009). This is often used to minimize the impact of unresolved complex mixtures on sample analysis. Therefore, SiAgNO3 is well suited for isolating saturated n -alkanes and n -alkanoic acids from unsaturated compounds that may form due to compound (bio)degradation.
NOTE : Although Basic Protocol 3 is used to isolate both n -alkanes and n -alkanoic acids, the “Acid” fraction must first be methylated. Thus, first perform Basic Protocol 4 on the “Acid” fraction, and then perform Basic Protocol 3.Basic Protocol 3 can be performed on the “Neutral” fraction right after aminopropyl column chromatography (Basic Protocol 2, step 13).
Materials
-
0.1 N silver nitrate solution (N/10, Certified ACS; Fisher Chemical cat. no. SS72)
-
100- to 200-mesh silica gel, high-purity grade (Davisil Grade 923), pore size 30 Å (Millipore Sigma cat. no. 214477)
-
Hexane (HEX), HPLC grade, 69°C boiling point, 0.1 polarity index (Millipore Sigma cat. no. 100795)
-
“Neutral” fraction from Basic Protocol 2, step 13
-
Dichloromethane (DCM), HPLC grade, 39.7°C boiling point, 3.1 polarity index (Millipore Sigma cat. no. DX0831)
-
Methanol (MeOH), HPLC grade, 64.7°C boiling point, 5.1 polarity index (Millipore Sigma cat. no. MX0488)
-
Compressed nitrogen gas (N2), 99.99% purity
-
14.6-cm (5 3/4-inch) and 22.9-cm (9-inch) borosilicate glass Pasteur pipets
-
Column rack or clamp stand
-
Stainless steel forceps (precleaned with DCM)
-
Glass wool
-
Dremel 290 engraver (optional)
-
Analytical balance
-
Weighing boats
-
4-ml borosilicate glass screw-thread vials with PTFE-lined caps
-
Pipettor (e.g., Eppendorf Research® Plus G Variable Volume Mechanical Pipette)
Column preparation
1.Place 14.6-cm (5 3/4-inch) pipets (henceforth referred to as 5″ pipets) in the column rack or clamp stand.
2.Wrap a small amount of glass wool around the ends of a pair of forceps and place it into the 5″ pipet.
3.Use a 9-inch (22.9-cm) pipet to gently push the glass wool to the bottom of the 5″ pipet where it narrows.
4.Weigh 0.5 g 10% SiAgNO3.
5.Gently pour the SiAgNO3 into the 5″ pipet.
6.Press the Dremel tool gently against the side of each pipet to pack the SiAgNO3.
7.Weigh 0.5 g deactivated silica gel.
8.Gently pour the silica gel into the 5″ pipet on top of the SiAgNO3.
9.Press the Dremel tool gently against the side of each pipet to pack the silica gel on top of the SiAgNO3.
10.This is the column used hereafter (Fig. 2). The columns do not need to be wrapped in aluminum foil, as the silver will not decompose in the time needed for extractions.

Column chromatography
11.For each sample, label three 4-ml vials with the sample name, date, and solvent fraction.
12.Label an additional set of vials “Waste.”
13.Add 3 ml hexane to the SiAgNO3 columns and collect in the waste vials.
14.Add 1 ml hexane to the “Neutral” fraction vials with a pipettor.
15.Using a separate pipet for each sample, rinse the inside of the TLE vial three times with the hexane.
16.Add the 1 ml hexane to the top of the SiAgNO3 column.
17.Replace the “Waste” vials with the “HEX” vials.
18.Add another 1 ml hexane to the “Neutral” vials and rinse the inside six times.
19.Add the 1 ml hexane to the top of the SiAgNO3 column.
20.Add another 1 ml hexane to the “Neutral” vials and thoroughly rinse the inside ten times.
21.Add the 1 ml hexane to the top of the SiAgNO3 column.
22.Add 1 ml hexane directly to the top of the SiAgNO3 column.
23.Add 1 ml DCM to the “Neutral” vials with a pipettor.
24.Use a separate pipet (can be the same pipet from step 5) to rinse the inside of the “Neutral” vials three times.
25.Add the 1 ml DCM to the top of the SiAgNO3 column.
26.Replace the “HEX” vials with the “DCM” vials.
27.Repeat steps 8-12 with DCM as the solvent.
28.Add 1 ml MeOH to the “Neutral” vials with a pipettor.
29.Use a separate pipet (can be the same from steps 5 and 14) to rinse the inside of the “Neutral” vials three times.
30.Add the 1 ml MeOH to the top of the SiAgNO3 column.
31.Replace the “DCM” vials with the “MeOH” vials.
32.Repeat steps 8-12 with MeOH as the solvent.
33.Evaporate all fractions to dryness with nitrogen gas, and store in a refrigerator. The n -alkanes are in the “Hex” fraction and will be analyzed by GC-MS.
Support Protocol 3: PREPARATION OF SILICA GEL INFUSED WITH 10% SILVER NITRATE
Typically, between 5% and 10% (w/w) silver nitrate (SiAgNO3) to silica gel is used when separating saturated from unsaturated hydrocarbons or reducing the impact from biodegradation. In the following protocol, 10 g activated silica gel and 59 ml of 0.1 N solution were mixed in order to get a SiAgNO3 concentration of 10% (w/w). However, the following equation can be used to adjust the percentage of silver nitrate to silica gel:
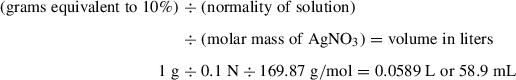
As silver nitrate is light sensitive, especially once it is infused into silica gel, only 10 g SiAgNO3 is prepared at a time. However, this, too, can be adjusted depending on the number of samples being processed, especially if it is more than 20 at a time. Always prepare the SiAgNO3 at least 3 days in advance.
Materials
-
100- to 200-mesh silica gel, high-purity grade (Davisil Grade 923), pore size 30 Å (Millipore Sigma cat. no. 214477)
-
0.1 N silver nitrate solution (N/10, Certified ACS; Fisher Chemical cat. no. SS72)
-
Oven-cleaned glass beaker
-
Combusted (i.e., oven cleaned for 8 hr at 500°C) aluminum foil
-
Analytical balance
-
Graduated cylinder
-
Drying oven (the door should not have a window that allows light to enter)
1.Completely cover an oven-cleaned glass beaker with aluminum foil.
2.Place the beaker on an analytical balance, and tare the balance so that it reads “0.”
3.Add 10 g silica gel.
4.Measure 59 ml silver nitrate solution using a graduated cylinder and add it to the beaker.
5.Cover the beaker with aluminum foil and gently mix the solution by hand.
6.Store the beaker in a drying oven set to 75°C for at least 1 day.
7.Once all water has evaporated, cover the beaker tightly and keep in the drying oven or in a desiccator.
Basic Protocol 4: METHYLATION OF n-ALKANOIC ACIDS
Free, underivatized fatty acids are difficult to analyze by GC mass spectrometry (GC-MS) due to their polarity, which leads to GC column adsorption issues. In order to make them GC amenable, the n -alkanoic acid fraction should be converted to fatty acid methyl esters (FAMEs), which are stable compounds that can be quantitatively analyzed using GC-MS. In this process, an oxygen atom of the carboxyl group on the n -alkanoic acid is protonated by hydrochloric acid, and then methanol is combined with the protonated acid to yield a methyl ester and water byproduct.
Materials
-
Methanol (MeOH), HPLC grade, 64.7°C boiling point, 5.1 polarity index (Millipore Sigma cat. no. MX0488-1)
-
37% hydrochloric acid (HCl), ACS reagent (Millipore Sigma cat. no. 320331)
-
“Acid” fraction from Basic Protocol 2, step 18
-
5% (w/v) sodium chloride (50‰), prepared from NaCl (Millipore Sigma cat. no. S7653) and reverse-osmosis-deionized water (RODi)
-
Hexane (HEX), HPLC grade, 69°C boiling point, 0.1 polarity index (Millipore Sigma cat. no. 100795)
-
Compressed nitrogen gas (N2), 99.99% purity
-
Drying oven
-
4-ml borosilicate glass screw-thread vials with PTFE-lined caps
-
14.6-cm (5 3/4-inch) borosilicate glass Pasteur pipets
-
Pipettor (e.g., Eppendorf Research® Plus G Variable Volume Mechanical Pipette)
Methylation
1.Make a 2% solution of HCl in MeOH by combining 94.5 ml MeOH with 5.5 ml 37% HCl.
2.Add 1 ml 2% HCl in MeOH to each of the 4-ml vials containing the “Acid” fraction (see Basic Protocol 2, step 18).
3.Cap the vials under nitrogen to force out oxygen.
4.Vortex mix the vials for 20 s.
5.Store in an oven set to 50°C for 20-24 hr.
Isolation of FAMEs
6.Remove the vials from the oven, and add 1 ml 5% (w/v) aqueous NaCl solution and 1 ml hexane. Cap the vials.
7.Shake (by hand) the vials for 5 s, and then vortex for 20 s.
8.Let the mixture settle for 30 s until the two phases (water and hexane) separate due to their differences in density.
9.Carefully pipet into a fresh oven-cleaned, labeled 4-ml vial the top (hexane) portion, which contains the FAMEs.
10.Add another 1 ml hexane to the 4-ml vial, shake, vortex, let settle, and pipet into the fresh vial. Repeat two additional times.
11.Evaporate the hexane-containing FAMEs in the 4-ml vials with N2.
12.Conduct Basic Protocol 3.The DCM fraction will contain the GC-amenable FAMEs.
Basic Protocol 5: GAS CHROMATOGRAPHY MASS SPECTROMETRY (GC-MS)
Gas chromatography mass spectrometry is an analytical technique for identifying and quantifying organic compounds in complex matrices. The gas chromatograph separates compounds by (i) volatilizing a sample in a heated inlet, (ii) using an inert carrier gas (e.g., helium) to transfer the sample from the inlet through a fused silica capillary column containing a stationary-phase coating, and (iii) passing the sample from the column into the mass spectrometer, which provides structural information on the injected compounds. The mass spectrometer ionizes the analytes eluting through the GC column as they enter the detector, and the ions are deflected by a magnetic field and finally distinguished electrically according to their mass-to-charge ratio (m/z). The fragmentation of the ions that represent the original molecule injected into the GC is then used to determine the structure of targeted compounds. The data resulting from GC-MS are known as “mass spectra” and represent the m/z value and abundance of a given molecular ion (M+).
