Device Fabrication Using Soft Lithography Technique V2
Jann Gamboa, Freeman Lan
Disclaimer
DISCLAIMER – FOR INFORMATIONAL PURPOSES ONLY; USE AT YOUR OWN RISK
The protocol content here is for informational purposes only and does not constitute legal, medical, clinical, or safety advice, or otherwise; content added to protocols.io is not peer reviewed and may not have undergone a formal approval of any kind. Information presented in this protocol should not substitute for independent professional judgment, advice, diagnosis, or treatment. Any action you take or refrain from taking using or relying upon the information presented here is strictly at your own risk. You agree that neither the Company nor any of the authors, contributors, administrators, or anyone else associated with protocols.io, can be held responsible for your use of the information contained in or linked to this protocol or any of our Sites/Apps and Services.
Abstract
Standard Operating ProcedureInstitute of Biomedical EngineeringUniversity of TorontoName of the procedures: Device Fabrication using Soft Lithography TechniqueLocation: MB308API: Dr. Freeman LanInstructor: Jann Gamboa
Steps
Potential Hazards
-
Wear cut resistant gloves when using the razor!
-
Always put the lid on when using the
Equipment
| Value | Label |
|---|---|
| SCK-300 | SCK-300P Spin Coater Kit |
| Spin Coater | TYPE |
| Intras Scientific | BRAND |
| SCK-300 | SCK-300P Spin Coater Kit |
Note: The wafer could come off and cut you, or
burns the eyes! Put on safety goggles when using
Equipment Preparation
Hot Plates and Oven
The first step consists of turning one hot plate to 95°C and another hot plate to ~200°C
Turn on the
Equipment
| Value | Label |
|---|---|
| BLACK+DECKER 4-Slice Convection Oven, Stainless Steel, TO1313SBD | NAME |
| Oven | TYPE |
| BLACK + DECKER HOME | BRAND |
| B00GGFHH4U | SKU |
by turning the timer to "stay on" and the temperature to 60°C.
Equipment Checklist:
-
Tweezers (2)
-
Pyrex dish for the
-
waste beaker -
waste beaker -
solid Waste Container -
Aluminum foil
-
Double sided tape
-
Glass Slabs (for exposure)
-
Timers
Equipment
| Value | Label |
|---|---|
| SCK-300 | SCK-300P Spin Coater Kit |
| Spin Coater | TYPE |
| Intras Scientific | BRAND |
| SCK-300 | SCK-300P Spin Coater Kit |
Ensure that the spin coater cover and base are covered with aluminum foil.
Place a piece of double sided tape on the spin coater.
The tape should cover the diameter of the spin coater stage and be centered.
Note: There may be times when the tape is not sticking to the spin coater stage. To mitigate this issue, clean the stage with
- Unscrew the stage portion of the spin coater
- Place it on a separate clean beaker.
- Pour
onto it, just enough to submerge the surface of the stage. You can obtain the either from the "Used PGMEA bottle" so long as the number of times that it has been recycled is <10. Otherwise, obtain it from the fresh PGMEA squeeze bottle. - Agitate the beaker gently for
0h 5m 0s - After
0h 5m 0s, pick up the stage using tweezers and wash it off for the last time with freshover the same beaker. - Wash the stage with
over the waste beaker. - Blow dry the spin coater stage using the Dyson technique.
- Proceed to place the double sided tape on the spin coater.
- Discard the
to the "Fully depleted PGMEA waste bottle".
Equipment
| Value | Label |
|---|---|
| Silicon Wafer | NAME |
| SU-8 Substrate | TYPE |
| University Wafer | BRAND |
| ID-452 | SKU |
Locate or prepare a beaker for the waste
Pick up the silicon wafer using the tweezer located in the fume hood.
Rinse the wafer with
Make sure that the
Subsequently, use compressed air to blow the alcohol off of the wafer (using the Dyson technique; ask Jann or Freeman if you are unsure)
Spin-coating and Soft Baking
Centre the silicon wafer on the spin-coater stage.
