Humanized Mouse Models for Immuno-oncology Drug Discovery
Rajendra Kumari, Rajendra Kumari, Gerold Feuer, Gerold Feuer, Ludovic Bourré, Ludovic Bourré
Abstract
Breakthroughs in cancer treatment with immunotherapeutics have provided long-term patient benefits for many different types of cancer. However, complete response is not achieved in many patients and tumor types, and the mechanisms underlying this lack of response are poorly understood. Despite this, numerous new targets, therapeutics, and drug combinations are being developed and tested in clinical trials. Preclinical models that recapitulate the complex human tumor microenvironment and the interplay between tumor and immune cells within the cancer-immunity cycle are needed to improve our understanding and screen new therapeutics for efficacy and safety/toxicity. Humanized mice, encompassing human tumors and human immune cells engrafted on immunodeficient mice, have been widely used for many years in immuno-oncology, with developments to improve both the humanization and the translational value central to the next generation of models. In this overview, we discuss recent advances in humanized models relevant to immuno-oncology drug discovery, the advantages and limitations of such models, the application of humanized models for efficacy and safety assessments of immunotherapeutics, and the potential opportunities. © 2023 Crown Bioscience. Current Protocols published by Wiley Periodicals LLC.
INTRODUCTION
Immunotherapy has revolutionized cancer treatment and prolonged patient survival across numerous cancer types. However, the benefits of immunotherapies remain limited because most patients are unresponsive or relapse (Chen & Mellman, 2017). The complex, dynamic interactions of the tumor and the immune system within the tumor microenvironment (TME) are also influenced by fibroblasts, epithelial and endothelial cells, and cancer therapeutics. Despite our improved understanding of the complexities of cancer and an increase in the number of immune-oncology (IO) targets, therapeutics, and combination approaches (Upadhaya et al., 2021), clinical trials are still dominated by immune checkpoint inhibitors (ICIs). There is an increased need to generate a reliable preclinical screening platform to evaluate novel immunotherapies and combination treatment strategies rationally and predictably to reduce attrition in the clinic and overcome patient relapse and resistance. Mouse models of cancer (Fig. 1A) are fundamental for understanding oncology and advancing preclinical cancer treatments. These models include xenograft, syngeneic/homograft, genetically engineered mouse models (GEMMs), chemically induced models, and humanized immune system (HIS) mouse models, each of which has specific utilities for IO based on their strengths and weaknesses (Q. X. Li et al., 2017). Human tumor xenograft models such as patient-derived xenografts (PDXs) reflect the heterogeneity of patient tumors, but the immunodeficient mice lack functional immune systems. Homograft models such as syngeneics or GEMMS are widely used to evaluate anticancer agents, but the murine immune biology poorly reflects humans. Unfortunately, no single model fully recapitulates a specific tumor type within its TME; indeed, each model only mirrors a component of tumor biology. Therefore, the selection of an appropriate mouse model for immunotherapeutic drug discovery depends on which models closely resemble the tumor biology of interest and, in some cases, may require more than one model. Thus, having a suite of preclinical tools becomes advantageous in progressing drug candidates forward. Early in vitro discovery research may facilitate the selection of in vivo models or the screening in 3D co-cultures of organoids with immune cells (Kumari et al., 2022; Xu et al., 2023) or, alternatively, ex vivo patient-derived tumor tissue (Beztsinna et al., 2022) may provide relevant human TME interactions, but these systems lack the complexity of in vivo biology. The HIS model, which comprises the engraftment of human immune cells in severely immunodeficient mice, and the co-engraftment of human tumors to establish a reconstituted TME, have become increasingly more attractive for the evaluation of immunotherapeutics (Fig. 1B) as they provide several advantages over other mouse models and have evolved and improved in the 30 years since the introduction of the NOD/scid mouse (Hesselton et al., 1995). Despite the advancements in HIS models, limitations to their application remain; thus, this review aims to survey the different mice strains and methodologies available to establish HIS, the applications for IO, the advantages and limitations of HIS models, and advances and future opportunities.
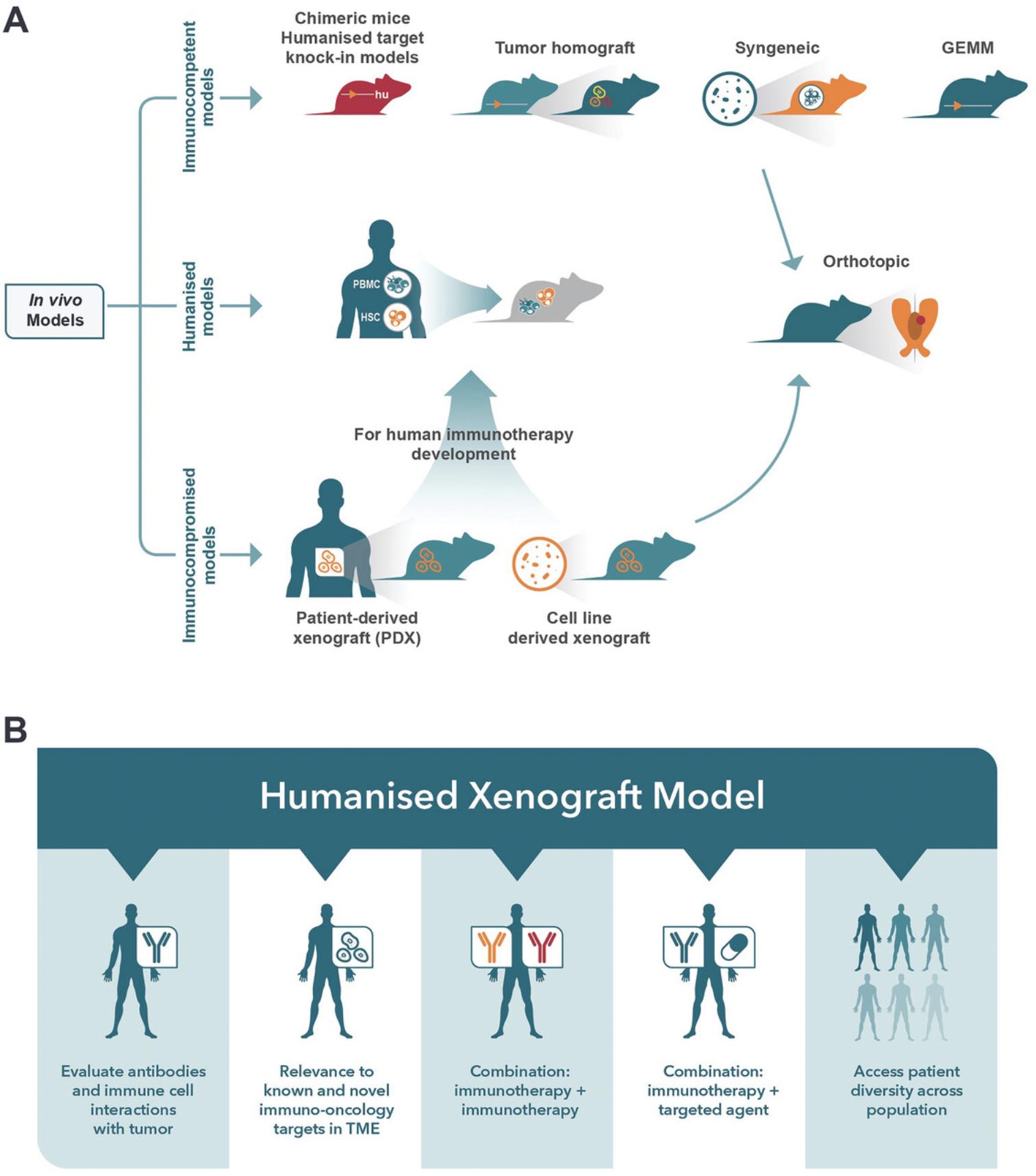
OVERVIEW OF IMMUNODEFICIENT MICE
Xenotransplantation of human tumors requires immunodeficient mouse strains. Successful xeno-engraftment of human tumors and hematopoietic malignancies has significantly increased with advancements in immunodeficient mouse strains (Table 1). A significant development in the abrogation of T- and B-cell functions was achieved by inactivating the gamma-chain of the IL-2 receptor (Ito et al., 2002; Traggiai et al., 2004), resulting in strains with defects in macrophage activity, complement-dependent hemolytic activity, and natural killer (NK) cell activity (Aspeslagh et al., 2016; Stewart et al., 1996).
| Mouse name | Strain | Features | Supplier | References |
|---|---|---|---|---|
| NOD-scid | NOD.Cg-Prkdcscid-/- | Lacks mature T and B cells with NOD genetic background reducing innate immune system function and complement activity | Multiple, e.g., Jackson Laboratory stock #001303 | (Shultz et al., 2007) |
| NSG™ | NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ | Also referred to as NOD scid gamma, NSG mice are severely immunodeficient and null for IL2rg, enabling more efficient engraftment of HSC, PBMCs, and PDXs or ASC and tissue | Jackson Laboratory stock #005557 | (Shultz et al., 2007; Shultz et al., 2005) |
| NSG B2m | NOD.Cg-B2mtm1Unc Prkdcscid Il2rgtm1Wjl/SzJ | b2m KO NSG triple mutant mice include NSG features plus MHC class I deficiency to decrease GvHD | Jackson Laboratory stock #010636 | (King et al., 2009) |
| NSG-SGM3 | NOD.Cg-Prkdcscid Il2rgtm1Wjl Tg(CMV-IL3,CSF2,KITLG)1Eav/MloySzJ | Transgenic NSG mouse altered to express human GM-CSF, SCF, and IL-3 cytokines to increase differentiation of human myeloid cells (macrophages and DCs) and engraftment of AML | Jackson Laboratory stock #013062 | (Billerbeck et al., 2011) |
| NRG | NOD.Cg-Rag1tm1Mom Il2rgtm1Wjl/SzJ | Also known as NOD rag gamma, these mice have a targeted knockout mutation in recombination activating gene 1 (Rag1) and IL2rgnull resulting in T, B, and NK cell deficiency to allow efficient engraftment of HSC, PDX, etc. NRG are more resistant to irradiation and genotoxic drugs than scid background mice. | Jackson Laboratory stock #007799 | (Pearson et al., 2008) |
| NOG | NOD.Cg-Prkdcscid Il2rgtm1Sug/ JicTac | Lacks mature T, B, and NK cells with truncated IL2rg, dysfunctional innate immune system, and reduced complement activity to enable more efficient engraftment | CIEA, Taconic | (Shultz et al., 2007) |
| NOG-EXL | NOD.Cg-Prkdcscid Il2rgtm1Sug Tg(SV40/HTLV-IL3,CSF2)10-7Jic/JicTac | hGM-CSF/hIL-3 NOG is an immunodeficient CIEA NOG mouse altered to express human GM-CSF and IL-3 cytokines to support differentiation of human myeloid lineages and HSC engraftment | CIEA, Taconic | (Ito et al., 2013) |
| hIL-15-NOG | NOD.Cg-Prkdcscid Il2rgtm1Sug Tg(CMV-IL2/IL15)1-1Jic/JicTac | NOG mouse altered to express human IL-15 cytokine to improve the engraftment and differentiation of NK cells compared to NOG mouse, suitable for ADCC evaluation | CIEA, Taconic | (Katano et al., 2017) |
| BRG | C.Cg-Rag2tm1Fwa Il2rgtm1Sug/JicTac | Also referred to as BALB/c Rag2-/– IL-2Rᵞc-/-, available from multiple suppliers, these mice have T, B, and NK cell deficiencies and are similar to NRG for engraftment, irradiation tolerance, and fully functional complement cascade | CIEA Taconic (also Genoway, Jackson Laboratory) | (Legrand et al., 2011) |
| BRGS | BALB/c Rag2tm1Fwa Il2rgtm1Cgn SirpaNOD | BRG mouse with polymorphism SIRPαNOD inhibiting murine “eat me” signals by macrophages | Genoway | (Legrand et al., 2011) |
| BRGSF | BALB/c Rag2tm1Fwa Il2rgtm1Cgn SirpaNOD Flt3tm1lrl | BRGS mice with mutation in fetal liver kinase-2 (Flk2-/-) to improve human DC maturation with hFlt3 ligand | Genoway | (Li et al., 2016) |
| NCG | NOD-Prkdcem26Cd52Il2rgem26Cd22/NjuCrl | NCG is a coisogenic, genetically engineered immunodeficient mouse created by sequential CRISPR-Cas9 editing of the Prkdc and Il2rg loci in the NOD/Nju mouse and carries a mutation in the Sirpa gene. NCG lacks functional/mature T, B, and NK cells with reduced macrophage and DC function and is suitable for HSC engraftment | Charles River | |
| NCG-M |
NOD/ShiLtJGpt-Prkdcem26Cd52Il2rgem26Cd22 Rosa26em1Cin(hCSF2&IL3&KITLG)/Gpt |
Also known as NCG-SGM3, the NCG mouse has been altered to express human GM-CSF, SCF, and IL-3 cytokines to promote the expansion of myeloid cells as well as T cells, B cells, and NK cells. | Gempharmatech | |
| NCG-IL-15 |
NOD/ShiLtJGpt-Prkdc em26Cd52Il2rg em26Cd22Il15 em1Cin(hIL15)/Gpt |
Human IL-15 NCG knock-in model to support colonization and activity of human NK cells | Gempharmatech | |
| MISTRG |
C;129S4-Rag2tm1.1Flv Csf1tm1(CSF1)Flv Csf2/Il3tm1.1(CSF2,IL3)Flv Thpotm1.1(TPO)Flv Il2rgtm1.1Flv Tg(SIRPA)1Flv/J |
MISTRG are Rag2-/Il2rg-deficient mice with human versions of GM-CSF, IL-3, M-CSF, TPO, and Sirpa to increase differentiation of macrophages, DCs, and development of NK cells | Discontinued | (Rongvaux et al., 2014) |
The improvements in immunodeficiency have allowed the xeno-engraftment of normal human peripheral blood lymphocytes and hematopoietic progenitor/stem cells (CD34+ HSCs). Transgenic mice designed to express human GM-CSF and IL-3 cytokines support the differentiation of human myeloid cell lineages, including plasmacyoid dendritic cells (pDC) and myeloid suppressor cells, resulting in a much broader profile of human immune reconstitution (Ito et al., 2013; Wunderlich et al., 2010).