Materials
-
“HEX” fraction containing the n -alkanes from Basic Protocol 3, step 12
-
“DCM” fraction containing the FAMEs from Basic Protocols 3 (step 17) and 4
-
Hexane (HEX), HPLC grade, 69°C boiling point, 0.1 polarity index (Millipore Sigma cat. no. 100795)
-
Compressed nitrogen gas (N2), 99.99% purity
-
Alkane standard solution C21-C40, 40 mg/L each (Millipore Sigma cat. no. 04071)
-
Even carbon saturated FAMEs standard solution C4-C24, 1000 μg/ml (Millipore Sigma cat. no. 49453-U)
-
14.6-cm (5 3/4-inch) borosilicate glass Pasteur pipets
-
2-ml screw-top GC vials and caps (Agilent part no. 5182-0715)
-
250-µl Agilent vial insert, glass with polymer feet (Agilent part no. 5181-1270)
-
Pipettor (e.g., Eppendorf Research® Plus G Variable Volume Mechanical Pipette)
-
Agilent 7890B gas chromatograph system
-
Agilent 5977A series mass selective detector (MSD)
-
HP-5 capillary column, 30 m length, 0.25 mm i.d., and 0.25 μm film (Agilent part no. 19091J-433)
-
Splitless, single-taper, ultra-inert inlet liner (Agilent part no. 5190-2293)
Preparing samples for GC analysis
1.Add 7-10 drops of hexane to the “biomarker” vials using a pipet.
2.With a fresh pipet (for each sample), gently rinse the biomarker vials with the hexane.
3.Transfer the hexane to an oven-cleaned, labeled 2-ml GC vial that has been fitted with a 250-µl insert.
4.Add an additional 7-10 drops of hexane to each biomarker vial, rinse, and transfer again to the 2-ml GC vial.
5.Repeat step 4 until the 250-µl insert is full.
6.Evaporate the hexane from the 2-ml GC vial (250-µl insert) using a gentle stream of N2.
7.Once evaporated, add 100 µl hexane to the 250-µl insert to resuspend the biomarkers. The samples are now ready for GC analysis.
Setting recommended parameters for GC-MS operation
8.Oven temperature program:
- Initial temperature: 60°C
- Hold time: 1 min
- Rate 1: 10°C/min
- Temperature 1: 150°C
- Hold time: 0 min
- Rate 2: 8°C/min
- Final temperature: 320°C
- Hold time: 10 min.
9.Front inlet:
- Mode: Pulsed splitless
- Initial temperature: 250°C
- Pulse time: 1 min
- Pressure: 11.5 psi
- Total flow: 104.3 ml/min
- Septum purge flow: 3 ml/min
- Purge flow to split vent: 100 ml/min at 1 min
- Injection volume: 1 μl.
10.Column:
- Pressure: 11.5 psi
- Flow: 1.1 ml/min
- Average velocity: 41.756 cm/sec
- Holdup time: 1.1974 min.
11.MS scan parameters:
- Low mass: 30 m/z
- High mass: 650 m/z
- MS source: 230°C
- MS quad: 150 C°C
- Ionization energy: 70 electron volts (eV).
Operating the GC-MS for sample analysis
12.Create a new method with the recommended parameters above using the Agilent GC-MS (MSD ChemStation) software.
13.Create a new sequence for the samples made ready in steps 1-7:
-
Make sure sample name and data file name correspond.
-
Select the newly created method (step 1) in the method data path.
-
Verify that each sample has a unique AutoSampler position number.
-
Include hexane blanks in the sequence to check for contamination.
-
Also include the alkane and FAMEs standard solutions.
14.Place sample vials in the numbered slots on the AutoSampler tray so that the vials correspond with the position number set in the sequence editor.
15.Fill the wash vials in AutoSampler positions A and B with DCM and MeOH, respectively.
16.Click “Run Sequence.”
Identifying compounds
17.Run the n -alkane and FAMEs standards on the GC-MS apparatus.
18.Run the prepared samples from steps 1-7 on the GC-MS apparatus.
19.Use the retention times and m/z ratios of the C21-C40 n -alkane or C4-C24 FAMEs standards to compare with retention times and m/z ratios of the unknown samples (Fig. 3).
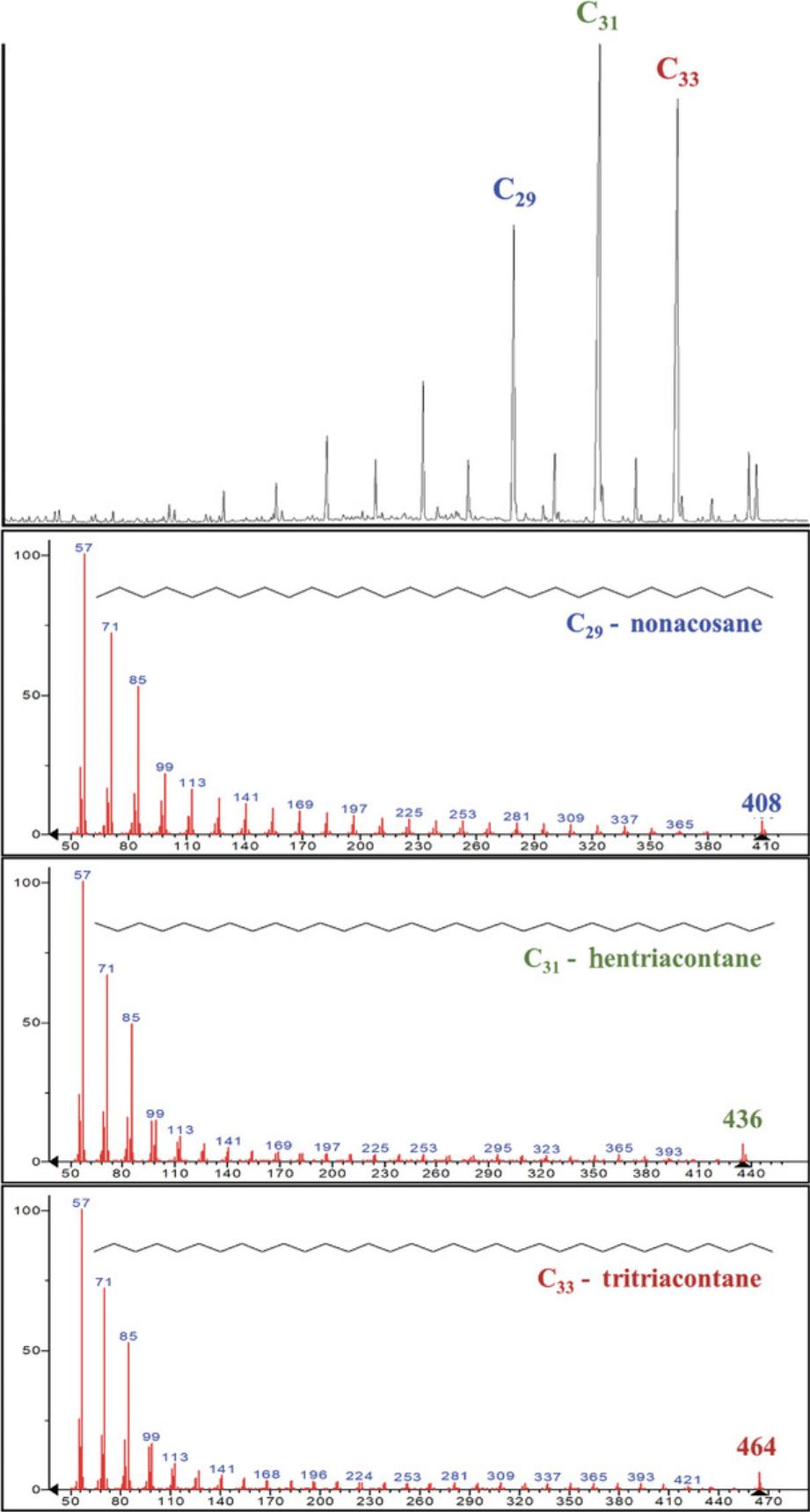
Basic Protocol 6: GAS CHROMATOGRAPHY ISOTOPE RATIO MASS SPECTROMETRY (GC-IRMS)
Gas chromatography isotope ratio mass spectrometry (GC-IRMS, or IRMS) follows the same principles as GC-MS, except that instead of yielding structural information by scanning fragmented ions, IRMS measures, with high precision, small variations in the relative abundances of carbon (13C/12C) and hydrogen (2H/1H or D/H) isotopes, as well as nitrogen (15N/14N), oxygen (18O/16O), sulfur (34S/32S), and other elements and their isotopes. Samples are first converted to gas (e.g., H2, CO2) before the mass spectrometer calculates the corresponding ratio of ions within the gas and monitors the mass-to-charge (m/z) ratios of the target compounds. For example, in the analysis of 13C to 12C, the mass spectrometer monitors the ions with m/z values of 44, 45, and 46, which correspond to the ions produced from CO2 molecules containing 12C and 13C, as well as 16O, 17O, and 18O in various combinations (Carter & Barwick, 2011). Therefore, minute variations in the natural abundance of the heavier isotope can be detected in the presence of larger amounts of the lighter isotope. This ratio—(R) abundance of heavy isotope/abundance of light isotope—is expressed using the delta (δ) notation in units of per mil (‰), which reports changes in isotopic abundance as deviations compared to a designated isotope standard. Isotope composition of a sample is expressed (see Equation 1):
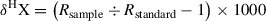
Materials
-
Hexane (HEX), HPLC grade, 69°C boiling point, 0.1 polarity index (Millipore Sigma cat. no. 100795)
-
Compressed nitrogen gas (N2), 99.99% purity
-
Schimmelmann n -Alkane Mixture B5 C16-C30 (Biogeochemical Laboratories, University of Indiana)
-
Schimmelmann Fatty Acid Ester Mixture F8-2 (Biogeochemical Laboratories, University of Indiana)
-
n -Alkanes from Basic Protocol 5
-
FAMEs from Basic Protocols 5
-
Elementar ionOS® Stable Isotope Software
-
Agilent 7890B gas chromatograph system
-
Elementar GC5 furnace system interface
-
Elementar Isoprime AnthrovisION mass spectrometer
-
HP-5 capillary column, 30 m length, 0.25 mm i.d. and 0.25 μm film (Agilent part no. 19091J-433)
-
Agilent splitless, single-taper, ultra-inert inlet liner (Agilent part no. 5190-2293)
-
Pipettor (e.g., Eppendorf Research® Plus G Variable Volume Mechanical Pipette)
Tuning the IRMS
1.Under the Isoprime visION tab of the ionOS® Stable Isotope Software, select, for example, CO2 from the dropdown list of species to configure the IRMS to CO2.
