Human Brain Sequential Extraction (Tau)
Michael X. Henderson
Abstract
This protocol details Human Brain Sequential Extraction (Tau). This protocol is an adaptation of the work of several labs.
Attachments
Steps
1 Day Before Extraction
Make sure glass 100 or 40 mL homogenizers and pestles (A and B) are cleaned with 70%
ethanol, wrapped in foil, and autoclaved.
Clean out ultracentrifuge tubes and caps with 70% ethanol and dry.
Transfer brain tissue to -20°C freezer the night before (speeds up thaw).
Ensure there are sufficient protease inhibitor, phosphatase inhibitor, and DTT.
Day 1 - Preparation
Turn on the ultracentrifuge ( Optima L-100 XP ), set the temperature for 4°C and turn the vacuum on. Make sure Ti-45 and Ti-70 rotors are available. They may be in room 5124.
Fill 2 buckets with wet ice.
Warm PMSF to Room temperature.
Record information about the tissue to be extracted. Note the weight of the dish to be used for
weighing the gray matter.
Fill a 15 mL conical with 10% neutral buffered formalin (NBF) and label with case number.
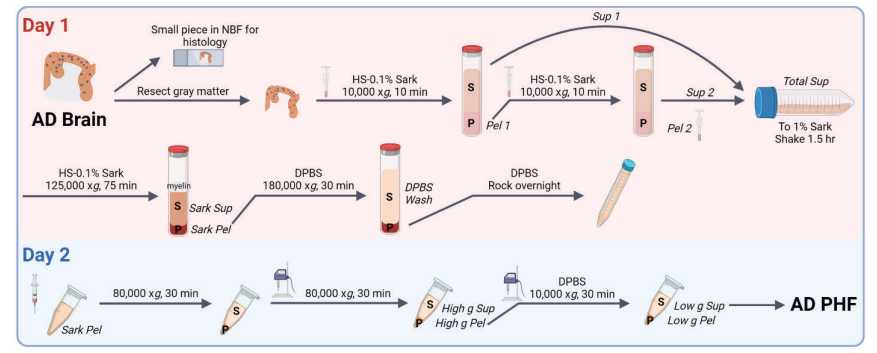
Day 1 - Extraction
Thaw bag(s) of brain tissue On ice.
Move brain to petri dish and weigh it.
Once tissue is thawed, remove meninges and blood vessels. Cut one thin, representative piece
off and transfer it to 10% neutral buffered formalin for fixation and subsequent IHC.
Carefully resect the remaining gray matter from white matter in ice-cold PBS. Transfer gray matter
to a petri dishOn ice to be weighed.
Weigh gray matter.
Prepare 30mL Extraction Buffer per gram gray matter. Add the following to ice-cold B ase
Buffer r: cOmplete protease inhibitor 1 tab b50mL ), phosphatase inhibitors (2&3) 1:100 0),0.1millimolar (mM) PMSF 1:5000 0),2millimolar (mM) DDT 1:500 0).
Add 9 volumes of Extraction Buffer to the homogenizer tube. Cut gray matter into small bits,
and use a buffer to transfer these bits to the tube.
Homogenize with pestle A until the pestle moves easily.
Homogenize with pestle B 3x 25 strokes. Save 200µL of this as Total Homogenate.
Pour homogenate evenly into Ti-45 tubes (fits ~50mL/tube). Balance tubes to within 0.1g.
Spin at 11300rpm,0h 0m 0s in the Ti-45 (10000x g,0h 0m 0s) for 0h 10m 0s at 4°C.
Pour supernatant into 50 mL conical by filtering it through a piece of kimwipe folded into two
layers placed in a funnel.
Add 9 volumes Extraction Buffer to the centrifuge tube and homogenizer. Transfer pellet in
buffer to homogenizer.
Homogenize with pestle A until the pestle moves easily.
Homogenize with pestle B 3x 25 strokes.
Pour homogenate evenly into Ti-45 tubes (fits ~50 mL/tube). Balance tubes to within 0.1g.
Spin at 11300rpm,0h 0m 0s in the Ti-45 (10000x g,0h 0m 0s) for 0h 10m 0s at 4°C.
During the spin, transfer Sup 1 to a beaker with a stir bar. Add sarkosyl to a final 1%.