HUMANIZATION METHODS
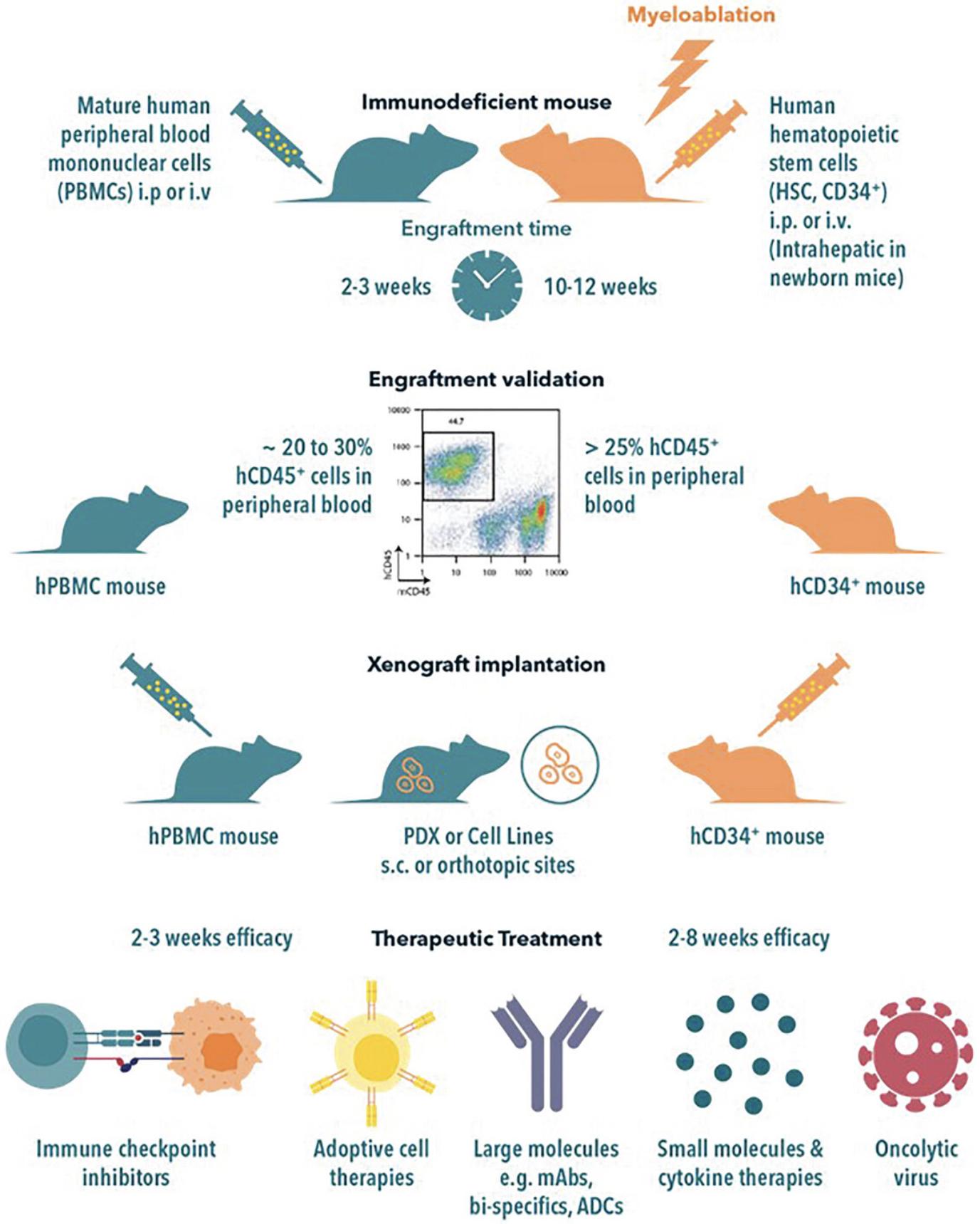
The engraftment of human immune system components into immunodeficient mice can be achieved via several strategies with varying benefits and limitations. No method can fully recapitulate the complex human TME within a mouse (Bareham et al., 2021; Medetgul-Ernar & Davis, 2022), so each methodology has limitations. Therefore, an in-depth understanding of the models and their advantages and limitations can facilitate the selection of the optimal humanization method and mouse strain to answer specific experimental questions. An overview of methods and the advantages and limitations of the two most common approaches to IO drug discovery are summarized in Table 2, and the workflow is shown in Figure 2.
| PBMC humanized mice | hCD34+ humanized mice | |
|---|---|---|
| Features |
|
|
| Advantages |
|
|
| Limitations |
|
|
hPBMC Model Generation
Humanization of mice using peripheral blood mononuclear cells (PBMCs) is the simplest and quickest model. PBMCs are typically derived from healthy donors, purified by Ficoll-Hypaque centrifugation, and administered by i.v. or i.p. injection. The ease of construction and rapid rate of humanization are clear advantages of the hPBMC model. Engraftment is validated by flow cytometry analysis for human CD45+ cells in the peripheral blood, which typically takes 2-3 weeks and is characterized by the proliferation of human T and B cells. The T cells have an activated and memory phenotype with an initial 1:1 ratio of CD4:CD8. Human T cells tend to predominate over time. This model is widely used to analyze human immune responses in autoimmunity and to gauge cytokine storm induction. Eventual lethal graft versus host disease (GvHD) due to the alloreactivity of the transplanted human CD4+ T cells is a significant impediment to long-term studies with extended dosing time-frames. Sublethal irradiation preconditioning of mice in this model has been discontinued as it was reported to accelerate GvHD. Mouse strain selection is also pivotal, as NOD scid gamma (NSG) mice have been shown to develop GvHD more rapidly than BALB/c Rag2 Il2rg (BRG) mice following humanization (Ali et al., 2012). Donor PBMCs show varying degrees of GvHD induction, but PBMC pre-screening can de-risk donor selection for graft versus tumor (GvT) in hu-PBMC mice and allows for more accurate testing of candidate drug efficacy. In vitro , screening multiple PBMC donors allows the identification of PBMCs with the least GvT activity while screening for minimal GvHD induction. In addition, in vitro pre-screening of PBMCs could include test compounds to determine efficacy. Intraperitoneal or subcutaneous implantation of PBMCs combined with tumor cells (“admix”) can also be used to establish the appropriate tumor:PBMC ratio that balances the in vivo GvT response. NSG strains with double knock-out (dKO) of MHC classes I and II (NSG-dKO and NOG-dKO) yield significantly lower levels of GvHD when reconstituted with PBMCs, although only a subset of human donors completely fails to induce GvHD (personal communications Jackson Laboratories; Brehm et al., 2019; Yaguchi et al., 2018). These results suggest that NSG-dKO and NOG-dKO mice may be advantageous in analyzing transferred human PBMCs with fewer xeno-GvHD effects.
CD34+ Humanized Mice
Multipotent CD34+ human hematopoietic stem cells (HSCs) can differentiate into mature lymphocytes, myeloid cells, erythrocytes, leukocytes, and platelets. CD34+ HSCs can be isolated from human bone marrow donors, umbilical cord blood, or human fetal liver (gestational age 18-21 weeks). CD34+ HSCs are generally purified using magnetic affinity columns, and purified cells (0.2-1.0 million cells) are injected i.v. into newborn or adult immunodeficient mice. The mice are typically sub-lethally irradiated or treated with busulfan (Hayakawa et al., 2009) to precondition the bone marrow. The human HSCs engraft in the murine bone marrow, yielding robust human hematopoiesis. Engraftment efficiencies depend on the source of the CD34+ cells and the age, sex, and strain of the mouse, but injection of CD34+ cells into NOG/NSG mice is a popular model for the development of multiple human cell lineages, including T and B lymphocytes, NK cells, myeloid cells, and erythroblasts (Shultz et al., 2005; Shultz et al., 2007). Since T cells mature on the murine thymus, GvHD in this model is significantly reduced and delayed. Intrahepatic injection of CD34+ cells into 2-day-old pups also results in robust humanization, as measured by hCD45+ cells in the peripheral blood (PB) (Grover et al., 2017). Human hematopoiesis is robust and stable, and human lymphocytes can often be detected 8-9 months post-inoculation. Although NK and myeloid cells develop in these mouse strains, they are relatively low in number. Further developments to express human cytokines and chemokines in mice have increased the number and maturation of myeloid cells. Examples of these models, such as NSG-SGM3, NOG-EXL, BRGSF, and MISTRG, are shown in Table 1 and summarized in the Challenges and Limitations section.
BLT Mice
The progenitor of humanized mouse models was initially the Thy/Liv SCID mouse model, which was generated by surgical implantation of human fetal thymus and liver (gestational age 18-21 weeks) under the kidney capsule (Aldrovandi et al., 1993; McCune et al., 1988). Hematopoietic progenitor cells are provided by the fetal liver and mature on the human thymus in the conjoint organ that is formed. Despite the role of the SCID-hu in deciphering HIV-1 infection and pathogenesis, the model was limited to human T-cell development and characterized by short survival times and relatively fast depletion of human T lymphoid cells. The Hu-BLT (bone marrow-liver-thymus) model was generated by combining the i.v. injection of human CD34+ HSCs, which localized to the murine bone marrow, with the Thy/Liv model to produce a more broad and robust human immune reconstitution, including generation of B, myeloid, and NK cells. A major advantage of the Hu-BLT mouse is the development of an adaptive immune response as well as human mucosal and secondary lymphoid tissues (Brainard et al., 2009; Stoddart et al., 2011; Wahl & Victor Garcia, 2014; Wege et al., 2014). BLT mice are commonly used for studies of tumor implantation, infectious disease (particularly HIV-1), and stem cell gene therapy (Brehm et al., 2013; Deruaz et al., 2017; Durost et al., 2018). Although BLT mice have a significant advantage over other humanized mouse models, the reliance on human fetal tissues is increasingly problematic.
HUMAN TUMOR IMPLANTATION AND UTILITY IN IO RESEARCH
HIS mice can support the growth of human tumor cell line-derived xenografts (CDXs) and patient-derived tumors (e.g., patient-derived xenografts (PDX)) to recapitulate the TME niche and evaluate human tumor immunity and immunotherapies (Q. X. Li et al., 2017; Wege, 2018). Human tumor cell lines to establish CDXs are widely available from commercial cell banks, and engraftment in immunodeficient mice is generally efficient, robust, and well-documented. However, expansion, continuous passaging, and adaption to 2D in vitro growth significantly increase genetic drift from the original patient tumor (Gillet et al., 2013; Guo et al., 2016). In comparison, PDXs are more reflective of human cancers, retaining the original tumor histo- and molecular pathology and heterogeneity, and mimicking the patient response to treatments (Guo et al., 2016; Tentler et al., 2012). These models are generated by transplanting patient tumor tissue directly into immunodeficient mice and expanding the tumor material in subsequent passages in vivo , thus removing the need for in vitro 2D culture (Gao et al., 2015; Yang et al., 2013). Despite the broad adoption of PDX models and their translational value, these can be costly, time-consuming, and low throughput. To mimic clinical studies, PDX can be enrolled as single-mouse surrogate subjects in population-based mouse clinical trials (MCTs), enabling the screening of large cohorts of patient tumors (Gao et al., 2015). This reduces the size and cost of PDX translational studies, increases the opportunity for biomarker identification and patient stratification (Guo et al., 2019), and provides opportunities for personalized medicine by way of patient “avatars” (Abdolahi et al., 2022). Thus, the application of PDX to HIS models is an extremely attractive opportunity, though several limitations must be considered before employing PDX models, as discussed in the next section.