2.In the task list, select Auto Detect Peak from the method dropdown and press start.
3.In the task list, select Autotune from the method dropdown and press start.
4.In the task list, select Auto EX Scan from the method dropdown and press start.
5.In the task list, select Background Scan from the method dropdown and press start.
6.In the task list, select Stability from the method dropdown and press start.
7.In the task list, select Linearity from the method dropdown and press start.
Setting recommended parameters for GC-IRMS operation
The operation settings on the GC-IRMS should ideally be the same as those on the GC-MSD in order to identify target peaks by retention time and compare to the GC-MSD.
8.Oven temperature program:
- Initial temperature: 50°C
- Initial time: 1 min
- Rate 1: 10°C/min
- Temperature 1: 150°C
- Hold time: 0 min
- Rate 2: 8°C/min
- Final temperature: 310°C
- Hold time: 10 min.
9.Front inlet:
- Mode: Splitless
- Initial temperature: 250°C
- Pressure: 12.969 psi
- Purge time: 2 min
- Purge flow: 15 ml/min
- Total flow: 20 ml/min
- Septum purge: 3.0
- Septum purge mode: Standard
- Injection volume: 1 μl.
10.Column:
- Dimensions: 30 m
- Pressure: 12.969 psi
- Flow: 1.1 ml/min
- Average velocity: 27.3 cm/sec
- Mode: Constant flow.
Operating the GC-IRMS apparatus for sample analysis
11.Create a new GC method with the recommended parameters above using the ionOS® Stable Isotope Software.
-
Select Manage under the 7890 GC tab, and enter values for Oven Temperature, Front or Back Inlet (whichever is in use), Front or Back Injector, and Columns.
-
Name and save the new GC method.
Note that some variables, such as flow rates and pressures, will vary from instrument to instrument.
12.Create a new ionOS® method:
-
Set the method from step 11 as the GC Method.
-
Set Solvent Delay to 8 min.
-
Leave Heart Split off.
-
Set Use Autosampler to True.
-
Set Injector to Front or Back, whichever is in use.
-
Set Vial Number to 1, but make sure that it is editable in the task list.
-
Set Volume to 1 µl.
-
Set Start and End Pulses to 3 each.
-
Set the Source Inlet Position to GC.
-
Name and Save the new ionOS®method.
-
Upload new method to the GC system.
13.Enter the samples (and standards) in the task list, and select the ionOS® method from the Method dropdown list.
14.Make sure all samples and standards have a unique vial number.
15.Place sample and standard vials in the numbered slots on the AutoSampler tray so that the vials correspond with the vial numbers set in the task list.
16.Fill the wash vials in AutoSampler positions A and B with DCM and MeOH, respectively.
17.Run a blank to make sure that there is no contamination on the column or in the system.
18.Run a Schimmelmann standard to make sure that the isotope values of the standard mixture are matched.
19.Schedule all samples and click Start.
COMMENTARY
Background Information
Plant wax lipids are organic compounds known as biomarkers, or biologically specific marker molecules. That is because their distinct chemical structures can be used as diagnostic tools for identifying their biosynthetic origins (Eglinton & Hamilton, 1967; Peters, Walters, & Moldowan, 1993). When an organism dies or loses a certain physical component (e.g., leaves falling to the ground), biomarkers are dispersed into the local environment and in archaeological sites, or transported long distances by wind and water and deposited in lacustrine or marine sediments (Eglinton & Eglinton, 2008). Biomarker analysis of distinct organic molecules (e.g., lipids, nucleic acids, proteins) seeks to identify molecular fragments and their precursor biomolecules in order to taxonomically identify the organisms that produced them (Briggs, Evershed, & Lockheart, 2000). Some biomarkers, like Silurian-dated chitin-protein complexes (Cody et al., 2011), n -alkanes from the Eocene (Pagani et al., 2006), and enamel proteomes of Pleistocene Homo (Welker et al., 2020), attest to the persistence of such molecules over geologic time and their applicability to the study of past organisms and environments. One of the major goals of biomarker research is to identify molecular fragments and their precursor biomolecules (e.g., lipids, nucleic acids, proteins) to taxonomically identify the organisms that produced them (Briggs et al., 2000). Long-chain n -alkanes (C27-C35) for example, are diagnostic of terrestrial plants (Eglinton & Hamilton, 1967), whereas short-chain n -alkanes (C17-C21) characterize aquatic algae (Cranwell, Eglinton, & Robinson, 1987), and mid-chain compounds (C21-C25 n -alkanes) are abundant in submerged and floating aquatic macrophytes (Barnes & Barnes, 1978; Cranwell, 1984; Ficken, Li, Swain, & Eglinton, 2000). Although there can be overlap (Aichner, Herzschuh, & Wilkes, 2010; Aichner, Herzschuh, Wilkes, Vieth, & Böhner, 2010; Sachse, Radke, & Gleixner, 2006), the dominance of certain n -alkanes (e.g., C29, C31) in samples isolated from total lipid extracts highlight the potential of plant waxes as biomarkers for ancient environments and climates. The application of isotopic measurements of these compounds has generated entirely new research opportunities, such as exploring global climate changes and the ensuing impacts on environments over time.
The biochemical approach for paleoclimate reconstructions is much more widespread now that techniques for extracting and analyzing plant wax lipid biomarkers are becoming more precise. The replacement, albeit minimal, of Soxhlet by accelerated or pressurized solvent extraction has sped up extraction times from days to hours, reduced total solvent volume, and improved recovery by increasing temperature but simultaneously preventing thermal degradation of temperature-sensitive compounds (Richter et al., 1996). Stationary-phase column chromatography using silica gel has been the standard method for separating lipids into individual, polarity-defined fractions for over 50 years (Crider, Alaupovic, Hillsberry, Yen, & Bradford, 1964), and silver nitrate is a proven addition for preventing unsaturated compounds (i.e., hydrocarbons containing multiple bonds) from interfering with GC analysis (De Vries, 1962; Nikolova-Damyanova, 2009). Constant improvements in GC and MS technology have resulted in the reliable measurement of organic compounds as well as small variations in the relative abundance of lighter and heavier isotopes (Carter & Barwick, 2011).
Traditionally, the most common isotope used as both an environmental and dietary indicator is δ13C (ratio between 13C and 12C). This is due, in part, to its effectiveness as an environmental proxy, which enables us to trace plant type, plant water-use efficiency, and relative paleotemperature based on the distinct ratio between the 13C and 12C isotopes found within plant lipid compounds (Castañeda & Schouten, 2011; Collister, Rieley, Stern, Eglinton, & Fry, 1994; Farquhar, Hubick, Condon, & Richards, 1989; Lockheart, van Bergen, & Evershed, 1997). The stable carbon isotopic composition of all higher plants is a function of their specific photosynthetic pathway (C3, C4, or Crassulacean acid metabolism), the carbon isotopic composition of atmospheric CO2 (δ13CCO2), the ratio of atmospheric CO2 partial pressure (p CO2) inside leaves relative to atmospheric p CO2 (Farquhar et al., 1989; O'Leary, 1981), and, in mangrove ecosystems, water use efficiency and salinity (Ladd & Sachs, 2013).
Analysis using stable hydrogen isotopes, on the other hand, is a relatively new approach for reconstructing paleoecology (Liu, Yang, & Li, 2006; Yang & Huang, 2003). Over the past 20 years, improvements in gas chromatography isotype ratio mass spectrometry (GC-IRMS) have made the molecular examination of hydrogen isotopes more accurate and routine (Burgoyne & Hayes, 1998; Hilkert, Douthitt, & Schluter, 1999; Scrimgeour, Begley, & Thomason, 1999). Tracing paleohydrology is achievable because stable hydrogen isotope values, expressed as δD (the ratio between deuterium and hydrogen, written as D/H or 2H/1H), record an integrated signal of evapotranspiration, precipitation water, relative humidity, moisture source, altitude, latitude, temperature, and degree of continentality (i.e., distance from the coast; Dansgaard, 1964; Hou, D'Andrea, MacDonald, & Huang, 2007; Liu & Yang, 2008). Moreover, differences between algal-derived C17 and leaf wax δD can be used to reconstruct terrestrial evapotranspiration due to evaporative enrichment of deuterium in the leaf water (Sachse, Radke, & Gleixner, 2004). When coupled, δ13C and δD present a unique opportunity to analyze both paleoatmospheric and hydrological conditions as well as C3- vs. C4-dominance in ecosystems.