Note: It is imperative to centre the silicon wafer onto the spin coater stage as it can lead to uneven coating. If this occurs, proceed to wash off the 0h 5m 0s using the instructions above over a Pyrex dish, not the beaker.
Obtain the
Place about 1mL to 2mL (the size of a quarter coin) of the
Note 1: DO NOT let the
Note 2: Note that
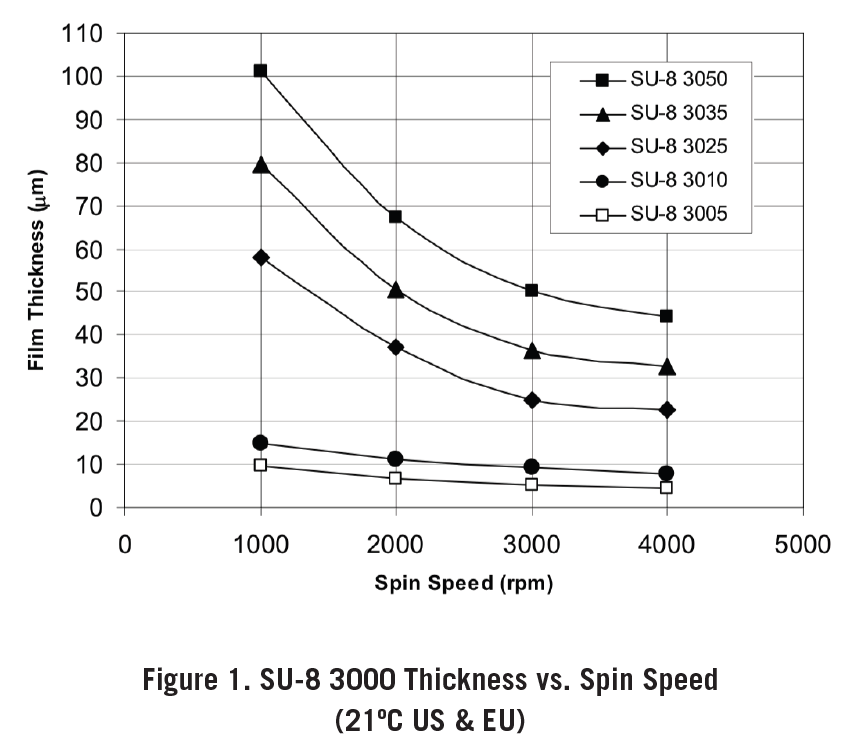
Spin the wafer according to the appropriate rpm (1000 for 0h 0m 10s and refer to Figure 1 for the appropriate rpm for 0h 0m 30s)
After spinning, pick up the wafer on its edge and place it on the 95°C hot plate for 0h 10m 0s
Note 1: Make sure that the double sided tape from the spin coater is not stuck on the bottom of the wafer before placing it on the hot plate.
Note 2: Adjust the soft bake times according to the thickness of the channel
#尊敬的用户,由于网络监管政策的限制,部分内容暂时无法在本网站直接浏览。我们已经为您准备了相关原始数据和链接,感谢您的理解与支持。
https://lh7-us.googleusercontent.com/docsz/AD_4nXcRDrlyh8cvpbw6jWijoJsHU6NdDCpvlWgIo2sQ8WuVa7iQOTmZ_Dq1Q8OK4DKi8-fHG9sqPNJGmjH4T_RZXFbrKmNxIRPTWSqraPPEEt8cfk7siGStVJxRAXpvriaHSwpTORffKSlaoHDwwcUXNhKvqBMG?key=7qYbjium-XapHcBAOGPKnQ
Photolithographic Exposure
Place the wafer and the print out of the mask in between two pieces of glass such that the order is:
Glass | wafer | mask | Glass <––– UV Light
Note 1: Make sure that the mask is facing up – that is, the description (writings) on the mask is facing up. You can tell that the mask is facing up if you can read the description.
Important: Wear the UV glasses.
Ensure that the
Equipment
| Value | Label |
|---|---|
| UV Flashlight | NAME |
| UV Flashlight | TYPE |
| Alonefire | BRAND |
| B09J885Q6N | SKU |
is on full battery, that is, 4/4 bars.