Orthotopic or metastatic implantation of PDX or CDX tumors generates a more organ-specific TME (Fig. 1A); however, establishing these models is complicated, requiring regular imaging, such as optical imaging for bioluminescent-tagged xenograft models or MRI to track tumor growth, typically under anesthesia. Consequently, the adoption of simpler subcutaneous or systemic implantation by intravenous injection is more routine for HIS models. However, valuable insights into metastasis and TME can be gained with HIS models. Voillet et al. demonstrated a potential role for human macrophages in a melanoma CDX metastasis model established in a hCD34+ HSC MISTRG. However, this phenomenon was not observed in mice that lacked M-CSF (Voillet et al., 2022). In another study, orthotopic hepatocellular carcinoma (HCC) humanized mice were established by intrasplenic (i.s.) injection of tumor cells into hCD34+ HSC humanized NSG mice (Zhao et al., 2021) to create a more relevant TME for evaluation of human-specific monoclonal antibodies (mAbs) like pembrolizumab in combination with other mAbs such as bevacizumab and a small molecule STAT3 inhibitor.
A wide selection of tumor models is available for HIS modeling. Identifying the most relevant model can be facilitated by online model databases for CDX, PDX, and organoids (see internet resources) that have been profiled for genetic features, histopathology, and pharmacological characteristics. Alternatively, in vitro co-culture screens using immune cells with cell lines or organoids derived from PDXs (PDXOs) treated with immunotherapeutics could also identify models to progress to in vivo studies (Kumari et al., 2022; Xu et al., 2023) and facilitate the design of HIS models.
CHALLENGES AND LIMITATIONS OF HIS MODELS
Selection of the most appropriate immunodeficient mouse, humanization method, and tumor model are all essential for immunotherapeutic evaluation and achieving study aims (Martinov et al., 2021). The advantages and limitations are associated with complex features of the HIS model and must be considered before designing the study. One must first determine whether the functionality of the immune cells of interest has been validated after engraftment. It is then important to determine whether the longevity of the engrafted mice is sustainable. Finally, one must determine whether the reconstituted human immune system interacts with the tumor in a tumor-specific or alloreactive manner, or both. These factors determine the feasibility of an experimental approach and its translational potential for evaluating immunotherapeutics. Although not always reported, initial pilot studies in small cohorts of HIS mice can be conducted to characterize the selected mouse strain, humanization method, and tumor implantation protocols prior to immuno-therapeutic evaluation. The advantages and limitations of the huPBMC and CD34+ HSC models are summarized in Table 2.
Graft Versus Host Disease
Humanization-induced GvHD is a common limitation in the PBMC model and can impact the duration of a therapeutic study. However, the murine MHC class I deficient NSG B2m-/- mouse, which has a B2m (beta 2 immunoglobulin) deletion, presents a delayed GvHD onset, typically 2-3 weeks, in comparison to NSG mice (Brehm et al., 2019). As a result, the antigen-presenting cells (APCs) are deficient in murine MHC class I and limit the ability to stimulate human CD8+ T-cell proliferation and response and the CD4+ T-cell response. Although MHC class II deficiency also reduces APC stimulation of CD4+ T-cell proliferation, it does not confer resistance to GvHD; thus, MHC class I and II dKO enable murine tissue to remain undetected by human immune cells (Brehm et al., 2019; Yaguchi et al., 2018). In the BLT humanized model, T cells develop in a human thymic environment and recognize murine tissue, resulting in GvHD, limiting their use in long-term studies (Greenblatt et al., 2012).
Donor Variability
Allogeneic reactivity of donor hPBMCs can also lead to graft versus tumor (GvT) and GvHD. In vitro prescreening of donor PBMCs before establishing in vivo models may allow the identification of PBMCs with minimal alloreactive GvT to enable tumor establishment and target-related efficacy assessment. For instance, co-culture of multiple hPBMC donors across multiple tumors may identify which hPBMCs:tumor alloreactive combinations to avoid in vivo , thus saving time and resources and reducing, refining, and replacing (3Rs) any unnecessary animal work. However, an in vitro prescreen does not forecast the donor hPBMC impact on tumor growth kinetics or the variability of growth and therapeutic response, in which case a small in vivo pilot prescreen may be advisable before pursuing a larger study with multiple arms and donors. For example, we have observed slower growth kinetics for HCC827 NSCLC xenografts in hPBMC NSG mice compared to NSG mice. Pilot studies guided the determination of cohort size, optimal cell numbers for the HCC827 inoculum, and the timing and number of hPBMCs administered i.v. to optimize a study evaluating an immune checkpoint inhibitor (Zhang et al., 2020). HLA matching is an alternative option, although there is no consensus if this is the best approach.
Selecting a single hPBMC donor compared to multiple donors has different pros and cons. Using PBMCs from a single donor minimizes variability in tumor growth and therapeutic response and facilitates comparisons of response across multiple tumor models to represent tumor diversity and heterogeneity. However, a single donor may not represent immune diversity within a patient population and only a limited number of mice may be humanized based on the supply of PBMCs from a single patient. An ideal approach may be using multiple PBMC donors for each tumor model, and a lower number of tumor models may better represent immune diversity in therapeutic response, although the control and randomization of the humanized cohorts may be more complex. Either approach can be adopted based on the study objectives.
Similarly, for CD34+ HSCs obtained from either fetal liver or umbilical cord blood, donor-to-donor availability must be considered in study design, whereas the onset of GvHD is slower than the hPBMC model (Table 2), allowing slower-growing tumors like PDX to establish and grow to a minimum tumor size before randomization to treatment arms. Due to the limited population of CD34+ cells derived from a single source of human umbilical cord blood, typically, the number of mice that can be inoculated ranges from 10 to 15; therefore, multiple donors are required to establish the large cohorts needed for efficacy studies.
Advances in Modelling Innate Immunity
Both the PBMC and HSC models are limited in the reconstitution of the entire complement of human immune cell populations and their proportions and activity/maturation compared to humans. Most mouse strains, like the commonly used NSG and NOG, support the reconstitution of the human lymphoid compartment, but the lack of a human cytokine environment typically impairs the development of different lineages. For example, the PBMC model lacks myeloid engraftment and has a short-term natural killer (NK) and NK T-cell survival (Ye et al., 2020). In comparison, the HSC model has reasonable human T and B cell levels but low NK cell levels, perhaps due to the lack of human IL-2 and IL-15 essential for NK cell survival (Rongvaux et al., 2014). Thus, evaluating immunotherapeutics targeting the myeloid compartment requires alternative models.
Several mouse models expressing human cytokines have been developed to overcome the limitation of low myeloid cell engraftment (Table 1). The NSG-SGM3 mouse strain has been designed to express human IL-3, GM-CSF , and SCF transgenes which enable myeloid cell development following CD34+ cell engraftment (Billerbeck et al., 2011; Coughlan et al., 2016; Wunderlich et al., 2018). Humanized NSG-3GM mice, however, do suffer from shortened survival time and exhibit hematopoietic progenitor cell exhaustion. The hNOG EXL model, which also expresses human GM-CSF and IL-3 , engrafts human HSCs efficiently and is accompanied by the development of mast cells and granulocytes (Ito et al., 2013). Recent reports have shown that NOG-EXL HIS mice support myeloid, NK, and B cells, producing physiological IgG levels in contrast to humanized NOG mice (Maser et al., 2020). Notably, pDCs were detected in the tumor milieu of xenografted humanized NOG-EXL mice but not in humanized NOG mice. This suggests that human tumors can contribute to human cytokine expression, which shapes the functionality of human myeloid cell development in these models. Since pDC in solid tumors correlates with poor clinical outcomes, this model is important to investigate pro- and anti-tumoral human pDC activity in vivo. Other models that support the myeloid compartment include the BRGSF mouse, which, when boosted with the hFLT3 ligand, leads to enhanced human monocyte and DC development and increased human NK and T cells (Li et al., 2016). The MISTRG mouse was developed without the signal regulatory protein alpha (Sirpα) transgene and with human M-CSF, GM-CSF, IL-3 , and thrombopoietin (TPO) transgenes to enhance engraftment of HSCs and promote the development of human T and B lymphocytes and NK and myeloid cells (Rongvaux et al., 2014)
To improve human NK cell expansion, human IL-2 transgenic NOG mice (Katano et al., 2015) and human IL-7 and IL-15 double knock-in (dKI) NSG mice (Matsuda et al., 2019) have been produced for HSC-humanized mice. For PBMC-humanized models, human IL-15 transgenic NOG mice have been utilized to successfully produce mature NK cells (Katano et al., 2017). Transgenic expression of human IL-6 in NOG and MISTRG mice engrafted with HSCs enhanced immunosuppressive myeloid cell development (Hanazawa et al., 2018) and B cell class switching with antigen-specific IgG production (Yu et al., 2017).
IL-2 receptor common gamma chain (IL-2rg) is commonly knocked out in several mouse strains (Table 1) that lack functional lymph nodes. As a result, the adaptive immune response is impaired, which may impact the development of a functional adaptive immune response in humanized mice. To reestablish lymph node development, the NOG-pRORγt-yc/GM3 transgenic mouse expresses murine Il2rγ under the promoter of retinoic acid receptor-related orphan receptor gamma t (RORγt) together with the expression of human IL-2 and GM-CSF. This transgenic mouse shows improved T lymphocyte levels, antigen-specific IgG responses, and IL-21-producing CD4+ T cells in the lymph nodes in HSC models Takahashi et al., 2017).
PRECLINICAL APPLICATIONS OF HUMANIZED MICE FOR CANCER IMMUNOTHERAPY DRUG DISCOVERY
Despite the challenges highlighted above, progress has been made to improve HIS models and their translational value, yielding an abundant variety of HIS models from which to choose for immunotherapeutic studies, such as monoclonal antibodies, bi- or tri-specific T-cell engagers, adoptive cell therapies, small molecules, and oncolytic viruses.
Therapeutic Efficacy Studies
Despite their complexity and challenges, the PBMC and CD34+ HSC HIS models are attractive options for screening immunotherapeutic efficacy and evaluating antitumor immunity. Advances in HIS development have overcome many of these challenges and enabled the evaluation of different types of immunotherapeutic agents. In a typical efficacy evaluation, treatment is initiated when a tumor volume has been established, such as 80-200 mm3 for a subcutaneous tumor measured by calipers or a defined bioluminescent signal for optical imaging assessments. Intra-study tumor growth variability is common in HIS models due to the heterogeneity of the tumor models (in the case of PDX), model-based variability in tumor immunity, humanization method, and inter-donor variation. Due to the limited number of mice that can be inoculated per donor, randomization across treatment arms can be challenging. The most informative approaches to assigning mice to study arms are flow cytometry to determine baseline huCD45+ levels, tumor volume, and body weight measurements. Throughout treatment, the frequency of tumor size measurements should be consistent with the growth rate of the tumor and HIS models. For example, the huPBMC model will have a relatively short therapeutic window, therefore, 2-3 tumor measurements per week are appropriate. In contrast, the frequency for CD34+ HSC models will depend on the tumor growth rate. In parallel, body weight measurements should be taken frequently (2-3 times weekly), and animal health should be monitored daily to identify potential toxicities associated with immunotherapy, metastases, or onset of GvHD. Study endpoints should be predefined, such as maximum tumor size and body weight loss, according to local guidelines. For example, 2000 mm3 and no greater than 20% bodyweight loss or when a therapeutic response has been achieved if this occurs before a clinical endpoint. In addition to tumor burden assessment at termination, the peripheral immune response can be evaluated by flow cytometry analysis of the blood, bone marrow, and spleen. Within the TME, tumor-infiltrating immune cells can also be assessed by flow cytometry once the tumor is removed and dissociated or by IHC of FFPE tumors. However, this depends on the residual tumor size at termination. Flow cytometry quantitatively assesses different immune cells, whereas IHC provides a spatial evaluation of the immune cells relative to the surrounding cells. Both approaches provide insights into the responder or non-responder profile of the immunotherapeutic antitumor effect relative to the level of immune cell infiltration, activation, and proliferation.
Different HIS mouse models may respond differently to the same agents, such as specific ICI Abs, likely due to donor-to-donor variability in the immune cells used to reconstitute the models. Thus, the study design should include quantifying immune cell populations to correlate tumor immunity response with immune contexture. In addition, the therapeutic target and treatment modality will determine which model is most appropriate.
Immune checkpoint inhibitors and monoclonal antibodies
Human-specific mAbs, including the first-generation immune checkpoint inhibitors (ICI) targeting programmed cell death protein 1 (PD-1), its ligand PD-L1, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), and lymphocyte activation gene 3 (LAG3) have been evaluated in HIS models to determine their impact on both the immune system and the tumor, either alone as monotherapies or in combination. For example, pembrolizumab treatment targeting PD-1 significantly reduced NSCLC PDX and TNBC PDX/CDX tumor growth in CD34+ HSC NSG mice but not in NSG mice (Wang et al., 2018). Antibody depletion analysis demonstrated that the pembrolizumab response was mediated by hCD8+ T cells consistent with clinical observations. In a study using partially matched HCC-PDX, blockade of PD-1 by pembrolizumab or CTLA-4 with ipilimumab reactivated hCD8+ T cells, increased the M1/M2 macrophage ratio, and reduced tumor growth (Zhao et al., 2018).
Anti-PD-1 treatment showed similar efficacy in a model of hPBMCs NSG mice bearing NSCLC PDX (Pyo et al., 2019) and hCD34+ HIS models. Our labs have also extensively examined anti-PD-1 effects in multiple tumors and HIS models, such as SCC PDX in hCD34+ HSC NSG mice (Izadi et al., 2017) and huPBMC mice implanted with HCC827 NSCLC CDXs (Zhang et al., 2020). A panel of seven SCLC PDX models was inoculated into NSG mice humanized using five HSC donors in an n=1 MCT study design with pembrolizumab and a control arm (Izadi et al., 2017). Variable responses were seen across the cohorts of HSC donors and PDX, with an overall response rate comparable to clinical trial responses (Ott et al., 2015), highlighting the translational relevance of HIS models.