Plant wax lipid extraction and analysis is now being applied in archaeology contexts to investigate human-environment interactions (Brittingham et al., 2019; Colcord et al., 2018; Collins et al., 2017; Connolly et al., 2019; Lupien et al., 2018; Magill, Ashley, & Freeman, 2013a, 2013b; Magill, Ashley, Domínguez-Rodrigo, & Freeman, 2015; Patalano, 2019; Uno et al., 2016). Because n -alkanes and n -alkanoic acids record ancient environmental conditions through δ13C and δD ratios, they can track variations in hydroclimate, vegetation community and structure, and habitat type and be used to investigate human biological and cultural adaptive responses to ecological changes. This technique can also be applied in more recent archaeological contexts such as the study of plant domestication and animal husbandry strategies (Égüez & Makarewicz, 2018; Patalano et al., 2015). One issue that must be considered when conducting this type of work at archaeological sites, however, is human modification of the landscape and possible reworking of soil lipid archives. Approaches for extracting leaf wax lipids vary in terms of the technique used to separate total lipids from a sedimentary matrix (e.g., PSE, Soxhlet, ultrasonic), the adsorbents used in column chromatography (e.g., aminopropyl, silica gel, magnesium silicates, alumina), and even the compounds of interest (e.g., n -alkanes, FAMEs, n -alkanols). Nevertheless, the general protocol for isolating leaf waxes always includes solid-phase extraction column chromatography and the identification and quantification of components through GC-IRMS. Thus, the goals of the workflow (Fig. 4) presented in this article, which is designed to address archaeological related research questions, are to:
- Improve recoverability of leaf waxes from terrestrial sediments, especially ancient paleosols that may be lipid-poor;
- Target multiple biomarkers at the onset in case technical or taphonomic issues become apparent;
- Reduce analyte loss by combining SiAgNO3 and silica gel column chromatography, while moderating the time constraints inherent in separating compounds into different fractions;
- Limit the interference of degraded or unsaturated compounds, which negatively impact GC-MS and GC-IRMS analyses;
- Prioritize reproducibility across sample sets and laboratories.
Critical Parameters
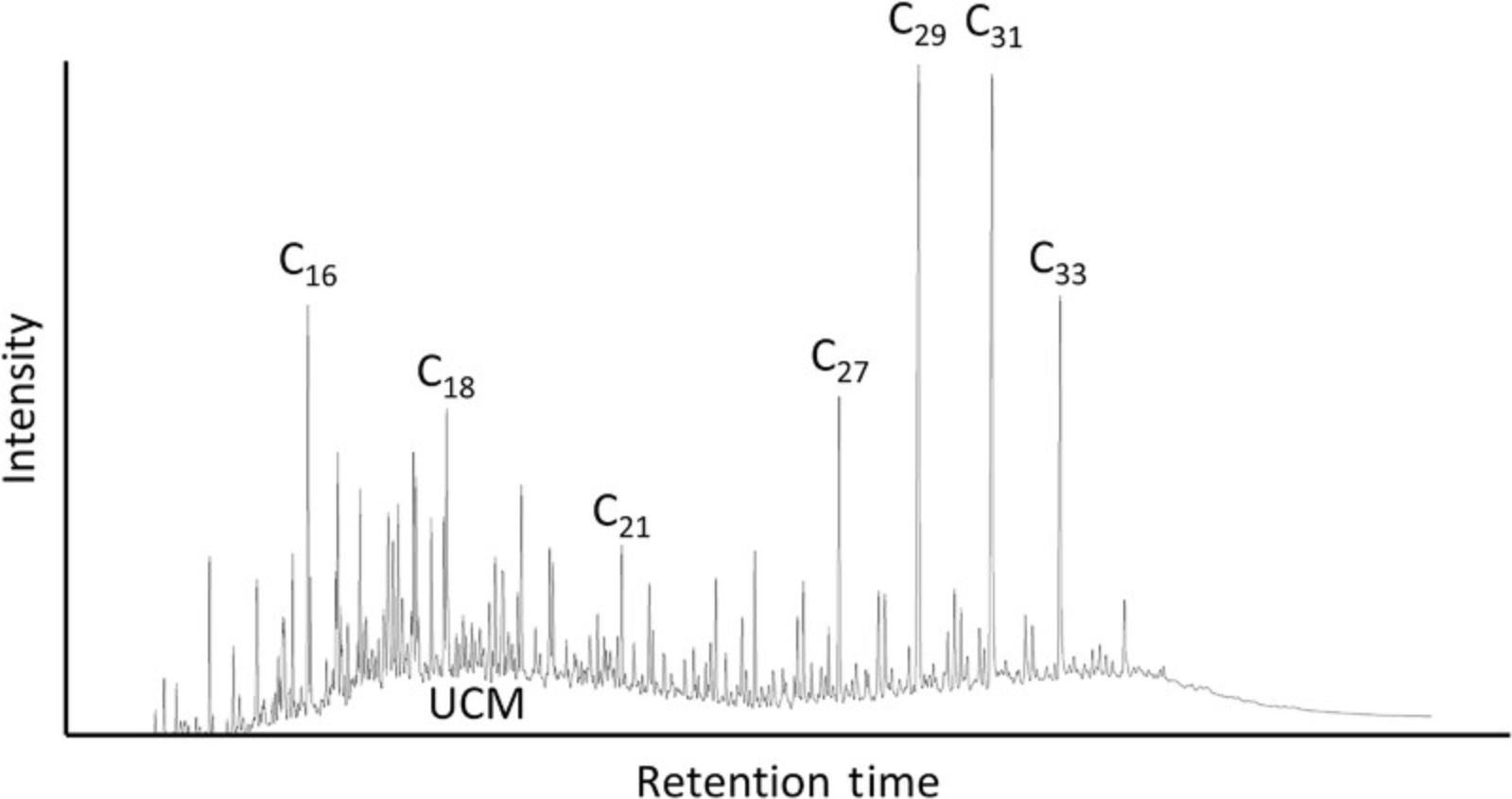
The archaeological applications of plant wax lipids are both inventive and exciting, but the future of this research depends on the taphonomic understanding of these compounds in terrestrial sediments. Although biomarkers have been recovered in ocean cores dated to the early Eocene (Pagani et al., 2006), there are complex interactions between lipid molecules, microbial (fungal and bacterial) communities, and soil chemistry that can result in the (bio)degradation of leaf waxes prior to and after burial. For example, some bacteria and filamentous fungi carry genes encoding n -alkane-degrading enzymes that damage these compounds soon after deposition (Brittingham, Hren, & Hartman, 2017; Grimalt, Torras, & Albaigés, 1988; Leahy & Colwell, 1990). As a result, breakdown products such as unresolved complex mixtures (UCMs) hinder the interpretability and quantification of leaf wax biomarkers and may cause post-depositional isotopic fractionation.
UCMs form when structurally complex organic compounds cannot be resolved (i.e., separated) by gas chromatography due to an overabundance of degraded hydrocarbons in terrestrial sediments (Bouloubassi & Saliot, 1993; Gough & Rowland, 1990; Fig. 5) or to petroleum contamination in marine sediments. Therefore, UCMs are indicative of the extent of degradation in terrestrial-derived samples or oil contamination in ocean depositional environments. Typically, microbial reworking of leaf waxes results in UCM distributions with chromatographic outputs between C16 and C22 (Grimalt et al., 1988). Fortunately, this does not overlap with the retention window for higher-chain n -alkanes, and many samples will have slight evidence for microbial activity that does not interfere with GC-MS/IRMS analyses; in fact, even relatively modern topsoils can exhibit UCMs (Patalano, 2019). In extensively reworked samples, on the other hand, the severity of the UCM may completely obscure the n -alkanes, making it impossible to obtain any reliable data. Yet multiple analytical techniques have been developed to diminish the impact of UCMs, including molecular sieves, urea adduction, and, as outlined in this protocol, silver nitrate column chromatography.
Infusing silica gel with silver nitrate is a comparatively straightforward procedure that, when the gel is then used in column chromatography, can limit the effect of UCMs on leaf waxes. This is because silver ions interact with degraded isomers and homologues of cyclic and branched hydrocarbons, as opposed to saturated straight-chain n -alkanes, and resolve complex lipid mixtures into simpler molecular species (Nikolova-Damyanova, 2009). Silver nitrate may not remove the entirety of the UCM hump, however. It is then necessary to use molecular sieves or urea adduction. Molecular sieves separate straight-chain molecules on the basis of their sizes and shapes but generally requires the use of hazardous hydrofluoric acid to digest and release compounds trapped within the sieve. Urea adduction separates straight-chain hydrocarbons by trapping them in a crystal matrix that is then dissolved with water. This method does not require hazardous chemicals but usually requires a certain amount of starting material (1-2 mg), which may be unavailable in ancient sedimentary samples. Ultimately, it is best to compare n -alkanes and n -alkanoic acids (as FAMEs) from the same sample because this will demonstrate the severity of compound decay and whether diagenesis has generated significant isotope fractionation at the molecular level.
Fatty acids are the biosynthetic precursors of the n -alkanes in plant cuticular wax, and it has been shown (Chikaraishi, Naraoka, & Poulson, 2004; Gao, Guimond, Thomas, & Huang, 2015) that n -alkanoic acids are depleted by 1.3‰ to 1.4‰ relative to the n -alkanes with the corresponding carbon numbers: that is, the C30 n -acid is slightly more depleted than the C29 n -alkane. Knowing this, both compounds can be extracted by first subjecting the TLEs to aminopropyl column chromatography; the “Acids” fraction can then be methylated, converting the n -alkanoic acids to FAMEs; and then both the n -alkanes (from the “Neutral” fraction) and the FAMEs can be isolated individually through SiAgNO3. Preparing both fractions allows one to use the two biomarkers to compare isotope values between corresponding carbon-numbered biomarkers, and if one fraction turns out to be heavily degraded, there is still an opportunity to obtain paleoenvironmental data with the other, potentially better-preserved separate fraction.
As noted above, one thing that should be taken into consideration is the potential for human modification of landscapes or the selectivity of specific plants and the resulting leaf wax input into archaeological sediments. In fact, leaf wax lipid biomarkers can be used to interpret human-modified environments, especially if domesticated crops supplanted natural vegetation. The potentially distinct isotope signals of crops from those of native plants can provide insight on the introduction of species to a given environment (Patalano et al., 2015). Additionally, waxes in cave sites could suggest human selectivity and usage of specific plants for bedding or firewood (Brittingham et al., 2019; Collins, Carr, Schefuß, Boom, & Sealy, 2017), or differences in the windborne deposition of waxes due to changing environmental parameters.