Place the UV light apparatus over on top of the glasses. Make sure that the box is covering the entirety of the glasses. Make sure that you line up the UV light on the centre of the wafer and the mask.
Inform everyone in the lab before turning on
Expose for 0h 0m 30s
Post-Exposure Baking (PEB)
Heat the wafer again by placing it on the 95°C hot plate for 0h 3m 0s to 0h 5m 0s
Note 1: At this point you should be able to see the outlines of the channel on the wafer. No visible latent image during or after PEB means that there was insufficient exposure, temperature or both.
Channel Development
For this step, you would need a compound called
WARNING 1:
WARNING 2:
Pour
If the
Cover the Pyrex dish using aluminum foil.
Manually agitate (gentle) the Pyrex dish every once in a while.
The development time depends on the thickness (height) of the channels. See the table below for guidance.
#尊敬的用户,由于网络监管政策的限制,部分内容暂时无法在本网站直接浏览。我们已经为您准备了相关原始数据和链接,感谢您的理解与支持。
https://lh7-us.googleusercontent.com/docsz/AD_4nXeUPiMLBcS75M9AZertjNqHIx9-1HErikPsfMC5B4sQBywUrl-1eWwX1yJ1soTwPx1-HY4c4kJtdb5r25AGdnlOS7TIMh4AJIOm1tltGXgAVa4udYj30-hEahwteguCDwVgwi9Ep7iAYuI-1BhntdAIxCUP?key=7qYbjium-XapHcBAOGPKnQ
After the appropriate development time has passed, take out the wafer using tweezers.
Rinse the wafer with fresh
Rinse the wafer with
If white precipitates arise, wash with fresh
Repeat steps 24-25
If
Recycle the
Note: If the number of lines has reached 10, discard the
Hard Baking
Place the wafer on the 200°C hot plate for 0h 2m 0s
At this point you should be able to view the channels on the device under the microscope. Place the device on a clean petri dish after baking and view the channel from there using the microscopes.
Label the petri dish with the dimensions of the channels, ID of the device, the date, your initials.
Molding a Device
Prepare the
For 30g, you would need 2.5g of the
Mix vigorously using the Dremel tool or manually using the mixing sticks until you get an opaque colour and a lot of bubbles.
Degas or desiccate until the
Equipment
| Value | Label |
|---|---|
| Vacuum Desiccator | NAME |
| Ted Pella | BRAND |
| 2246 | SKU |
| Any vacuum desiccator that will fit your samples will work | SPECIFICATIONS |
While waiting, place the wafer on a petri dish. Cover it so that it is free from dust.
Once the de-gassing is done, pour the
Make sure that the temperature of the oven is around 60°C. Place it in the oven for several hours or even 0h 2m 0s
Important: since the temperature of the oven fluctuates drastically, place the cover of the petri dish at the bottom by placing the device on top of it so that it does not melt.
Harvesting the Device
After several hours, take the device out of the oven.
IMPORTANT: Wear the cut resistant gloves.
Cut the device mold using a scalpel. Make sure that you are not cutting into the channels and don't push too hard on the wafer or it will splinter
Peel the device mold using tweezers or your hands
Note 1: It is very easy to break the silicon wafer so when you are cutting or peeling off the device, be very careful not to break the wafer.
Punch the inlet and outlets of the device using the manual puncher.
Tape the device so that it would be free from dust.
Plasma Bonding the Device
Speak to Jann or Freeman about this or refer to the SOP for the Harrick Plasma Cleaner PDG-32G from the Matsuura Lab
Aquapel Treatment
IMPORTANT: Wear safety glasses!
To make the device mold hydrophobic – cut a piece off of the aquapel syringe tubing.
Stick the tubing into the channel, push just enough to get the liquid into the droplet making a nozzle.
Push air through the channel to clear it out.
Put in the oven for 0h 5m 0s to dry.
Seal the aquapel syringe tubing by melting the tubing end for a FEW seconds on the hot plate or lamp and sealing it with your gloved fingers.