Nivolumab, also targeting PD-1, co-administered with urelumab (anti-CD137) slowed both CDX and PDX gastric cancer tumor models in an hPBMC model (Sanmamed et al., 2015). In another study, nivolumab did not affect osteosarcoma tumor growth in an hPBMC model but had a partial effect on lung metastasis due to increased CD8+ T-cell cytolytic activity in the lung, suggesting a possible role for nivolumab in treating metastatic osteosarcoma (Zheng et al., 2018).
Bi-specific antibodies T cell engagers
HIS models are also widely used to evaluate bi-specific and tri-specific antibodies targeting T cells to a tumor antigen. For example, CD-3 based bispecifics simultaneously bind CD3 on T cells, and tumor antigens such as CD20 expressed on lymphoma cells (Smith et al., 2015) or Claudin-6 on ovarian cells (Stadler et al., 2016), have been evaluated in hPBMC models. Similarly, hCD34+ HSC models were used to evaluate EpCAM/CD3 bispecific antibodies for colon carcinoma (Wulf-Goldenberg et al., 2011). A novel carcinoembryonic antigen T-cell bispecific antibody (CEA TCB) has been tested in huPBMC models, where they showed potent antitumor activity in poorly infiltrated, highly inflamed TME (Bacac et al., 2016). Targeting PMSA with CD3/PMSA bispecifics in prostate cancer using hPBMC humanized NSG mice eradicated prostate tumors and reduced lung metastases (Zekri et al., 2021). Another innovative bispecific co-targeting human PD-1 and PD-L1 was evaluated in hPBMC NGS mice implanted with lung cancer CDX, where a potent antitumor response was observed in comparison to monotherapy or combination therapy (Kotanides et al., 2020). Finally, a first-in-class bispecific HX009 targeting PD-1 and CD47 also showed enhanced killing in a lymphoma tumor in a hPBMC model compared to anti-PD-1 mAb (Ke et al., 2023).
NK cell and cytokine-based immunotherapy
Modulation of the adaptive immune system is a promising approach for immunotherapy. Early humanized models using NSG, NRG, NOG, and BRGS mice did not support the development of human innate immunity due to the lack of human cytokines and minimal cross-species reactivity of mouse cytokines in vivo. Several recent developments in the transgenic expression of human cytokines in mice have overcome this limitation and enabled the development of innate immunity in HIS models, such as natural killer (NK) cells, which are cytotoxic effector lymphocytes that produce proinflammatory cytokines and chemokines (Bald et al., 2020; Wolf et al., 2023). NK cells have enormous potential for antitumor immunity and progress is being made to stimulate both NK and NKT cells with mAb or cytokine therapies.
A critical mechanism of therapeutic antibody-mediated killing of tumor cells is antibody-dependent cellular cytotoxicity (ADCC), mediated predominately by NK cells comprising 10% to 20% of PBMCs. NK cells recognize the IgG1 antibody via Fc receptors and attack the target cell. ADCC activity can be measured in vitro by admixing human NK cells with tumor cells and therapeutic antibodies, but the mouse and human NK cell responses may differ in vivo ; therefore, humanization is required. The anti-CD52 human antibody alemtuzumab activates NK cell-mediated ADCC in hCD34+ HSC implanted with lymphoma (Leskov et al., 2013). Similarly, anti-CCR4 human antibody ADCC activity was demonstrated in both hPBMCs in NOG mice with lymphoma (Ito et al., 2009) and hCD34+ HSC in NOG IL-2 Tg mice with Hodgkin's lymphoma (Katano et al., 2015).
Proinflammatory cytokine administration boosts the antitumor function of effector immune cells to remove tumors. The numerous immunostimulatory cytokines include IL-2, IL-7, IL-12, IL-15, IL-18, IL-21, TNFα, IFNβ, and GM-CSF; thus, cytokine therapies can produce antitumor immunity via different mechanisms. A study in breast cancer CDX implanted in NGS hCD34+ HSC mice showed co-administration of trastuzumab and IL-15 enhanced tumor eradication. However, fatal adverse effects associated with T-cell hyperactivation due to trastuzumab-induced elevated CD44 expression were also observed with increased liver and lung metastases (Wege et al., 2017).
Novel IL-15-based trispecific killer engagers, which include a single chain scFV against CD16 and CD133, bridge NK cells and CD133+ myeloid targets, such as HL-60 leukemia, to enhance NK cell expansion, priming, and survival. This effect was demonstrated in NSG mice transplanted with allogeneic human NK cells purified from PBMCs, and the reduction in systemic HL-60 tumor burden was elegantly demonstrated via optical imaging following i.v. injection of bioluminescent cells (Vallera et al., 2016).
Oncolytic viruses
A wide range of oncolytic DNA and RNA viruses are in development for selective targeting of tumor cells for eradication via immunogenic cell death, releasing tumor-specific antigens and eliciting abscopal effects. Combinations with immunotherapy are actively pursued in clinical trials and preclinical studies (Shi et al., 2020). Talimogene laherparepvec was the first oncolytic virus approved by the FDA in 2017 for advanced metastatic melanoma. The approach relies on GM-CSF to recruit and mature antigen-presenting cells (APCs) like dendritic cells (DCs) (Corrigan et al., 2017). A recent study described arming Torque Teno oncolytic virus (TTV) with IL-21 in lung carcinoma tumors alone or in combination with chimeric antigen receptor T (CART) or invariant natural killer T (iNKT) cell therapy in a humanized mouse model. The results showed synergistic effects of the combination therapy (Chen et al., 2021). A combination of pembrolizumab with immunogenic oncolytic adenovirus ONCOS-102 exhibited synergistic antitumor and abscopal effects in an HSC humanized A2058 melanoma huNOG mouse model (Kuryk, Møller & Jaderberg, 2019, 2019b).
T cell editing and adoptive cellular therapy
Chimeric antigen receptors (CARs) are engineered cell surface-activating receptors that recognize specific antigens or markers on the surface of tumor cells. Patient T or NK cells can be isolated and engineered to express a CAR and then injected back into the patient as autologous adoptive cell therapies. The first CAR-T cell therapy, tisagenlecleucel, targeting the CD19 antigen, was approved in 2017 for the treatment of patients up to 25 years of age with relapsed and/or refractory B-cell precursor acute lymphoblastic leukemia (ALL) (Maude et al., 2018). This advancement was followed by axicabtagene ciloleucel a few months later, targeting CD19 in adults with relapsed and/or refractory large B-cell lymphoma (Neelapu et al., 2017). The efficacy of CAR-T cells targeting a variety of tumor markers is routinely evaluated in immunodeficient models that are implanted with human tumors and allogeneic human CAR T/NK cells and have been extensively reviewed elsewhere (Kumari et al., 2021; Mhaidly & Verhoeyen, 2020). Despite the success of CAR-T cell therapies in the clinic, resulting in durable responses in many patients with hematological cancers, limitations include poor infiltration in solid tumors, immune suppression, lack of long-term persistence, antigen escape, off-tumor toxicities, and cytokine release syndrome (CRS), requiring further investigation in preclinical models. However, adverse events, such as off-tumor toxicity, cytokine release syndrome, or neurotoxicity observed in the clinic, are not reproduced in xenograft mice, perhaps due to the lack of human target expression in normal mouse tissues, including other human immune cell populations. CAR-T evaluation in HIS models enables both efficacy and safety to be evaluated. The utility of HIS mice in safety and toxicology is discussed below. To refine the preclinical evaluation of CAR-T responses, Jin et al. established a humanized mouse model in NSG mice with genetically matched (autologous) primary acute B-lymphoblastic leukemia (B-ALL) that enabled CD19-targeted CAR T cell therapy to be evaluated in immunocompetent hosts without allogeneic or xenogeneic immune responses (Jin et al., 2019).
Preclinical Safety and Toxicology
Predictions of toxicity are critical in cancer drug discovery. Therapies targeting T cells, including cell therapies, can cause unwanted side effects such as immune-related adverse events (irAEs) that range from organ-specific toxicity to a systemic inflammatory response known as cytokine release syndrome (CRS) (Liu et al., 2023; Pallin et al., 2018; Tay et al., 2022). Typical irAEs include pneumonia, hepatitis, colitis, nephritis, dermatitis, encephalitis, and rheumatologic complications (Abdel-Wahab et al., 2018; Kazandjian et al., 2016; Weaver et al., 2019). Traditional mouse models and even non-human primates predominantly failed to predict these adverse events due to interspecies differences in their immune systems.
HIS mice have proven useful preclinical models to study and predict irAEs. For example, high-dose IL-2 (HDIL2) that was initially approved by the FDA for metastatic renal cell carcinoma (mRCC) and metastatic melanoma is associated with irAEs. Li et al. showed that clinical irAEs can be recapitulated in BRGS mice humanized with CD34+ cells, where HDIL2 treatment leads to severe morbidity and high mortality (Li et al., 2017). These investigators demonstrated that the IL-2 toxicity was mediated by the human T cells due to a decrease in Treg homeostasis and function in humanized mice. Kähkönen et al. observed that CD34+ humanized mice engrafted with human breast cancer and supplemented with estradiol suffered severe anemia, which was amplified under pembrolizumab (anti-PD-1) and impacted survival (Kähkönen et al., 2020). Anemia is among the most common adverse effects in patients receiving anti-PD-1 therapy (Kim et al., 2014; Langer et al., 2016). Weaver et al. studied nivolumab (anti-PD-1) irAEs in a BLT humanized mouse model in NOG and transgenic hGM-CSF and IL-3 NOG (NOG-EXL) mice (Weaver et al., 2019). Despite significantly reduced survival in BLT-NOG versus BLT-NOG-EXL mice, similar irAEs were observed in both humanized strains, similar to clinical observations.
Immunotoxicity and side effects of anti-CTLA4 (ipilimumab) have also been shown in CD34+ mice (Zhao et al., 2018) engrafted with HCC PDX models, consistent with clinical data. In this study, mice treated with ipilimumab showed significant weight loss, while the control and anti-PD-1 (pembrolizumab) groups remained healthy. The pathological analysis confirmed that the group treated with ipilimumab developed massive cell infiltration and liver, lung, and kidney damage that was not observed in the control and pembrolizumab-treated groups.
Among irAEs, life-threatening inflammatory toxicities such as CRS and immune effector cell-associated neurotoxicity syndrome (ICANS) are observed following the administration of antibodies and adoptive T-cell therapy (Morris et al., 2022; Siegler & Kenderian, 2020). An unfortunate example of under-prediction of toxicity was observed with the CD28 superagonist mAb TGN1412 (Eastwood et al., 2010). In this case, there was a lack of CD28 expression on the CD4+ effector memory T cells in non-human primates used for preclinical safety, thus failing to predict CRS. More recently, it was shown that CRS and lymphopenia occurred in PBMC humanized mice treated with TGN1412 and the anti-CD3, OKT3 (Weißmüller et al., 2016).
CRS induction upon activation with anti-CD3 antibodies has also been described in CD34+ BRGFS mice (Martin-Jeantet et al., 2022). Anti-hCD3 mAb (OKT3) administration in BRGSF-HIS mice induced a rapid release of human cytokines (i.e., IL-6, TNF-α, IFN-γ, IL-2) in serum. Pretreatment with Flt3L, enhancing the human myeloid compartment, boosted the production of cytokines. CRS clinical signs such as hypothermia and weight loss were reproduced in OKT3-injected BRGSF-HIS mice. Pretreatment with Tocilizumab (an anti-IL-6R) reduced cytokine production, suggesting a strong role of myeloid cells in the pathogenesis of CRS.
The safety of CAR-T cell therapy is frequently associated with treatment-related adverse reactions, including CRS and neurotoxicity (Schubert et al., 2021). Monocyte-derived IL-1 and IL-6 appear to be required for CRS and neurotoxicity in CAR-T therapies (Norelli et al., 2018; Xue et al., 2021), underlining the importance of the myeloid cell compartment in CRS, which was also described in the BRGSF-HIS mice boosted with Flt3L.
Norelli et al. showed that the major toxicities induced by CD19 CAR T cells in humans, including long-lasting B cell aplasia, severe CRS, and lethal neurotoxicity, can be recapitulated in CD34+ NSG-SGM3 humanized mice (Norelli et al., 2018). These investigators tested CD19 CAR T and CAR T specific for CD44v6, an antigen overexpressed on acute myeloid leukemia (AML) and multiple myeloma (MM), as well as on circulating monocytes. CD19 CAR T and CD44v6 CAR T produced similarly severe toxicity, including CRS and neurotoxicity, driven by IL-1 and IL-6, reversed by anti-IL-1 treatment.