Troubleshooting
The major issues associated with Basic Protocol 1 typically involve pressure disparities at the interface between the PSE and extraction cells. During extraction, the cells are held in place with an upper and lower tightening device, with the upper section housing the solvent mixture divider that distributes solvent to each activated cell position. If the cells are improperly loaded (i.e., filled too high with sample), the upper tightening device will not close correctly, which may cause a system error in which the set pressure (103 bar) cannot be reached. This is likely to cause the extraction process to pause or abort. If this problem persists even when samples are properly loaded, check that the cup seals on both the upper and lower tightening device are not degraded. Cup seals have a lifetime of about 100 extractions, but contamination or residual sand and dust can shorten their operational efficiency and require them to be replaced earlier. If there is a persistent pressure issue, check the integrity of the seals. A quick indication that a seal needs to be replaced is when there is a leak and the extraction solvents can be smelled. If a leak is detected, make sure to run blanks after fixing any seals to make sure contaminants have not entered the system.
Depending on how fine-grained samples are, there is also the possibility that they may clog the extraction cell such that solvent will not pass into the collection vessels. If working with extremely silty or clayey samples, make sure to thoroughly mix the sample with Ottawa sand; it may be beneficial to fill the bottom of the extraction cell first with 1 g of Ottawa sand, followed by the sand/sample mix, and finally another top layer of sand. Clogged samples can cause blowback in which the sample, sand, and solvent are ejected from the top of the cell. If this occurs, it is best to carefully clean the heating block (after cooling) as well as the solvent mixture divider, and then flush the system.
With column chromatography (Basic Protocols 2 and 3), the biggest concern typically involves preventing the column from becoming dry during the process. When constructing columns with 5″ pipets, it is not possible to stop the flow of the solvent as it elutes through the silica. Thus, before starting an extraction, make sure that the solvent level can be monitored throughout the process and that solvent can be added to the top of the silica when needed. An extraction manifold that includes a stopcock valve will resolve this problem and allow more samples to be processed at once. Another issue that can manifest is when lipid-rich samples added to the column slow the elution of the solvent. Sometimes, the columns become partially clogged by the TLE, which reduces the speed at which solvent is collected in the different fraction vials. To increase the elution speed, use a pipet bulb to gently force the solvent through the column, but be careful not to draw the solvent and silica gel upward when depressing the bulb.
The biggest potential setback linked to preparing and using silver nitrate is that the silver ion (Ag+) is light sensitive and can decompose into elemental silver. To identify whether silver nitrate has been exposed to light for too long, specifically once infused in silica gel, look for discoloration (blackening or purpling). Typically, this happens when preparing the SiAgNO3, so make sure to arrange it in a beaker covered with aluminum foil and dry in an oven that remains dark with the door shut. If the SiAgNO3 retains its white coloring after oven-drying, then it is safe to use in column chromatography; the silver ions will not decompose in the column in the amount of time needed to extract samples, so there is no need to wrap the pipets in aluminum foil. If the SiAgNO3 does become discolored, however, discard the first round and prepare it once again. Because of this concern, it is best to start with smaller amounts of silica gel (10 g), so that if the SiAgNO3 is unusable, excessive silica gel will not have been wasted.
Troubleshooting GC-MS and GC-IRMS can be incredibly complex and is beyond the scope of this protocol. However, many resources exist on servers like ISOGEOCHEM that provide information on methods in stable isotope geochemistry. Here, we outline a few common problems that can occur and how they might be resolved. Septa (e.g., Agilent part no. 5183-4761) and syringe should be regularly changed, specifically at the interface between the inlet liner and syringe/injector assembly, as they often break down with repeated injections. Also, the liner should be changed regularly because septa particles can accumulate on the glass wool in the liner. If column bleed, or the thermal breakdown of the stationary phase (which is characterized by a steady rise in the baseline), becomes excessive and interferes with sample analyses, either condition the column by temperature ramping to the column's maximum isothermal temperature (325°C for an Agilent HP-5 capillary column) for 30 min, trim the end of the column that leads to the injector by at least 20 cm, or replace the column. When there is a suspected leak in the system, perform a leak check to locate it, and then tighten or replace the ferrule. If all chromatogram peaks are reduced in height or area, check the syringe for blockages, adjust the gas flow rates, and make sure the purge flow or split ratio are not too high. Also, check that the injector or oven temperatures are not too low to detect higher-molecular-weight compounds. In addition, when working in splitless mode, if peaks are reduced, ensure that the split vent is opened for an adequate amount of time or that the initial column temperature is not too high. If only some peaks are reduced, this can indicate that the inlet liner is contaminated or that there is a problem with the column or a potential leak; in that case, clean or replace the inlet liner, cut the column for 50 cm at the injector end, locate and repair leaks, and adjust gas flow; replace the column if needed. Improperly installed columns or columns that are overloaded with sample will have chromatogram peaks with “fronting” or “shark-fin”-shaped asymmetry. Reinstall the column, reduce the injection volume, or dilute the samples by adding more solvent to the sample, or run in split mode. Defective or clogged syringes, clogged columns, or leaks at the inlet will result in chromatograms with just one air peak or without any peaks. Finally, if there are changes in retention time relating to when known peaks emerge, check the column flow, as there is likely to be a leak that needs to be fixed, often at the septum between the inlet liner and syringe/injector assembly.
If no sample peaks are detected on the IRMS, verify that the furnace tube is installed correctly, that the furnace is set to the correct operating temperature (850° for carbon and 1450° for hydrogen), the column is not broken, the syringe is not blocked, and the filament is not damaged. If the system is producing poor stable isotopic data, introduce monitoring gas pulses into the IRMS and measure their stability and linearity. If stability and linearity are not the problem , clean the IRMS source.
Statistical Analysis
The carbon preference index (CPI) examines the odd-over-even (n -alkanes) or even-over-odd (n -alkanoic acids) carbon number predominance, which, in terrestrial sediments, acts as an indicator for hydrocarbon maturity (Duan & Xu, 2012). Alkanes and n -acids deriving from land plants display CPI >5.0, whereas mature or heavily degraded samples are characterized by considerably lower CPI values of ≤1.0. Although the CPI reflects the degree of plant wax preservation, the wide range observed in living plants (between 2.1 and 16.7) precludes its use as a single metric on which to base sample integrity assessments (Bush & McInerney, 2013). CPI is calculated with the following formulae—see Equations 2 and 3 for n -alkanes (top) and FAMEs (bottom): 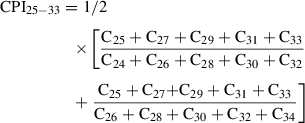
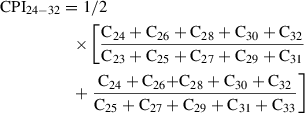
The compositions of n -alkanes and fatty acids are often compared using the average chain length (ACL), or the weight-averaged number of carbon homologues, in higher plants and sedimentary samples. ACL can be used (cautiously) as a proxy for both vegetation and climate, as it has been shown to be higher in C4 grasses (Rommerskirchen, Plader, Eglinton, Chikaraishi, & Rullkötter, 2006), but also correlates with higher growing season temperature and aridity. ACL, however, cannot distinguish graminoids from woody plants or fatty acids originating from terrestrial vs. submerged plants (but can be used to differentiate algal from plant sources; Bush & McInerney, 2013; Liu & Liu, 2017). Typically, plants growing in hot, dry environments such as the wooded grasslands of East Africa will have higher ACL values, regardless of plant type. Low ACL values, on the other hand, can be used as a qualitative measure for aquatic and emergent plant and bacterial input of biomarkers into sedimentary records. ACL is calculated with the following formulae for n -alkanes (top) and FAMEs (bottom; see Equations 4 and 5): 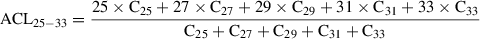
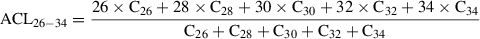
Understanding Results
Generally, short-chain n -alkanes (C17-C21) characterize aquatic algae, mid-chain n -alkanes (C21-C25) distinguish macrophytes, and long-chain n -alkanes (C27-C35) typify terrestrial vegetation. Furthermore, terrestrial plants generally contain a higher abundance of C26-C32 n -alkanoic acids (Chikaraishi & Naraoka, 2007; Gao, Hou, Toney, MacDonald, & Huang, 2011), whereas macrophytes display more C20-C24 n -alkanoic acids (Wang & Liu, 2012) and C14-C18 n -alkanoic acids derive from algae (Liu & Liu, 2017). However, both aquatic and terrestrial plants contain extensive C16 and C18 acid components (Huang, Shuman, Wang, & Webb, 2002) as these comprise the two major monomer families of cutin in the aerial surfaces of plant leaves.
Plants that use the C3 photosynthetic pathway usually have either C27, C29, or C31 as the prevailing n -alkane homologue, whereas C4 grasses and herbs commonly have C31 or C33 as their most abundant compounds. Although leaf wax distribution can be used to estimate biosynthetic sourcing, environmental variables play an important role in determining the distribution of n -alkanes, as plant communities adapted to warmer and drier conditions have higher amounts of longer-chain molecules regardless of photosynthetic pathway (Bush & McInerney, 2015; Dodd & Rafii, 1998). Furthermore, there can be differential degradation, with longer chains being preferentially degraded by microbial action, which is evidenced by different n -alkane homologs (i.e., ≥C24) appearing homogenous in terms of δ13C (Nguyen Tu, Derenne, Largeau, Bardoux, & Mariotti, 2004). Therefore, the best approach for distinguishing between C3 and C4 plants is through δ13C data.
Leaf wax lipid δ13C values of n -alkanes and n -alkanoic acids vary between –27‰ and –39‰ in C3 vegetation and between –14‰ and –26‰ in C4 plants (Bi, Sheng, Liu, Li, & Fu, 2005; Collister et al., 1994; O'Leary, 1981; Rommerskirchen et al., 2006; Vogts, Moossen, Rommerskirchen, & Rullkötter, 2009). It is the greater discrimination against atmospheric 13C by C3 photosynthesis that accounts for this isotopic distinction; C4 plants do not discriminate as significantly against 13C, hence their higher δ13C values (Ehleringer, 1989). Therefore, δ13C values can be used to identify the biosynthetic source of leaf wax lipids which can, indirectly, be used to determine broader plant community composition and environments. For example, in the tropics, C3 plants dominate forest and woodland ecosystems, whereas C4 grasses and sedges dominate more open settings (Bush & McInerney, 2013; Rommerskirchen et al., 2006; Vogts et al., 2009). Meanwhile, along altitudinal gradients of grassland environments, C4 grasses dominate at warmer, lower elevations, with C3 grasses becoming more common at higher elevations (Roberts, Lee-Thorp, Mitchell, & Arthur, 2013; Smith, Lee-Thorp, & Sealy, 2002). In temperate areas, the intensity and duration of sunlight, canopy structure, wax production, plant taxonomy, season, and the total concentration of atmospheric CO2 all contribute to leaf wax biosynthesis and δ13C signature.