Pour supernatant into 50 mL conical by filtering it through a piece of kimwipe folded into two
layers placed in a funnel.
Combine Sup 1 and Sup 2 in a beaker with a stir bar. Add sarkosyl to a final 1% concentration
(1/27).
Stir Total Sup for 1- 1h 30m 0s at Room temperature.
Add the Extraction Buffer to the centrifuge tube and transfer the pellet to the homogenizer. You can use the same vol as Sup 2 for homogenization.
Add Total Sup to Ti-45 tubes. Balance tubes to within 0.1g. Spin at 40000rpm,0h 0m 0s in the Ti-45 (125000x g,0h 0m 0s) for 1h 15m 0s at 4°C.
Pipet out supernatant into a beaker. Myelin will have floated. Remove this with a pipette.
Use the vacuum to aspirate remaining liquid around the Sark Pellet.
Add 10mL DPBS to each centrifuge tube to wash the Sark Pellet. Use the vacuum to remove DPBS.
Add 2mL DPBS to wash Sark Pellet a second time. Use vacuum to remove DPBS.
Add 1mL DPBS to each tube. Pipette liquid towards the Sark Pellet with a cut P1000 tip until the
pellet has loosened. Use a transfer pipette to transfer the pellet to a Ti-70 centrifuge tube.
Use a cut P1000 tip to pipette up and down to resuspend the pellet.
Add DPBS into the centrifuge tube to reach maximum volume. Balance tubes to within 0.1g.
Spin Sark Pel at 50000rpm,0h 0m 0s in the Ti-70 (18000x g,0h 0m 0s) for 0h 30m 0s at 4°C.
Save 200µL of the supernatant as DPBS wash. Remove the remaining supernatant by vacuum.
Add 100µL DPBS/ 1 g tissue to the pellet. Use a cut P1000 tip to first loosen the pellet, then
break it up as much as possible without pipetting up and down. Transfer to 1.5 mL tubes.
Vortex briefly and spin in a tabletop centrifuge at 1000x g,0h 0m 0s for 0h 1m 0s.
Rock the tube at 4°C .
Day 1 - Cleanup
Bleach all homogenizers, tools and tubes, but use only soap for metal lids.
Rinse centrifuge tubes for 0h 10m 0s after bleaching.
Bleach out ice bucks.
Bleach vacuum flask and rinse out.
Day 2
Move fixed brain to leaching buffer and cassette for paraffin embedding.
Spin in a tabletop centrifuge at 1000x g,0h 0m 0s for 0h 1m 0s at 4°C.
Pass the suspension repeatedly through a 27G ½ needle to homogenize.
Transfer the resuspended Sark Pel to an autoclaved 1.5 mL Beckman ultracentrifuge tube.
Add 100µL DPBS to the centrifuge tube and resuspend well.
Balance tubes to within 0.1g. Spin in OptimaMAX-TL (room 5124) ( TLA100.3 rotor with adaptors) at 45000rpm,0h 0m 0s (80000x g,0h 0m 0s) for 0h 30m 0s at 4°C.
Remove supernatant and add 100µL DPBS/1 g tissue to the pellet.
Vortex to mix. Freeze or continue to process Sark Pellet.
Day 2 or 3 – Further Purification
Thaw the Sark Pellet.
Sonicate the Sark Pellet with 20x 1 sec pulses with hand sonicator.
Balance tubes to within 0.1g. Spin in OptimaMAX-TL (room 5124) ( TLA100.3 rotor with
adaptors) at45000rpm,0h 0m 0s 80000x g,0h 0m 0s ) for0h 30m 0s at4°C .
Transfer supernatant into another tube as High g Sup using sterile pipette tips.
Resuspend the pellet with 20% of the volume of DPBS (e.g. for 700µL Sup, add 140µL).
Sonicate High g Pellet for 90-120x 0h 0m 1s pulses with hand sonicator On ice to break up the pellet.
Transfer the resuspended High g Pellet to a 1.5 mL tube. Spin in tabletop centrifuge at 10000x g,0h 0m 0s for 0h 30m 0s at 4°C.
Transfer supernatant using a sterile pipette tip to a 1.5 mL tube.
Add an equal volume of DPBS to the pellet and sonicate 30x 0h 0m 1s pulses with hand sonicator to resuspend the pellet.