On-target off-tumor effects are also a major safety concern for CAR-T targeting the central nervous system (CNS). For example, CAR T cells targeting GD2, a ganglioside antigen expressed on the surface of several solid cancer such as neuroblastoma, glioma, cervical cancer, and sarcoma, showed good efficacy in clinical studies without significant safety concerns (Del Bufalo et al., 2023; Majzner et al., 2022). However, GD2 is also expressed at low levels in healthy neurons, skin melanocytes, and peripheral nerves (Doronin et al., 2014), increasing the risk of CNS toxicity. Richman et al. used an enhanced version of GD2 CAR-T by introducing a single point mutation in the anti-GD2 scFv, improving antitumor efficacy against GD2+ human neuroblastoma in NSG mice (Richman et al., 2018). Despite the enhanced efficacy, CAR-T infiltration and proliferation were associated with neuronal destruction. Although this study was conducted in a non-humanized mouse model, this observation illustrates that the ability to study on-target off-tumor effects would be enhanced in the context of humanized mice. However, a lack of fully functional crosstalk between human immune cells and mouse non-immune cells can undermine endothelium and stromal cell contributions in CRS and neurotoxicity.
FUTURE PERSPECTIVES
The advances in humanized mouse models have generated powerful preclinical tools for assessing the efficacy and safety of immunotherapeutics. However, the establishment of HSC humanized mice continues to rely on the isolation of allogeneic primary human CD34+ hematopoietic progenitor cells, which are generally isolated from human bone marrow, cord blood, or fetal liver, and the objective of generating a fully functional human T cell compartment remains particularly ambitious. The challenges of using primary stem cells in constructing these HIS mice include matching HLA antigens to prevent alloreactivity, GvHD disease, and viral/fungal contamination of primary HSCs. HIS mice engrafted with autologous human immune cells (PBMCs) and matched PDXs could create a suitable “avatar” approach with HLA-restricted tumor-specific T-cell responses that could predict a patient's response to immunotherapies for a personalized treatment approach, which has been demonstrated for ICI in a patient with melanoma (Jespersen et al., 2017). Unfortunately, the number of patients who will survive long enough to benefit from their avatar is relatively low, as the time to establish PDX does not correlate favorably with patient survival. Induced pluripotent stem cells (iPSCs) are created by genetically reprogramming somatic differentiated cells (Takahashi et al., 2007; Yu et al., 2007). Utilizing embryonic stem cells (ESCs) and iPSCs to generate hematopoietic stem cells (HSCs) would circumvent many technical and ethical issues associated with the isolation of human HSCs and would allow the generation of a renewable HSC source of a defined genetic background potentially generating an autologous system for PDX studies if both the tumor and skin biopsy were taken from the same patient. Although the generation of human HSCs iPSCs/ESCs has been elusive, recent reports have demonstrated success in iPSC-based models (Zeleniak et al., 2022). T cells engrafted with iPSC-derived thymus organoids generated both cellular and humoral responses, including induction of proinflammatory responses and inhibition of allogeneic tumor graft growth (Zeleniak et al., 2022). Human intestinal organoids (HIOs) derived from pluripotent stem cells that were subsequently engrafted under the kidney capsule of humanized mice created a microenvironment resembling human intestinal lymphoid follicles containing T and B cells and GALT-like structures (Bouffi et al., 2023). Intestine-immune crosstalk was detected in these mice and microbial exposure activated immune cells. HIO-humanized mouse models enable the study of intestinal infections, food allergies, and the development of mucosal vaccines.
Further challenges in humanized mouse models can arise when it is necessary to achieve orthotopic tumor implantation, model advanced metastatic disease stages, or evaluate the efficacy of combination therapies, all of which require additional knowledge and experience in executing meaningful studies. Evaluation of ICIs, such as the anti-PD-L1 mAb durvalumab, with radiotherapy has yielded promising and safe results in patients with advanced NSCLC, indicating radiotherapy is a potent immunomodulator that enhances the antitumor response of ICI (Altorki et al., 2021). Radiotherapy combination studies in HIS models have been limited but could help guide effective combination strategies for immunotherapeutics, radiotherapy, and biomarker identification. Ames et al. effectively demonstrated a multi-modal approach using adoptive NK cell therapy and radiotherapy to treat tumors and resident CSCs, leading to significant and durable responses in various solid tumors and metastases as assessed by bioluminescent imaging (Ames et al., 2015). Recapitulating the primary or metastatic TME in humanized mouse models has great potential in understanding resistance mechanisms and disease progression and assessing abscopal effects in preclinical oncology studies.
CONCLUDING REMARKS
The utility of HIS models has increased in the last decade due to immunotherapeutic discoveries and development, but these require preclinical models that capture the human immune-tumor interaction more faithfully than simple immunocompetent and immune-deficient systems. Reconstituted HIS models are complex and require careful consideration and implementation specific to the immunotherapy under preclinical evaluation. Understanding these limitations has led to a new generation of models, providing better insights into the therapeutic modulation of lymphoid and myeloid compartments, the TME, and identifying irAEs. No single HIS model can fully replicate the patient in a mouse, but a wider selection of HIS models from and continuous advancements position humanized mice as promising tools for accelerating immunotherapeutic drug discovery, providing valuable insights into translational efficacy and toxicity, and perhaps advancing personalized medicine approaches in oncology.
Author Contributions
Rajendra Kumari : Conceptualization, data curation, original draft writing, review, and editing; Gerold Feuer : Conceptualization, original draft writing; Ludovic Bourré : Conceptualization, original draft writing, review, and editing.
Conflict of Interest
All authors are employees of Crown Bioscience, Inc.
Open Research
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Literature Cited
- Abdel-Wahab, N., Shah, M., Lopez-Olivo, M. A., & Suarez-Almazor, M. E. (2018). Use of Immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: A systematic review. Annals of Internal Medicine , 168(2), 121–130. https://doi.org/10.7326/m17-2073
- Abdolahi, S., Ghazvinian, Z., Muhammadnejad, S., Saleh, M., Asadzadeh Aghdaei, H., & Baghaei, K. (2022). Patient-derived xenograft (PDX) models, applications and challenges in cancer research. Journal of Translational Medicine , 20(1), 206. https://doi.org/10.1186/s12967-022-03405-8
- Aldrovandi, G. M., Feuer, G., Gao, L., Jamieson, B., Kristeva, M., Chen, I. S. Y., & Zack, J. A. (1993). The SCID-hu mouse as a model for HIV-1 infection. Nature , 363(6431), 732–736. https://doi.org/10.1038/363732a0
- Ali, N., Flutter, B., Sanchez Rodriguez, R., Sharif-Paghaleh, E., Barber, L. D., Lombardi, G., & Nestle, F. O. (2012). Xenogeneic graft-versus-host-disease in NOD-scid IL-2Rγnull mice display a T-effector memory phenotype. PLoS ONE , 7(8), e44219. https://doi.org/10.1371/journal.pone.0044219
- Altorki, N. K., Mcgraw, T. E., Borczuk, A. C., Saxena, A., Port, J. L., Stiles, B. M., Lee, B. E., Sanfilippo, N. J., Scheff, R. J., Pua, B. B., Gruden, J. F., Christos, P. J., Spinelli, C., Gakuria, J., Uppal, M., Binder, B., Elemento, O., Ballman, K. V., & Formenti, S. C. (2021). Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: A single-centre, randomised phase 2 trial. The Lancet Oncology , 22(6), 824–835. https://doi.org/10.1016/s1470-2045(21)00149-2
- Ames, E., Canter, R. J., Grossenbacher, S. K., Mac, S., Smith, R. C., Monjazeb, A. M., Chen, M., & Murphy, W. J. (2015). Enhanced targeting of stem-like solid tumor cells with radiation and natural killer cells. Oncoimmunology , 4(9), e1036212. https://doi.org/10.1080/2162402x.2015.1036212
- Aspeslagh, S., Postel-Vinay, S., Rusakiewicz, S., Soria, J. C., Zitvogel, L., & Marabelle, A. (2016). Rationale for anti-OX40 cancer immunotherapy. European Journal of Cancer , 52, 50–66. https://doi.org/10.1016/j.ejca.2015.08.021
- Bacac, M., Klein, C., & Umana, P. (2016). CEA TCB: A novel head-to-tail 2:1 T cell bispecific antibody for treatment of CEA-positive solid tumors. Oncoimmunology , 5(8), e1203498. https://doi.org/10.1080/2162402x.2016.1203498
- Bald, T., Krummel, M. F., Smyth, M. J., & Barry, K. C. (2020). The NK cell–cancer cycle: Advances and new challenges in NK cell–based immunotherapies. Nature Immunology , 21(8), 835–847. https://doi.org/10.1038/s41590-020-0728-z
- Bareham, B., Georgakopoulos, N., Matas-Céspedes, A., Curran, M., & Saeb-Parsy, K. (2021). Modeling human tumor-immune environments in vivo for the preclinical assessment of immunotherapies. Cancer Immunology, Immunotherapy , 70(10), 2737–2750. https://doi.org/10.1007/s00262-021-02897-5
- Beztsinna, N., Meesters, N., Daszkiewicz, L., Grillet, F., van der Meer, D., Yan, K., Spanjaard, E., Vader, W., & Price, L. (2022). Abstract 2059: Immunotherapy testing in 3D Ex vivo patient tissue platform with preserved tumor microenvironment. Cancer Research , 82(12_Supplement), 2059. https://doi.org/10.1158/1538-7445.Am2022-2059
- Billerbeck, E., Barry, W. T., Mu, K., Dorner, M., Rice, C. M., & Ploss, A. (2011). Development of human CD4+FoxP3+ regulatory T cells in human stem cell factor-, granulocyte-macrophage colony-stimulating factor-, and interleukin-3-expressing NOD-SCID IL2Rγ(null) humanized mice. Blood , 117(11), 3076–3086. https://doi.org/10.1182/blood-2010-08-301507
- Bouffi, C., Wikenheiser-Brokamp, K. A., Chaturvedi, P., Sundaram, N., Goddard, G. R., Wunderlich, M., Brown, N. E., Staab, J. F., Latanich, R., Zachos, N. C., Holloway, E. M., Mahe, M. M., Poling, H. M., Vales, S., Fisher, G. W., Spence, J. R., Mulloy, J. C., Zorn, A. M., Wells, J. M., & Helmrath, M. A. (2023). In vivo development of immune tissue in human intestinal organoids transplanted into humanized mice. Nature Biotechnology , 41, 824–831. https://doi.org/10.1038/s41587-022-01558-x
- Brainard, D. M., Seung, E., Frahm, N., Cariappa, A., Bailey, C. C., Hart, W K., Shin, H. S., Brooks, S. F., Knight, H. L., Eichbaum, Q., Yang, Y. G., Sykes, M., Walker, B. D., Freeman, G J., Pillai, S., Westmoreland, S. V., Brander, C., Luster, A. D., & Tager, A. M. (2009). Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. Journal of Virology , 83(14), 7305–7321. https://doi.org/10.1128/jvi.02207-08
- Brehm, M. A., Jouvet, N., Greiner, D. L., & Shultz, L. D. (2013). Humanized mice for the study of infectious diseases. Current Opinion in Immunology , 25(4), 428–435. https://doi.org/10.1016/j.coi.2013.05.012