Comparing δD and δ13C can help to reveal the climatic and environmental aspects dictating plant composition and structure in temperate, subtropical, and tropical zones. Source water hydrogen is the primary signal recorded in the δD data of leaf wax lipids from terrestrial plants. In the tropics, the dominant mechanism influencing precipitation δD is the “amount effect” (Dansgaard, 1964), in which rainfall intensity and evaporation control the isotopic depletion of rain. This leads to more depleted δD values in plants growing in areas with high precipitation, and more enriched δD in areas with lower precipitation and greater evaporation (Sachse et al., 2004). Also, because water availability is often the dominant factor dictating true forest or woodland development (Sankaran et al., 2005), soil δ13C reflects plant community composition and structure can indirectly reflect rainfall amount. For example, leaf wax and soil δ13C is enriched in open, dry environments dominated by C4 grasses. Alternatively, in both high altitudes and latitudes, precipitation δD is influenced by changes in temperature, with depleted δD values recorded in colder climates. Furthermore, plant type and ecological lifeform, leaf shape, and water-use efficiency induce changes in leaf water δD (Hou et al., 2007; Liu & Yang, 2008).
In soils, relative to plant matter, there is a +4‰ to +6‰ enrichment in n -alkane and n -alkanoic acid δ13C relative to plant matter, which is attributed to the combination of the Suess effect (≤2‰), and microbial 13C contribution to soil isotope archives (2-4‰; Nguyen Tu et al., 2004; Wu, West, & Feakins, 2019). With this knowledge, the distinct δ13C signatures of both C3 and C4 plants allow the estimation of their relative contribution to sedimentary carbon archives.
Time Considerations
Pressurized solvent extraction involves multiple timed steps defined by the user. Therefore, depending on the number of cycles and the hold and discharge times for each cycle, lipid extraction times can vary considerably. However, with Basic Protocol 1, which uses three cycles, hold and discharge times of 10 and 2 min, respectively, and solvent and gas flushing of 2 min each, a complete extraction takes roughly 50 min (not including sample heat-up time). Sample preparation can take ∼1 hr if extracting six samples owing to the time needed to weigh them, mix with sand, and fill extraction cells. Remember that samples should be freeze dried for 24 hr or oven dried for at least 48 hr before extraction. Depending on the degree of lithification of sediments, it can take a considerable amount of time to completely homogenize them with mortar and pestle. Include an additional 1.5 to 2 hr for solvent evaporation and another 30 min to transfer TLEs to new vials (note: this assumes evaporation with a Büchi Syncore® Analyst Vacuum Evaporator, which can evaporate twelve samples at a time. Total lipids are extracted in two groups of six, and then the twelve total samples are evaporated at once). It takes 72 hr to extract lipids using the Soxhlet apparatus (Alternate Protocol 1.1) and one additional day to clean the glassware. Consequently, it can take a full 5 days to extract one sample set; if possible, extract in batches of 6 or 12. As for Ultrasonic Extraction (Alternate Protocol 1.2), the sonication, centrifugation, and solvent transferal can take between 2.5 and 4 hr depending on the number of sample being processed at one time. Include an additional hour for solvent evaporation when using two 40-ml vials for each sample.
Aminopropyl column chromatography can be completed in under 45 min when extracting five samples simultaneously. The wider surface area and greater headspace of the Agilent Bond Elut NH2 cartridge allows faster elution rates and the addition of more solvent/TLE at once. With 5″ pipet columns, on the other hand, the limited headspace between the top of the silica and the top of the pipet, and the smaller surface area, result in slower elution times and smaller solvent volumes being added at a time. Therefore, SiAgNO3 column chromatography generally takes about 1 hr per five samples with three solvent fractions. To accelerate this process, use rubber pipet bulbs to gently force the solvent through the columns.
Converting n -alkanoic acids to GC amenable FAMEs takes 20-24 hr (Basic Protocol 4), and the timing for the hexane removal of FAMEs from the 5% NaCl aqueous solution depends on the number of samples being processed at once. Processing twelve samples in four groups of three takes about 0.5 hr to complete. Avoid transferring any of the NaCl solution along with the FAMEs.
Analyzing one sample on the GC-MS with the parameters outlined in Basic Protocol 5 takes 48 min, so it is best to load as many samples as possible into a sequence and run throughout the day and overnight. Make sure that gas supply and wash solvent volume are at levels that will not deplete when running overnight. Because sample preparation involves transferring n -alkanes or FAMEs to 2-ml GC vials with 250-µl inserts and then evaporating with N2, set aside at least 1 hr (20 min for transferring, 30 min for evaporating, 10 min for resuspending samples in 100 µl hexane and creating a sequence) to prepare samples for GC-MS. An IRMS analysis takes slightly longer (60-70 min) because it includes three reference gas injections at the start and end of each sample measurement. Solvent volume within the GC vials can often be the same as that used for the GC-MS (i.e., 100 µl), but if biomarker amounts are low, it is necessary to evaporate the solvent to increase the concentration (ng/µl). Include an additional 15-20 min prep time to evaporate and concentrate samples and an extra 10 min to create a sequence on the IRMS. Samples and standards should be run in duplicate or triplicate for both δ13C and δD to maintain a tracking of contamination and precision. Also take into consideration the time (1-2 days) required for changing the Elementar GC5 Furnace System and conditioning the IRMS when switching between carbon and hydrogen isotope analysis.
A rough estimate of the overall time requirements for extracting (Basic Protocol 1 or Alternative Protocols 1 or 2), separating (Basic Protocols 2, 3, and 4), and analyzing (Basic Protocols 5 and 6) 50 samples from start to finish is 7 weeks. This assumes overlap between the protocols (i.e. samples that have already been PSE extracted can be separated into individual fractions while a second sample set is being extracted with the PSE). Week 1 is dedicated to Basic Protocol 1 and part of Basic Protocol 2. Basic Protocol 2 and Basic Protocol 4 are the focus of week 2, and Basic Protocol 3 is started at the end of week 2 and finished in week 3; when analyzing both n -alkanes and FAMEs simultaneously, an additional week may be necessary. Mass spectrometry, that is both GC-MS (Basic Protocols 5) and carbon GC-IRMS (Basic Protocol 6), are conducted in weeks 4 and 5, with week 6 being used to conduct hydrogen GC-IRMS. Reserve an additional week (week 7) for troubleshooting or machine maintenance that may become necessary during any of the stages.
Acknowledgments
For financial support, we thank the Canadian Social Sciences and Humanities Research Council for a grant under its Partnership Grant Program (no. 895-2016-1017) awarded to Dr. Julio Mercader, the University of Calgary, the Explorers Club Exploration Fund (grant to R.P.), the Ruggles-Gates Fund for Biological Anthropology (grant to R.P.), and the Max Planck Society. P.R. also received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement no. 850709) for this project. For research conducted in Tanzania, we thank the Tanzanian Ministry of Natural Resources and Tourism, through its Antiquities Division, for permission to carry out our work (14/2017/2018), the Ngorongoro Conservation Area for permission to enter its protected area (BE.504/620/01/53), the Antiquities division for the Tanzanian export license for the materials (EA.150/297/01: 5/2018/2019), and the Tanzanian Executive Secretary from the Mining Commission (00001258), who provided authorization for exploration to the University of Calgary. Open access funding enabled and organized by Projekt DEAL.
Literature Cited
- Aichner, B., Herzschuh, U., & Wilkes, H. (2010). Influence of aquatic macrophytes on stable carbon isotope signatures of sedimentary organic matter in lakes on the Tibetan Plateau. Organic Geochemistry , 41, 706–718. doi: 10.1016/j.orggeochem.2010.02.002.
- Aichner, B., Herzschuh, U., Wilkes, H., Vieth, A., & Böhner, J. (2010). δD values of n -alkanes in Tibetan lake sediments and aquatic macrophytes—A surface sediment study and application to a 16 ka record from Lake Koucha. Organic Geochemistry , 41, 779–790. doi: 10.1016/j.orggeochem.2010.05.010.
- Barnes, M. A., & Barnes, W. C. (1978). Organic compounds in lake sediments. In A. Lerman (Ed.), Lakes: Chemistry, geology, physics (pp. 127–152). Berlin: Springer-Verlag.
- Bi, X., Sheng, G., Liu, X., Li, C., & Fu, J. (2005). Molecular and carbon and hydrogen isotopic composition of n -alkanes in plant leaf waxes. Organic Geochemistry , 36, 1405–1417. doi: 10.1016/j.orggeochem.2005.06.001.
- Bouloubassi, I., & Saliot, A. (1993). Investigation of anthropogenic and natural organic inputs in estuarine sediments using hydrocarbon markers (NAH, LAB, PAH). Oceanologica Acta , 16(2), 145–161.
- Briggs, D. E. G., Evershed, R. P., & Lockheart, M. J. (2000). The biomolecular paleontology of continental fossils. Paleobiology , 26(4), 169–193. doi: 10.1017/S0094837300026920.
- Brittingham, A., Hren, M. T., & Hartman, G. (2017). Microbial alteration of the hydrogen and carbon isotopic composition of n -alkanes in sediments. Organic Geochemistry , 107, 1–8. doi: 10.1016/j.orggeochem.2017.01.010.
- Brittingham, A., Hren, M. T., Hartman, G., Wilkinson, K. N., Mallol, C., Gasparyan, B., & Adlet, D. S. (2019). Geochemical evidence for the control of fire by middle palaeolithic hominins. Nature Scientific Reports , 9(1), 15368. doi:10.1038/s41598-019-51433-0.
- Burgoyne, T. W., & Hayes, J. M. (1998). Quantitative production of H2 by pyrolysis of gas chromatographic effluents. Analytical Chemistry , 70, 5136–5140. doi: 10.1021/ac980248v.