- Brehm, M. A., Kenney, L. L., Wiles, M. V., Low, B. E., Tisch, R. M., Burzenski, L., Mueller, C., Greiner, D. L., & Shultz, L. D. (2019). Lack of acute xenogeneic graft- versus-host disease, but retention of T-cell function following engraftment of human peripheral blood mononuclear cells in NSG mice deficient in MHC class I and II expression. Faseb Journal , 33(3), 3137–3151. https://doi.org/10.1096/fj.201800636R
- Chen, D. S., & Mellman, I. (2017). Elements of cancer immunity and the cancer-immune set point. Nature , 541(7637), 321–330. https://doi.org/10.1038/nature21349
- Chen, T., Ding, X., Liao, Q., Gao, N., Chen, Y., Zhao, C., Zhang, X., & Xu, J. (2021). IL-21 arming potentiates the anti-tumor activity of an oncolytic vaccinia virus in monotherapy and combination therapy. Journal for ImmunoTherapy of Cancer , 9(1), e001647. https://doi.org/10.1136/jitc-2020-001647
- Corrigan, P. A., Beaulieu, C., Patel, R. B., & Lowe, D. K. (2017). Talimogene laherparepvec: An oncolytic virus therapy for melanoma. Annals of Pharmacotherapy , 51(8), 675–681. https://doi.org/10.1177/1060028017702654
- Coughlan, A. M., Harmon, C., Whelan, S., O'brien, E. C., O'reilly, V. P., Crotty, P., Kelly, P., Ryan, M., Hickey, F. B., O'farrelly, C., & Little, M. A. (2016). Myeloid engraftment in humanized mice: Impact of granulocyte-colony stimulating factor treatment and transgenic mouse strain. Stem Cells and Development , 25(7), 530–541. https://doi.org/10.1089/scd.2015.0289
- Del Bufalo, F., de Angelis, B., Caruana, I., Del Baldo, G., de Ioris, M. A., Serra, A., Mastronuzzi, A., Cefalo, M. G., Pagliara, D., Amicucci, M., Li Pira, G., Leone, G., Bertaina, V., Sinibaldi, M., Di Cecca, S., Guercio, M., Abbaszadeh, Z., Iaffaldano, L., Gunetti, M., … Locatelli, F. (2023). GD2-CART01 for relapsed or refractory high-risk neuroblastoma. New England Journal of Medicine , 388(14), 1284–1295. https://doi.org/10.1056/NEJMoa2210859
- Deruaz, M., Murooka, T. T., Ji, S., Gavin, M. A., Vrbanac, V. D., Lieberman, J., Tager, A. M., Mempel, T. R., & Luster, A. D. (2017). Chemoattractant-mediated leukocyte trafficking enables HIV dissemination from the genital mucosa. JCI Insight , 2(7), e88533. https://doi.org/10.1172/jci.insight.88533
- Doronin, I. I., Vishnyakova, P. A., Kholodenko, I. V., Ponomarev, E. D., Ryazantsev, D. Y., Molotkovskaya, I. M., & Kholodenko, R. V. (2014). Ganglioside GD2 in reception and transduction of cell death signal in tumor cells. BMC Cancer , 14(1), 295. https://doi.org/10.1186/1471-2407-14-295
- Durost, P. A., Aryee, K. E., Manzoor, F., Tisch, R. M., Mueller, C., Jurczyk, A., Shultz, L. D., & Brehm, M. A. (2018). Gene therapy with an adeno-associated viral vector expressing human interleukin-2 alters immune system homeostasis in humanized mice. Human Gene Therapy , 29(3), 352–365. https://doi.org/10.1089/hum.2017.072
- Eastwood, D., Findlay, L., Poole, S., Bird, C., Wadhwa, M., Moore, M., Burns, C., Thorpe, R., & Stebbings, R. (2010). Monoclonal antibody TGN1412 trial failure explained by species differences in CD28 expression on CD4+ effector memory T-cells. British Journal of Pharmacology , 161(3), 512–526. https://doi.org/10.1111/j.1476-5381.2010.00922.x
- Gao, H., Korn, J. M., Ferretti, S., Monahan, J. E., Wang, Y., Singh, M., Zhang, C., Schnell, C., Yang, G., Zhang, Y., Balbin, O. A., Barbe, S., Cai, H., Casey, F., Chatterjee, S., Chiang, D. Y., Chuai, S., Cogan, S. M., Collins, S. D., … Sellers, W. R. (2015). High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nature Medicine , 21(11), 1318–1325. https://doi.org/10.1038/nm.3954
- Gillet, J. P., Varma, S., & Gottesman, M. M. (2013). The clinical relevance of cancer cell lines. JNCI: Journal of the National Cancer Institute , 105(7), 452–458. https://doi.org/10.1093/jnci/djt007
- Greenblatt, M. B., Vbranac, V., Tivey, T., Tsang, K., Tager, A. M., & Aliprantis, A. O. (2012). Graft versus host disease in the bone marrow, liver and thymus humanized mouse model. PLoS ONE , 7(9), e44664. https://doi.org/10.1371/journal.pone.0044664
- Grover, A., Troy, A., Rowe, J., Troudt, J. M., Creissen, E., Mclean, J., Banerjee, P., Feuer, G., & Izzo, A. A. (2017). Humanized NOG mice as a model for tuberculosis vaccine-induced immunity: A comparative analysis with the mouse and guinea pig models of tuberculosis. Immunology , 152(1), 150–162. https://doi.org/10.1111/imm.12756
- Guo, S., Jiang, X., Mao, B., & Li, Q. X. (2019). The design, analysis and application of mouse clinical trials in oncology drug development. BMC Cancer , 19(1), 718. https://doi.org/10.1186/s12885-019-5907-7
- Guo, S., Qian, W., Cai, J., Zhang, L., Wery, J. P., & Li, Q. X. (2016). Molecular pathology of patient tumors, patient-derived xenografts, and cancer cell lines. Cancer Research , 76(16), 4619–4626. https://doi.org/10.1158/0008-5472.CAN-15-3245
- Hanazawa, A., Ito, R., Katano, I., Kawai, K., Goto, M., Suemizu, H., Kawakami, Y., Ito, M., & Takahashi, T. (2018). Generation of human immunosuppressive myeloid cell populations in human interleukin-6 transgenic NOG mice. Frontiers in Immunology , 9, 152. https://doi.org/10.3389/fimmu.2018.00152
- Hayakawa, J., Hsieh, M. M., Uchida, N., Phang, O., & Tisdale, J. F. (2009). Busulfan produces efficient human cell engraftment in NOD/LtSz-Scid IL2RγNull Mice. Stem Cells , 27(1), 175–182. https://doi.org/10.1634/stemcells.2008-0583
- Hesselton, R. M., Greiner, D. L., Mordes, J. P., Rajan, T. V., Sullivan, J. L., & Shultz, L. D. (1995). High levels of human peripheral blood mononuclear cell engraftment and enhanced susceptibility to human immunodeficiency virus type 1 infection in NOD/LtSz-scid/scid mice. Journal of Infectious Diseases , 172(4), 974–982. https://doi.org/10.1093/infdis/172.4.974
- Ito, A., Ishida, T., Yano, H., Inagaki, A., Suzuki, S., Sato, F., Takino, H., Mori, F., Ri, M., Kusumoto, S., Komatsu, H., Iida, S., Inagaki, H., & Ueda, R. (2009). Defucosylated anti-CCR4 monoclonal antibody exercises potent ADCC-mediated antitumor effect in the novel tumor-bearing humanized NOD/Shi-scid, IL-2Rgamma(null) mouse model. Cancer Immunology, Immunotherapy , 58(8), 1195–1206. https://doi.org/10.1007/s00262-008-0632-0
- Ito, M., Hiramatsu, H., Kobayashi, K., Suzue, K., Kawahata, M., Hioki, K., Ueyama, Y., Koyanagi, Y., Sugamura, K., Tsuji, K., Heike, T., & Nakahata, T. (2002). NOD/SCID/γcnull mouse: An excellent recipient mouse model for engraftment of human cells. Blood , 100(9), 3175–3182. https://doi.org/10.1182/blood-2001-12-0207
- Ito, R., Takahashi, T., Katano, I., Kawai, K., Kamisako, T., Ogura, T., Ida-Tanaka, M., Suemizu, H., Nunomura, S., Ra, C., Mori, A., Aiso, S., & Ito, M. (2013). Establishment of a human allergy model using human IL-3/GM-CSF-transgenic NOG mice. Journal of Immunology , 191(6), 2890–2899. https://doi.org/10.4049/jimmunol.1203543
- Izadi, H., Broudy, T., Yan, D., & Thatte, J. (2017). Abstract 4707: Evaluation of efficacy and immune response to PD1 checkpoint inhibition in human immune-reconstituted mice using patient-derived xenograft models. Cancer Research , 77(13_Supplement), 4707–4707. https://doi.org/10.1158/1538-7445.Am2017-4707
- Jespersen, H., Lindberg, M. F., Donia, M., Söderberg, E. M. V., Andersen, R., Keller, U., Ny, L., Svane, I. M., Nilsson, L. M., & Nilsson, J. A. (2017). Clinical responses to adoptive T-cell transfer can be modeled in an autologous immune-humanized mouse model. Nature Communications , 8(1), 707. https://doi.org/10.1038/s41467-017-00786-z
- Jin, C. H., Xia, J., Rafiq, S., Huang, X., Hu, Z., Zhou, X., Brentjens, R. J., & Yang, Y. G. (2019). Modeling anti-CD19 CAR T cell therapy in humanized mice with human immunity and autologous leukemia. EBioMedicine , 39, 173–181. https://doi.org/10.1016/j.ebiom.2018.12.013
- Kähkönen, T. E., Halleen, J. M., & Bernoulli, J. (2020). Immunotherapies and metastatic cancers: Understanding utility and predictivity of human immune cell engrafted mice in preclinical drug development. Cancers , 12(6), 1615. https://doi.org/10.3390/cancers12061615
- Katano, I., Nishime, C., Ito, R., Kamisako, T., Mizusawa, T., Ka, Y., Ogura, T., Suemizu, H., Kawakami, Y., Ito, M., & Takahashi, T. (2017). Long-term maintenance of peripheral blood derived human NK cells in a novel human IL-15- transgenic NOG mouse. Scientific Reports , 7(1), 17230. https://doi.org/10.1038/s41598-017-17442-7
- Katano, I., Takahashi, T., Ito, R., Kamisako, T., Mizusawa, T., Ka, Y., Ogura, T., Suemizu, H., Kawakami, Y., & Ito, M. (2015). Predominant development of mature and functional human NK cells in a novel human IL-2-producing transgenic NOG mouse. Journal of Immunology , 194(7), 3513–3525. https://doi.org/10.4049/jimmunol.1401323
- Kazandjian, D., Suzman, D. L., Blumenthal, G., Mushti, S., He, K., Libeg, M., Keegan, P., & Pazdur, R. (2016). FDA Approval Summary: Nivolumab for the treatment of metastatic non-small cell lung cancer with progression on or after platinum-based chemotherapy. The Oncologist , 21(5), 634–642. https://doi.org/10.1634/theoncologist.2015-0507
- Ke, H., Zhang, F., Wang, J., Xiong, L., An, X., Tu, X., Chen, C., Wang, Y., Mao, B., Guo, S., Ju, C., He, X., Sun, R., Zhang, L., O'connor, O. A., & Li, Q. X. (2023). HX009, a novel BsAb dual targeting PD1 x CD47, demonstrates potent anti-lymphoma activity in preclinical models. Scientific Reports , 13(1), 5419. https://doi.org/10.1038/s41598-023-32547-y
- Kim, A., Rivera, S., Shprung, D., Limbrick, D., Gabayan, V., Nemeth, E., & Ganz, T. (2014). Mouse models of anemia of cancer. PLoS ONE , 9(3), e93283. https://doi.org/10.1371/journal.pone.0093283
- King, M. A., Covassin, L., Brehm, M. A., Racki, W., Pearson, T., Leif, J., Laning, J., Fodor, W., Foreman, O., Burzenski, L., Chase, T. H., Gott, B., Rossini, A. A., Bortell, R., Shultz, L. D., & Greiner, D. L. (2009). Human peripheral blood leucocyte non-obese diabetic-severe combined immunodeficiency interleukin-2 receptor gamma chain gene mouse model of xenogeneic graft-versus-host-like disease and the role of host major histocompatibility complex. Clinical and Experimental Immunology , 157(1), 104–118. https://doi.org/10.1111/j.1365-2249.2009.03933.x
- Kotanides, H., Li, Y., Malabunga, M., Carpenito, C., Eastman, S. W., Shen, Y., Wang, G., Inigo, I., Surguladze, D., Pennello, A. L., Persaud, K., Hindi, S., Topper, M., Chen, X., Zhang, Y., Bulaon, D. K., Bailey, T., Lao, Y., Han, B., … Kalos, M. (2020). Bispecific Targeting of PD-1 and PD-L1 Enhances T-cell Activation and Antitumor Immunity. Cancer Immunology Research , 8(10), 1300–1310. https://doi.org/10.1158/2326-6066.Cir-20-0304
- Kumari, R., Ouyang, X., Wang, J., Xu, X., Zheng, M., An, X., & Li, Q. X. (2021). Preclinical pharmacology modeling of chimeric antigen receptor T therapies. Current Opinion in Pharmacology , 61, 49–61. https://doi.org/10.1016/j.coph.2021.08.008
- Kumari, R., Xu, X., & Li, H. Q. X. (2022). Translational and clinical relevance of PDX-derived organoid models in oncology drug discovery and development. Current Protocols , 2(7), e431. https://doi.org/10.1002/cpz1.431
- Kuryk, L., Møller, A.-S. W., & Jaderberg, M. (2019a). Abscopal effect when combining oncolytic adenovirus and checkpoint inhibitor in a humanized NOG mouse model of melanoma. Journal of Medical Virology , 91(9), 1702–1706. https://doi.org/10.1002/jmv.25501
- Kuryk, L., Møller, A. S. W., & Jaderberg, M. (2019b). Combination of immunogenic oncolytic adenovirus ONCOS-102 with anti-PD-1 pembrolizumab exhibits synergistic antitumor effect in humanized A2058 melanoma huNOG mouse model. Oncoimmunology , 8(2), e1532763. https://doi.org/10.1080/2162402x.2018.1532763
- Langer, C. J., Gadgeel, S. M., Borghaei, H., Papadimitrakopoulou, V. A., Patnaik, A., Powell, S. F., Gentzler, R. D., Martins, R. G., Stevenson, J. P., Jalal, S. I., Panwalkar, A., Yang, J. C. H., Gubens, M., Sequist, L. V., Awad, M. M., Fiore, J., Ge, Y., Raftopoulos, H., & Gandhi, L. (2016). Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: A randomised, phase 2 cohort of the open-label KEYNOTE-021 study. The Lancet Oncology , 17(11), 1497–1508. https://doi.org/10.1016/s1470-2045(16)30498-3
- Legrand, N., Huntington, N. D., Nagasawa, M., Bakker, A. Q., Schotte, R., Strick-Marchand, H., de Geus, S. J., Pouw, S. M., Böhne, M., Voordouw, A., Weijer, K., Di Santo, J. P., & Spits, H. (2011). Functional CD47/signal regulatory protein alpha (SIRP(alpha)) interaction is required for optimal human T- and natural killer- (NK) cell homeostasis in vivo. Proceedings of the National Academy of Sciences of the United States of America , 108(32), 13224–13229. https://doi.org/10.1073/pnas.1101398108
- Leskov, I., Pallasch, C. P., Drake, A., Iliopoulou, B. P., Souza, A., Shen, C. H., Schweighofer, C. D., Abruzzo, L., Frenzel, L. P., Wendtner, C. M., Hemann, M. T., & Chen, J. (2013). Rapid generation of human B-cell lymphomas via combined expression of Myc and Bcl2 and their use as a preclinical model for biological therapies. Oncogene , 32(8), 1066–1072. https://doi.org/10.1038/onc.2012.117
- Li, Q. X., Feuer, G., Ouyang, X., & An, X. (2017). Experimental animal modeling for immuno-oncology. Pharmacology & Therapeutics, 173, 34–46. https://doi.org/10.1016/j.pharmthera.2017.02.002
- Li, Y., Mention, J. J., Court, N., Masse-Ranson, G., Toubert, A., Spits, H., Legrand, N., Corcuff, E., Strick-Marchand, H., & Di Santo, J. P. (2016). A novel Flt3-deficient HIS mouse model with selective enhancement of human DC development. European Journal of Immunology , 46(5), 1291–1299. https://doi.org/10.1002/eji.201546132
- Li, Y., Strick-Marchand, H., Lim, A. I., Ren, J., Masse-Ranson, G., Dan, L., Jouvion, G., Rogge, L., Lucas, S., Bin, L., & Di Santo, J. P. (2017). Regulatory T cells control toxicity in a humanized model of IL-2 therapy. Nature Communications , 8(1), 1762. https://doi.org/10.1038/s41467-017-01570-9
- Liu, L. L., Skribek, M., Harmenberg, U., & Gerling, M. (2023). Systemic inflammatory syndromes as life-threatening side effects of immune checkpoint inhibitors: Case report and systematic review of the literature. Journal for ImmunoTherapy of Cancer , 11(3), e005841. https://doi.org/10.1136/jitc-2022-005841
- Majzner, R. G., Ramakrishna, S., Yeom, K. W., Patel, S., Chinnasamy, H., Schultz, L. M., Richards, R. M., Jiang, L., Barsan, V., Mancusi, R., Geraghty, A. C., Good, Z., Mochizuki, A. Y., Gillespie, S. M., Toland, A. M. S., Mahdi, J., Reschke, A., Nie, E. H., Chau, I. J., … Monje, M. (2022). GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature , 603(7903), 934–941. https://doi.org/10.1038/s41586-022-04489-4
- Martin-Jeantet, P., Renart-Depontieu, F., Bourre, L., Martin, G., Pappalardo, A., Campbell, D O., Doerner, A., Cherifi, Y., Sônego, F., & Thiam, K. (2022). Abstract 4036: BRGSF-HIS and CD3Ε humanized mice: Translatable preclinical mouse models for assessment of T-cell engagers-induced CRS. Cancer Research , 82(12_Supplement), 4036. https://doi.org/10.1158/1538-7445.Am2022-4036
- Martinov, T., Mckenna, K. M., Tan, W. H., Collins, E. J., Kehret, A. R., Linton, J. D., Olsen, T. M., Shobaki, N., & Rongvaux, A. (2021). Building the next generation of humanized hemato-lymphoid system mice. Frontiers in Immunology , 12, 643852. https://doi.org/10.3389/fimmu.2021.643852
- Maser, I. P., Hoves, S., Bayer, C., Heidkamp, G., Nimmerjahn, F., Eckmann, J., & Ries, C. H. (2020). The tumor milieu promotes functional human tumor-resident plasmacytoid dendritic cells in humanized mouse models. Frontiers in Immunology , 11, 2082. https://doi.org/10.3389/fimmu.2020.02082
- Matsuda, M., Ono, R., Iyoda, T., Endo, T., Iwasaki, M., Tomizawa-Murasawa, M., Saito, Y., Kaneko, A., Shimizu, K., Yamada, D., Ogonuki, N., Watanabe, T., Nakayama, M., Koseki, Y., Kezuka-Shiotani, F., Hasegawa, T., Yabe, H., Kato, S., Ogura, A., … Ishikawa, F. (2019). Human NK cell development in hIL-7 and hIL-15 knockin NOD/SCID/IL2rgKO mice. Life Science Alliance , 2(2), e201800195. https://doi.org/10.26508/lsa.201800195
- Maude, S. L., Laetsch, T. W., Buechner, J., Rives, S., Boyer, M., Bittencourt, H., Bader, P., Verneris, M. R., Stefanski, H. E., Myers, G. D., Qayed, M., de Moerloose, B., Hiramatsu, H., Schlis, K., Davis, K. L., Martin, P. L., Nemecek, E. R., Yanik, G. A., Peters, C., … Grupp, S. A. (2018). Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. New England Journal of Medicine , 378(5), 439–448. https://doi.org/10.1056/NEJMoa1709866
- Mccune, J., Namikawa, R., Kaneshima, H., Shultz, L., Lieberman, M., & Weissman, I. (1988). The SCID-hu mouse: Murine model for the analysis of human hematolymphoid differentiation and function. Science , 241(4873), 1632–1639. https://doi.org/10.1126/science.241.4873.1632
- Medetgul-Ernar, K., & Davis, M. M. (2022). Standing on the shoulders of mice. Immunity , 55(8), 1343–1353. https://doi.org/10.1016/j.immuni.2022.07.008
- Mhaidly, R., & Verhoeyen, E. (2020). Humanized mice are precious tools for preclinical evaluation of CAR T and CAR NK Cell therapies. Cancers , 12(7), 1915. https://doi.org/10.3390/cancers12071915
- Morris, E. C., Neelapu, S. S., Giavridis, T., & Sadelain, M. (2022). Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nature Reviews Immunology , 22(2), 85–96. https://doi.org/10.1038/s41577-021-00547-6
- Neelapu, S. S., Locke, F. L., Bartlett, N. L., Lekakis, L. J., Miklos, D. B., Jacobson, C. A., Braunschweig, I., Oluwole, O. O., Siddiqi, T., Lin, Y., Timmerman, J. M., Stiff, P. J., Friedberg, J. W., Flinn, I. W., Goy, A., Hill, B. T., Smith, M. R., Deol, A., Farooq, U., … Go, W. Y. (2017). Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. New England Journal of Medicine , 377(26), 2531–2544. https://doi.org/10.1056/NEJMoa1707447
- Norelli, M., Camisa, B., Barbiera, G., Falcone, L., Purevdorj, A., Genua, M., Sanvito, F., Ponzoni, M., Doglioni, C., Cristofori, P., Traversari, C., Bordignon, C., Ciceri, F., Ostuni, R., Bonini, C., Casucci, M., & Bondanza, A. (2018). Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nature Medicine , 24(6), 739–748. https://doi.org/10.1038/s41591-018-0036-4
- Ott, P. A., Fernandez, M. E. E., Hiret, S., Kim, D. W., Moss, R. A., Winser, T., Yuan, S., Cheng, J. D., Piperdi, B., & Mehnert, J. M. (2015). Pembrolizumab (MK-3475) in patients (pts) with extensive-stage small cell lung cancer (SCLC): Preliminary safety and efficacy results from KEYNOTE-028. Journal of Clinical Oncology , 33(15_suppl), 7502. https://doi.org/10.1200/jco.2015.33.15_suppl.7502
- Pallin, D. J., Baugh, C. W., Postow, M. A., Caterino, J. M., Erickson, T. B., & Lyman, G. H. (2018). Immune-related adverse events in cancer patients. Academic Emergency Medicine , 25(7), 819–827. https://doi.org/10.1111/acem.13443
- Pearson, T., Shultz, L. D., Miller, D., King, M., Laning, J., Fodor, W., Cuthbert, A., Burzenski, L., Gott, B., Lyons, B., Foreman, O., Rossini, A. A., & Greiner, D. L. (2008). Non-obese diabetic-recombination activating gene-1 (NOD-Rag1 null) interleukin (IL)-2 receptor common gamma chain (IL2r gamma null) null mice: A radioresistant model for human lymphohaematopoietic engraftment. Clinical and Experimental Immunology , 154(2), 270–284. https://doi.org/10.1111/j.1365-2249.2008.03753.x
- Pyo, K. H., Kim, J. H., Lee, J. M., Kim, S. E., Cho, J. S., Lim, S. M., & Cho, B. C. (2019). Promising preclinical platform for evaluation of immuno-oncology drugs using Hu-PBL-NSG lung cancer models. Lung Cancer , 127, 112–121. https://doi.org/10.1016/j.lungcan.2018.11.035
- Richman, S. A., Nunez-Cruz, S., Moghimi, B., Li, L. Z., Gershenson, Z. T., Mourelatos, Z., Barrett, D. M., Grupp, S. A., & Milone, M. C. (2018). High-affinity GD2-specific CAR T Cells induce fatal encephalitis in a preclinical neuroblastoma model. Cancer Immunology Research , 6(1), 36–46. https://doi.org/10.1158/2326-6066.Cir-17-0211
- Rongvaux, A., Willinger, T., Martinek, J., Strowig, T., Gearty, S. V., Teichmann, L. L., Saito, Y., Marches, F., Halene, S., Palucka, A. K., Manz, M. G., & Flavell, R. A. (2014). Development and function of human innate immune cells in a humanized mouse model. Nature Biotechnology , 32(4), 364–372. https://doi.org/10.1038/nbt.2858
- Sanmamed, M. F., Rodriguez, I., Schalper, K. A., Oñate, C., Azpilikueta, A., Rodriguez-Ruiz, M. E., Morales-Kastresana, A., Labiano, S., Pérez-Gracia, J. L., Martín-Algarra, S., Alfaro, C., Mazzolini, G., Sarno, F., Hidalgo, M., Korman, A. J., Jure-Kunkel, M., & Melero, I. (2015). Nivolumab and Urelumab enhance antitumor activity of human T lymphocytes engrafted in Rag2-/-IL2Rγnull immunodeficient mice. Cancer Research , 75(17), 3466–3478. https://doi.org/10.1158/0008-5472.Can-14-3510
- Schubert, M. L., Schmitt, M., Wang, L., Ramos, C. A., Jordan, K., Müller-Tidow, C., & Dreger, P. (2021). Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Annals of Oncology , 32(1), 34–48. https://doi.org/10.1016/j.annonc.2020.10.478
- Shi, T., Song, X., Wang, Y., Liu, F., & Wei, J. (2020). Combining oncolytic viruses with cancer immunotherapy: establishing a new generation of cancer treatment. Frontiers in Immunology , 11, 683. https://doi.org/10.3389/fimmu.2020.00683
- Shultz, L. D., Ishikawa, F., & Greiner, D. L. (2007). Humanized mice in translational biomedical research. Nature Reviews Immunology , 7(2), 118–130. https://doi.org/10.1038/nri2017
- Shultz, L. D., Lyons, B. L., Burzenski, L. M., Gott, B., Chen, X., Chaleff, S., Kotb, M., Gillies, S. D., King, M., Mangada, J., Greiner, D. L., & Handgretinger, R. (2005). Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. Journal of Immunology , 174(10), 6477–6489. https://doi.org/10.4049/jimmunol.174.10.6477
- Siegler, E. L., & Kenderian, S. S. (2020). Neurotoxicity and cytokine release syndrome after chimeric antigen receptor T Cell therapy: Insights into mechanisms and novel therapies. Frontiers in Immunology , 11, 1973. https://doi.org/10.3389/fimmu.2020.01973
- Smith, E. J., Olson, K., Haber, L. J., Varghese, B., Duramad, P., Tustian, A. D., Oyejide, A., Kirshner, J. R., Canova, L., Menon, J., Principio, J., Macdonald, D., Kantrowitz, J., Papadopoulos, N., Stahl, N., Yancopoulos, G. D., Thurston, G., & Davis, S. (2015). A novel, native-format bispecific antibody triggering T-cell killing of B-cells is robustly active in mouse tumor models and cynomolgus monkeys. Scientific Reports , 5, 17943. https://doi.org/10.1038/srep17943
- Stadler, C R., Bähr-Mahmud, H., Plum, L M., Schmoldt, K., Kölsch, A C., Türeci, Ö., & Sahin, U. (2016). Characterization of the first-in-class T-cell-engaging bispecific single-chain antibody for targeted immunotherapy of solid tumors expressing the oncofetal protein claudin 6. Oncoimmunology , 5(3), e1091555. https://doi.org/10.1080/2162402x.2015.1091555