- Bush, R. T., & McInerney, F. A. (2013). Leaf wax n -alkane distributions in and across modern plants: Implications for paleoecology and chemotaxonomy. Geochimica et Cosmochimica Acta , 117, 161–179. doi: 10.1016/j.gca.2013.04.016.
- Bush, R. T., & McInerney, F. A. (2015). Influence of temperature and C4 abundance on n -alkane chain length distributions across the central USA. Organic Geochemistry , 79, 65–73. doi: 10.1016/j.orggeochem.2014.12.003.
- Carter, J. F., & Barwick, V. J. (2011). Good practice guide for isotope ratio mass spectrometry. FIRMS. ISBN 978-0-948926-31-0.
- Castañeda, I. S., & Schouten, S. (2011). A review of molecular organic proxies for examining modern and ancient lacustrine environments. Quaternary Science Reviews , 30, 2845–2891. doi: 10.1016/j.quascirev.2011.07.009.
- Chikaraishi, Y., & Naraoka, H. (2007). δ13C and δD relationships among three n -alkyl compound classes (n -alkanoic acid, n -alkane and n -alkanol) of terrestrial higher plants. Organic Geochemistry , 38, 198–215. doi: 10.1016/j.orggeochem.2006.10.003.
- Chikaraishi, Y., Naraoka, H., & Poulson, S. R. (2004). Hydrogen and carbon isotopic fractionations of lipid biosynthesis among terrestrial (C3, C4 and CAM) and aquatic plants. Phytochemistry , 65, 1369–1381. doi: 10.1016/j.phytochem.2004.03.036.
- Cody, G. D., Gupta, N. S., Briggs, D. E. G., Kilcoyne, A. L. D., Summons, R. E., Kenig, F., … Scott, A. C. (2011). Molecular signature of chitin-protein complex in Paleozoic arthropods. Geology , 39, 255–258. doi: 10.1130/G31648.1.
- Colcord, D. E., Shilling, A. M., Sauer, P., Freeman, K. H., Njau, J. K., Stanistreet, I. G., … Brassel, S. C. (2018). Sub-Milankovitch paleoclimatic and paleoenvironmental variability in East Africa recorded by Pleistocene lacustrine sediments from Olduvai Gorge, Tanzania. Palaeogeography, Palaeoclimatology, Palaeoecology , 495, 284–291. doi: 10.1016/j.palaeo.2018.01.023.
- Collins, J. A., Carr, A. S., Schefuß, E., Boom, A., & Sealy, J. (2017). Investigation of organic matter and biomarkers from Diepkloof Rock Shelter, South Africa: Insights into Middle Stone Age site usage and palaeoclimate. Journal of Archaeological Science , 85, 51–65. doi: 10.1016/j.jas.2017.06.011.
- Collister, J. W., Rieley, G., Stern, B., Eglinton, G., & Fry, B. (1994). Compound-specific δ13C analyses of leaf lipids from plants with differing carbon dioxide metabolims. Organic Geochemistry , 21(6–7), 619–627. doi: 10.1016/0146-6380(94)90008-6.
- Connolly, R., Jambrina-Enríquez, M., Herrera-Herrera, A. V., Vidal-Matutano, P., Fagoaga, A., Marquina-Blasco, R., … Mallol, C. (2019). A multiproxy record of palaeoenvironmental conditions at the Middle Palaeolithic site of Abric del Pastor (Eastern Iberia). Quaternary Science Reviews , 225, 106023. doi: 10.1016/j.quascirev.2019.106023.
- Cranwell, P. A. (1984). Lipid geochemistry of sediments from Upton Broad, a small productive lake. Organic Geochemistry , 7, 25–37. doi: 10.1016/0146-6380(84)90134-7.
- Cranwell, P. A., Eglinton, G., & Robinson, N. (1987). Lipids of aquatic organisms as potential contributors to lacustrine sediments. Organic Geochemistry , 11, 513–527. doi: 10.1016/0146-6380(87)90007-6.
- Crider, Q. C., Alaupovic, P., Hillsberry, J., Yen, C., & Bradford, R. H. (1964). Separation of lipids by Silica Gel G column chromatography. Journal of Lipid Research , 5, 479–481.
- Dansgaard, W. (1964). Stable isotopes in precipitation. Tellus , 16, 436–468. doi: 10.3402/tellusa.v16i4.8993.
- De Vries, B. (1962). Quantitative separations of lipid materials by column chromatography on silica impregnated with silver nitrate. Journal of the Society of Chemical Industry , 84, 1049–1050.
- Dodd, R. S., & Rafii, Z. A. (1998). Ecotypic adaptation in Austrocedrus chilensis in cuticular hydrocarbon composition. New Phytologist , 138, 699–708. doi: 10.1046/j.1469-8137.1998.00142.x.
- Duan, Y., & Xu, L. (2012). Distributions of n -alkanes and their hydrogen isotopic composition in plants from Lake Qinghai (China) and the surrounding area. Applied Geochemistry , 27, 806–814. doi: 10.1016/j.apgeochem.2011.12.008.
- Eglinton, G., & Hamilton, R. J. (1967). Leaf epicuticular waxes. Science , 156, 1322–1335. doi: 10.1126/science.156.3780.1322.
- Eglinton, T. I., & Eglinton, G. (2008). Molecular proxies for paleoclimatology. Earth and Planetary Science Letters , 275, 1–16. doi: 10.1016/j.epsl.2008.07.012.
- Égüez, N., & Makarewicz, C. A. (2018). Carbon isotope ratios of plant n-alkanes and microstratigraphy analyses of dung accumulations in a pastoral nomadic winter campsite (Eastern Mongolia). Ethnoarchaeology , 10(2), 141–158. doi: 10.1080/19442890.2018.1510614.
- Ehleringer, J. R. (1989). Carbon isotope ratios and physiological processes in aridland plants. In P. W. Rundel, J. R. Ehleringer, & K. A. Nagy (Eds.), Stable Isotopes in Ecological Research (Vol. 68, pp. 525). New York: Springer-Verlag.
- Farquhar, G. D., Hubick, K. T., Condon, A. G., & Richards, R. A. (1989). Carbon isotope fractionation and plant water use efficiency. In P. W. Rundel, J. R. Ehleringer, & K. A.Nagy (Eds.), Stable isotopes in ecological research (Vol. 68, pp. 525). New York: Springer-Verlag.
- Ficken, K. J., Li, B., Swain, D. L., & Eglinton, G. (2000). An n -alkane proxy for the sedimentary input of submerged/floating freshwater aquatic macrophytes. Organic Geochemistry , 31, 745–749. doi: 10.1016/S0146-6380(00)00081-4.
- Gao, L., Guimond, J., Thomas, E., & Huang, Y. (2015). Major trends in leaf wax abundance, δ2H and δ13C values along leaf venation in five species of C3 plants: Physiological and geochemical implications. Organic Geochemistry , 78, 144–152. doi: 10.1016/j.orggeochem.2014.11.005.
- Gao, L., Hou, J., Toney, J., MacDonald, D., & Huang, Y. (2011). Mathematical modeling of the aquatic macrophyte inputs of mid-chain n -alkyl lipids to lake sediments: Implications for interpreting compound specific hydrogen isotopic records. Geochimica et Cosmochimica Acta , 75, 3781–3791. doi: 10.1016/j.gca.2011.04.008.
- Gough, M. A., & Rowland, S. J. (1990). Characterization of unresolved complex mixtures of hydrocarbons in petroleum. Nature , 344, 648–650. doi: 10.1038/344648a0.
- Grimalt, J. O., Torras, E., & Albaigés, J. (1988). Bacterial reworking of sedimentary fipids during sample storage. Advances in Organic Geochemistry , 13(4-6), 741–746. doi: 10.1016/0146-6380(88)90096-4.
- Hilkert, A. W., Douthitt, C. B., & Schluter, H. J. (1999). Isotope ratio monitoring gas chromatography/mass spectrometry of D/H by high temperature conversion isotope ration mass spectrometry. Communications in Mass Spectrometry , 13, 1226–1230. doi: 10.1002/(SICI)1097-0231(19990715)13:13<1226::AID-RCM575>3.0.CO;2-9.
- Hou, J., D'Andrea, W. J., MacDonald, D., & Huang, Y. (2007). Hydrogen isotopic variability in leaf waxes among terrestrial and aquatic plants around Blood Pond, Massachusetts (USA). Organic Geochemistry , 38, 977–984. doi:10.1016/j.orggeochem.2006.12.009.
- Huang, Y., Shuman, B., Wang, Y., & Webb, III, T. (2002). Hydrogen isotope ratios of palmitic acid in lacustrine sediments record late Quaternary climate variations. Geology , 30(12), 1103–1106. doi: 10.1130/0091-7613(2002)030<1103:HIROPA>2.0.CO;2.
- Ladd, S. N., & Sachs, J. P. (2013). Positive correlation between salinity and n -alkane δ13C values in the mangrove Avicennia marina. Organic Geochemistry , 64, 1–8. doi: 10.1016/j.orggeochem.2013.08.011.
- Leahy, J. G., & Colwell, R. R. (1990). Microbial degradation of hydrocarbons in the environment. Microbiological Reviews , 54, 305–315. doi: 10.1128/MMBR.54.3.305-315.1990.
- Liu, H., & Liu, W. (2017). Concentration and distributions of fatty acids in algae, submerged plants and terrestrial plants from the northeastern Tibetan Plateau. Organic Geochemistry , 113, 17–26. doi: 10.1016/j.orggeochem.2017.08.008.
- Liu, W., & Yang, H. (2008). Multiple controls for the variability of hydrogen isotopic compositions in higher plant n -alkanes from modern ecosystems. Global Change Biology , 14, 2166–2177. doi: 10.1111/j.1365-2486.2008.01608.x.
- Liu, W., Yang, H., & Li, L. (2006). Hydrogen isotopic compositions of n -alkanes from terrestrial plants correlate with their ecological life forms. Oecologia , 150, 330–338. doi: 10.1007/s00442-006-0494-0.