- Stewart, S. A., Feuer, G., Jewett, A., Lee, F. V., Bonavida, B., & Chen, I. S. Y. (1996). HTLV-1 gene expression in adult T-cell leukemia cells elicits an NK cell response in vitro and correlates with cell rejection in SCID mice. Virology , 226(2), 167–175. https://doi.org/10.1006/viro.1996.0643
- Stoddart, C. A., Maidji, E., Galkina, S. A., Kosikova, G., Rivera, J. M., Moreno, M. E., Sloan, B., Joshi, P., & Long, B. R. (2011). Superior human leukocyte reconstitution and susceptibility to vaginal HIV transmission in humanized NOD-scid IL-2Rγ(-/-) (NSG) BLT mice. Virology , 417(1), 154–160. https://doi.org/10.1016/j.virol.2011.05.013
- Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K., & Yamanaka, S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell , 131(5), 861–872. https://doi.org/10.1016/j.cell.2007.11.019
- Takahashi, T., Katano, I., Ito, R., Goto, M., Abe, H., Mizuno, S., Kawai, K., Sugiyama, F., & Ito, M. (2017). Enhanced antibody responses in a novel NOG transgenic mouse with restored lymph node organogenesis. Frontiers in Immunology , 8, 2017. https://doi.org/10.3389/fimmu.2017.02017
- Tay, S. H., Toh, M. M. X., Thian, Y. L., Vellayappan, B. A., Fairhurst, A. M., Chan, Y. H., Aminkeng, F., Bharwani, L. D., Huang, Y., Mak, A., & Wong, A. S. C. (2022). Cytokine release syndrome in cancer patients receiving immune checkpoint inhibitors: A case series of 25 patients and review of the literature. Frontiers in Immunology , 13, 807050. https://doi.org/10.3389/fimmu.2022.807050
- Tentler, J. J., Tan, A. C., Weekes, C. D., Jimeno, A., Leong, S., Pitts, T. M., Arcaroli, J. J., Messersmith, W. A., & Eckhardt, S. G. (2012). Patient-derived tumour xenografts as models for oncology drug development. Nature Reviews Clinical Oncology , 9(6), 338–350. https://doi.org/10.1038/nrclinonc.2012.61
- Traggiai, E., Chicha, L., Mazzucchelli, L., Bronz, L., Piffaretti, J. C., Lanzavecchia, A., & Manz, M G. (2004). Development of a human adaptive immune system in cord blood cell-transplanted mice. Science , 304(5667), 104–107. https://doi.org/10.1126/science.1093933
- Upadhaya, S., Neftelino, S. T., Hodge, J. P., Oliva, C., Campbell, J. R., & Yu, J. X. (2021). Combinations take centre stage in PD1/PDL1 inhibitor clinical trials. Nature Reviews Drug Discovery , 20(3), 168–169. https://doi.org/10.1038/d41573-020-00204-y
- Vallera, D. A., Felices, M., Mcelmurry, R., Mccullar, V., Zhou, X., Schmohl, J. U., Zhang, B., Lenvik, A. J., Panoskaltsis-Mortari, A., Verneris, M. R., Tolar, J., Cooley, S., Weisdorf, D. J., Blazar, B. R., & Miller, J. S. (2016). IL15 Trispecific Killer Engagers (TriKE) make natural killer cells specific to CD33+ targets while also inducing persistence, in vivo expansion, and enhanced function. Clinical Cancer Research , 22(14), 3440–3450. https://doi.org/10.1158/1078-0432.Ccr-15-2710
- Voillet, V., Berger, T R., Mckenna, K. M., Paulson, K. G., Tan, W. H., Smythe, K. S., Hunter, D. S., Valente, W. J., Weaver, S., Campbell, J. S., Kim, T. S., Byrd, D. R., Bielas, J. H., Pierce, R. H., Chapuis, A. G., Gottardo, R., & Rongvaux, A. (2022). An in vivo model of human macrophages in metastatic melanoma. Journal of Immunology , 209(3), 606–620. https://doi.org/10.4049/jimmunol.2101109
- Wahl, A., & Victor Garcia, J. (2014). The use of BLT humanized mice to investigate the immune reconstitution of the gastrointestinal tract. Journal of Immunological Methods , 410, 28–33. https://doi.org/10.1016/j.jim.2014.06.009
- Wang, M., Yao, L. C., Cheng, M., Cai, D., Martinek, J., Pan, C.-X., Shi, W., Ma, A. H., Vere White, R. W., Airhart, S., Liu, E. T., Banchereau, J., Brehm, M. A., Greiner, D. L., Shultz, L. D., Palucka, K., & Keck, J. G. (2018). Humanized mice in studying efficacy and mechanisms of PD-1-targeted cancer immunotherapy. Faseb Journal , 32(3), 1537–1549. https://doi.org/10.1096/fj.201700740R
- Weaver, J. L., Zadrozny, L. M., Gabrielson, K., Semple, K. M., Shea, K. I., & Howard, K. E. (2019). BLT-immune humanized mice as a model for nivolumab-induced immune-mediated adverse events: Comparison of the NOG and NOG-EXL strains. Toxicological Sciences , 169(1), 194–208. https://doi.org/10.1093/toxsci/kfz045
- Wege, A. K. (2018). Humanized mouse models for the preclinical assessment of cancer immunotherapy. Biodrugs , 32(3), 245–266. https://doi.org/10.1007/s40259-018-0275-4
- Wege, A. K., Huber, B., Wimmer, N., Männel, D. N., & Hehlgans, T. (2014). LTβR expression on hematopoietic cells regulates acute inflammation and influences maturation of myeloid subpopulations. Innate Immunity , 20(5), 461–470. https://doi.org/10.1177/1753425913497242
- Wege, A. K., Weber, F., Kroemer, A., Ortmann, O., Nimmerjahn, F., & Brockhoff, G. (2017). IL-15 enhances the anti-tumor activity of trastuzumab against breast cancer cells but causes fatal side effects in humanized tumor mice (HTM). Oncotarget , 8(2), 2731–2744. https://doi.org/10.18632/oncotarget.13159
- Weißmüller, S., Kronhart, S., Kreuz, D., Schnierle, B., Kalinke, U., Kirberg, J., Hanschmann, K. M., & Waibler, Z. (2016). TGN1412 induces lymphopenia and human cytokine release in a humanized mouse model. PLoS ONE , 11(3), e0149093. https://doi.org/10.1371/journal.pone.0149093
- Wolf, N. K., Kissiov, D. U., & Raulet, D. H. (2023). Roles of natural killer cells in immunity to cancer, and applications to immunotherapy. Nature Reviews Immunology , 23(2), 90–105. https://doi.org/10.1038/s41577-022-00732-1
- Wulf-Goldenberg, A., Eckert, K., & Fichtner, I. (2011). Intrahepatically transplanted human cord blood cells reduce SW480 tumor growth in the presence of bispecific EpCAM/CD3 antibody. Cytotherapy , 13(1), 108–113. https://doi.org/10.3109/14653249.2010.515577
- Wunderlich, M., Chou, F. S., Sexton, C., Presicce, P., Chougnet, C. A., Aliberti, J., & Mulloy, J. C. (2018). Improved multilineage human hematopoietic reconstitution and function in NSGS mice. PLoS ONE , 13(12), e0209034. https://doi.org/10.1371/journal.pone.0209034
- Wunderlich, M., Chou, F. S., Link, K. A., Mizukawa, B., Perry, R. L., Carroll, M., & Mulloy, J. C. (2010). AML xenograft efficiency is significantly improved in NOD/SCID-IL2RG mice constitutively expressing human SCF, GM-CSF and IL-3. Leukemia , 24(10), 1785–1788. https://doi.org/10.1038/leu.2010.158
- Xu, X., Kumari, R., Zhou, J., Chen, J., Mao, B., Wang, J., Zheng, M., Tu, X., An, X., Chen, X., Zhang, L., Tian, X., Wang, H., Dong, X., Bao, Z., Guo, S., Ouyang, X., Shang, L., Wang, F., … Li, Q. X. (2023). A living biobank of matched pairs of patient-derived xenografts and organoids for cancer pharmacology. PLoS ONE , 18(1), e0279821. https://doi.org/10.1371/journal.pone.0279821
- Xue, L., Yi, Y., Xu, Q., Wang, L., Yang, X., Zhang, Y., Hua, X., Chai, X., Yang, J., Chen, Y., Tao, G., Hu, B., & Wang, X. (2021). Chimeric antigen receptor T cells self-neutralizing IL6 storm in patients with hematologic malignancy. Cell Discovery , 7(1), 84. https://doi.org/10.1038/s41421-021-00299-6
- Yaguchi, T., Kobayashi, A., Inozume, T., Morii, K., Nagumo, H., Nishio, H., Iwata, T., Ka, Y., Katano, I., Ito, R., Ito, M., & Kawakami, Y. (2018). Human PBMC-transferred murine MHC class I/II-deficient NOG mice enable long-term evaluation of human immune responses. Cellular & Molecular Immunology, 15(11), 953–962. https://doi.org/10.1038/cmi.2017.106
- Yang, M., Shan, B., Li, Q., Song, X., Cai, J., Deng, J., Zhang, L., Du, Z., Lu, J., Chen, T., Wery, J. P., Chen, Y., & Li, Q. (2013). Overcoming erlotinib resistance with tailored treatment regimen in patient-derived xenografts from naive Asian NSCLC patients. International Journal of Cancer , 132(2), E74–84. https://doi.org/10.1002/ijc.27813
- Ye, C., Yang, H., Cheng, M., Shultz, L. D., Greiner, D. L., Brehm, M. A., & Keck, J. G. (2020). A rapid, sensitive, and reproducible in vivo PBMC humanized murine model for determining therapeutic-related cytokine release syndrome. The FASEB Journal , 34(9), 12963–12975. https://doi.org/10.1096/fj.202001203R
- Yu, H., Borsotti, C., Schickel, J. N., Zhu, S., Strowig, T., Eynon, E. E., Frleta, D., Gurer, C., Murphy, A. J., Yancopoulos, G. D., Meffre, E., Manz, M. G., & Flavell, R. A. (2017). A novel humanized mouse model with significant improvement of class-switched, antigen-specific antibody production. Blood , 129(8), 959–969. https://doi.org/10.1182/blood-2016-04-709584
- Yu, J., Vodyanik, M. A., Smuga-Otto, K., Antosiewicz-Bourget, J., Frane, J. L., Tian, S., Nie, J., Jonsdottir, G. A., Ruotti, V., Stewart, R., Slukvin, I. I., & Thomson, J. A. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science , 318(5858), 1917–1920. https://doi.org/10.1126/science.1151526
- Zekri, L., Vogt, F., Osburg, L., Müller, S., Kauer, J., Manz, T., Pflügler, M., Maurer, A., Heitmann, J. S., Hagelstein, I., Märklin, M., Hörner, S., Todenhöfer, T., Calaminus, C., Stenzl, A., Pichler, B., la Fougère, C., Schneider, M. A., Rammensee, H.-G., … Jung, G. (2021). An IgG-based bispecific antibody for improved dual targeting in PSMA-positive cancer. EMBO Molecular Medicine , 13(2), e11902. https://doi.org/10.15252/emmm.201911902
- Zeleniak, A., Wiegand, C., Liu, W., Mccormick, C. K. R., Alavi, A., Guan, H., Bertera, S., Lakomy, R., Tajima, A., Cohen, H., Wong, S., Balikani, L., Mizerak, B., Bar-Joseph, Z., Trucco, M., Banerjee, I., & Fan, Y. (2022). De novo construction of T cell compartment in humanized mice engrafted with iPSC-derived thymus organoids. Nature Methods , 19(10), 1306–1319. https://doi.org/10.1038/s41592-022-01583-3
- Zhang, J., Huang, Y., Xi, G., & Zhang, F. (2020). HX008: A humanized PD-1 blocking antibody with potent antitumor activity and superior pharmacologic properties. MAbs , 12(1), 1724751. https://doi.org/10.1080/19420862.2020.1724751
- Zhao, Y., Shuen, T. W. H., Toh, T. B., Chan, X. Y., Liu, M., Tan, S. Y., Fan, Y., Yang, H., Lyer, S. G., Bonney, G. K., Loh, E., Chang, K. T. E., Tan, T. C., Zhai, W., Chan, J. K. Y., Chow, E. K. H., Chee, C. E., Lee, G. H., Dan, Y. Y., … Chen, Q. (2018). Development of a new patient-derived xenograft humanised mouse model to study human-specific tumour microenvironment and immunotherapy. Gut , 67(10), 1845–1854. https://doi.org/10.1136/gutjnl-2017-315201
- Zhao, Y., Wang, J., Liu, W. N., Fong, S. Y., Shuen, T. W. H., Liu, M., Harden, S., Tan, S. Y., Cheng, J. Y., Tan, W. W. S., Chan, J. K. Y., Chee, C. E., Lee, G. H., Toh, H. C., Lim, S. G., Wan, Y., & Chen, Q. (2021). Analysis and validation of human targets and treatments using a hepatocellular carcinoma-immune humanized mouse model. Hepatology , 74(3), 1395–1410. https://doi.org/10.1002/hep.31812
- Zheng, B., Ren, T., Huang, Y., Sun, K., Wang, S., Bao, X., Liu, K., & Guo, W. (2018). PD-1 axis expression in musculoskeletal tumors and antitumor effect of nivolumab in osteosarcoma model of humanized mouse. Journal of Hematology & Oncology, 11(1), 16. https://doi.org/10.1186/s13045-018-0560-1
- Internet Resources.
- https://www.crownbio.com/oncology/oncology-databases/hubase
- PDX models are characterized in vivo and annotated in an online PDX database.
- https://www.crownbio.com/databases/xenobase
- Cell line models are characterized in vivo and annotated in an online xenograft database.
- https://www.crownbio.com/ob-registration
- Online organoid database for PDXO and PDO models.