- Lockheart, M. J., van Bergen, P. F., & Evershed, R. P. (1997). Variations in the stable carbon isotope compositions of individual lipids from the leaves of modern angiosperms: Implications for the study of higher land plant-derived sedimentary organic matter. Organic Geochemistry , 26, 137–153. doi: 10.1016/S0146-6380(96)00135-0.
- Lupien, R. L., Russell, J. M., Feibel, C., Beck, C., Castañeda, I. S., Deino, A., & Cohen, A. (2018). A leaf wax biomarker record of early Pleistocene hydroclimate from West Turkana, Kenya. Quaternary Science Reviews , 186, 225–235. doi: 10.1016/j.quascirev.2018.03.012.
- Magill, C. R., Ashley, G. M., Domínguez-Rodrigo, M., & Freeman, K. (2015). Dietary options and behavior suggested by plant biomarker evidence in an early human habitat. Proceedings of the National Academy of Science , 113(11), 2874–2879. doi: 10.1073/pnas.1507055113.
- Magill, C. R., Ashley, G. M., & Freeman, K. H. (2013a). Ecosystem variability and early human habitats in eastern Africa. Proceedings of the National Academy of Science , 110(4), 1167–1174. doi: 10.1073/pnas.1206276110.
- Magill, C. R., Ashley, G. M., & Freeman, K. H. (2013b). Water, plants, and early human habitats in eastern Africa. Proceedings of the National Academy of Science , 110(4), 1175–1180. doi: 10.1073/pnas.1209405109.
- McWilliams, K. M., & Angelici, R. J. (2016). Batchwise extraction of methyl linolenate (18:3, ALA) from fatty acid methyl esters derived from soybean and canola oils using silver nitrate/silica gel. European Journal of Lipid Science and Technology , 118, 252–261. doi: 10.1002/ejlt.201500084.
- Nguyen Tu, T. T., Derenne, S., Largeau, C., Bardoux, G., & Mariotti, A. (2004). Diagenesis effects on specific carbon isotope composition of plant n -alkanes. Organic Geochemistry , 35, 317–329.
- Nikolova-Damyanova, B. (2009). Retention of lipids in silver ion high-performance liquid chromatography: Facts and assumptions. Journal of Chromatography A , 1216, 1815–1824. doi: 10.1016/j.chroma.2008.10.097.
- O'Leary, M. (1981). Carbon isotope fractionation in plants. Phytochemistry , 20(4), 553–567. doi: 10.1016/0031-9422(81)85134-5.
- Pagani, M., Pedentchouk, N., Huber, M., Sluijs, A., Schouten, S., Brinkhuis, H., … Scientists, E. (2006). Arctic hydrology during global warming at the Palaeocene/Eocene thermal maximum. Nature , 442(10), 671–675. doi: 10.1038/nature05043.
- Patalano, R. (2019). The Environmental Context of the Earliest Acheulean at Olduvai Gorge, Tanzania (PhD in Archaeology). University of Calgary, Calgary, Alberta, Canada.
- Patalano, R., Wang, Z., Leng, Q., Liu, W., Zheng, Y. F., Sun, G. P., & Yang, H. (2015). Hydrological changes facilitated early rice farming in the lower Yangtze River Valley in China: A molecular isotope analysis. Geology , 43(7), 639–642. doi: 10.1130/G36783.1.
- K. Peters, C. Walters, & J. Moldowan (Eds.). (1993). The Biomarker guide. Cambridge: Cambridge University Press.
- Richter, B. E., Jones, B. A., Ezell, J. L., & Porter, N. L. (1996). Accelerated solvent extraction: A technique for sample preparation. Analytical Chemistry , 68, 1033–1039. doi: 10.1021/ac9508199.
- Roberts, P., Lee-Thorp, J. A., Mitchell, P. J., & Arthur, C. (2013). Stable carbon isotopic evidence for climate change across the late Pleistocene to early Holocene from Lesotho, southern Africa. Journal of Quaternary Science , 28, 360–369. doi:10.1002/jqs.2624.
- Rommerskirchen, F., Plader, A., Eglinton, G., Chikaraishi, Y., & Rullkötter, J. (2006). Chemotaxonomic significance of distribution and stable carbon isotopic composition of long-chain alkanes and alkan-1-ols in C4 grass waxes. Organic Geochemistry , 37, 1303–1332. doi: 10.1016/j.orggeochem.2005.12.013.
- Sachse, D., Radke, J., & Gleixner, G. (2004). Hydrogen isotope ratios of recent lacustrine sedimentary n -alkanes record modern climate variability. Geochim Cosmochim Acta , 68(23), 4877–4889. doi: 10.1016/j.gca.2004.06.004.
- Sachse, D., Radke, J., & Gleixner, G. (2006). δD values of individual n -alkanes from terrestrial plants along a climatic gradient—implications for the sedimentary biomarker record. Organic Geochemistry , 37, 469–483. doi: 10.1016/j.orggeochem.2005.12.003.
- Sankaran, M., Hanan, N. P., Scholes, R. J., Ratnam, J., Augustine, D. J., Cade, B. S., … Zambatis, N. (2005). Determinants of woody cover in African savannas. Nature , 438(7069), 846–849. doi:10.1038/nature04070.
- Scrimgeour, C. M., Begley, I. S., & Thomason, M. L. (1999). Measurement of deuterium incorporation into fatty acids by gas chromatography/isotope ratio mass spectrometry. Rapid Commun Mass Spectrom , 13, 271–274. doi: 10.1002/(SICI)1097-0231(19990228)13:4<271::AID-RCM468>3.0.CO;2-6.
- Simoneit, B. R. T. (2004). Biomarkers (molecular fossils) as geochemical indicators of life. Advances in Space Research , 33, 1255–1261. doi: 10.1016/j.asr.2003.04.045.
- Smith, J. M., Lee-Thorp, J. A., & Sealy, J. (2002). Stable carbon and oxygen isotopic evidence for late Pleistocene to middle Holocene climatic fluctuations in the interior of southern Africa. Journal of Quaternary Science , 17(7), 683–695. doi: 10.1002/jqs.687.
- Uno, K. T., Polissar, P. J., Kahle, E., Feibel, C., Harmand, S., Roche, H., & deMenocal, P. B. (2016). A Pleistocene palaeovegetation record from plant wax biomarkers from the Nachukui Formation, West Turkana, Kenya. Philosophical Transactions of the Royal Society B , 371(20150235), 1–10.
- Vogts, A., Moossen, H., Rommerskirchen, F., & Rullkötter, J. (2009). Distribution patterns and stable carbon isotopic composition of alkanes and alkan-1-ols from plant waxes of African rain forest and savanna C3 species. Organic Geochemistry , 40, 1037–1054. doi: 10.1016/j.orggeochem.2009.07.011.
- Wang, Z., & Liu, W. (2012). Carbon chain length distribution in n -alkyl lipids: A process for evaluating source inputs to Lake Qinghai. Organic Geochemistry , 50, 36–43. doi: 10.1016/j.orggeochem.2012.06.015.
- Welker, F., Ramos-Madrigal, J., Gutenbrunner, P., Mackie, M., Tiwary, S., Rakownikow Jersie-Christensen, R., … Cappellini, E. (2020). The dental proteome of Homo antecessor. Nature , 580(7802), 235–238. doi:10.1038/s41586-020-2153-8.
- Wu, M. S., West, A. J., & Feakins, S. J. (2019). Tropical soil profiles reveal the fate of plant wax biomarkers during soil storage. Organic Geochemistry , 128, 1–15. doi: 10.1016/j.orggeochem.2018.12.011.
- Yang, H., & Huang, Y. S. (2003). Preservation of lipid hydrogen isotope ratios in Miocene lacustrine sediments and plant fossils at Clarkia, northern Idaho, USA. Organic Geochemistry , 34, 413–423. doi: 10.1016/S0146-6380(02)00212-7.
Citing Literature
Number of times cited according to CrossRef: 6
- Mohammad Tahsin Karimi Nezhad, Pavel Šamonil, Pavel Daněk, Jakub Jaroš, Michal Hájek, Petra Hájková, Stanislav Jabinski, Travis B. Meador, Jan Roleček, Lipid biomarkers and stable isotopes uncover paleovegetation changes in extremely species-rich forest-steppe ecosystems, Central Europe, Environmental Research, 10.1016/j.envres.2024.119564, 259 , (119564), (2024).
- Cezary Kabala, Bogdan Gądek, Monika Mętrak, Karol Szymczak, Małgorzata Suska-Malawska, The record of paleolake sediments in soil catena in the arid steppe, Kyzylkum Desert, Uzbekistan, CATENA, 10.1016/j.catena.2024.108433, 246 , (108433), (2024).
- Deepak Kumar Jha, Robert Patalano, Jana Ilgner, Hema Achyuthan, Abdullah M. Alsharekh, Simon Armitage, James Blinkhorn, Nicole Boivin, Paul S. Breeze, Ravindra Devra, Nicholas Drake, Huw S. Groucutt, Maria Guagnin, Patrick Roberts, Michael Petraglia, Preservation of plant‐wax biomarkers in deserts: implications for Quaternary environment and human evolutionary studies, Journal of Quaternary Science, 10.1002/jqs.3597, 39 , 3, (349-358), (2024).
- Tammy Buonasera, Alison Damick, Daniel Shoup, Not up in smoke: Lipid and phytolith evidence for the function of combustion features at CA-ALA-11, a San Francisco Bay area shellmound, Journal of Archaeological Science: Reports, 10.1016/j.jasrep.2023.104133, 51 , (104133), (2023).
- Małgorzata Suska-Malawska, Małgorzata Kot, Anna Gręzak, Monika Mętrak, Mukhiddin Khudjanazarov, Karol Szymczak, Potential impact of Holocene climate changes on camel breeding practices of Neolithic pastoralists in the Central Asian drylands: A preliminary assessment, The Holocene, 10.1177/09596836221114289, 32 , 11, (1132-1143), (2022).
- Robert Patalano, Patrick Roberts, Nicole Boivin, Michael D. Petraglia, Julio Mercader, Plant wax biomarkers in human evolutionary studies, Evolutionary Anthropology: Issues, News, and Reviews, 10.1002/evan.21921, 30 , 6, (385-398), (2021).

