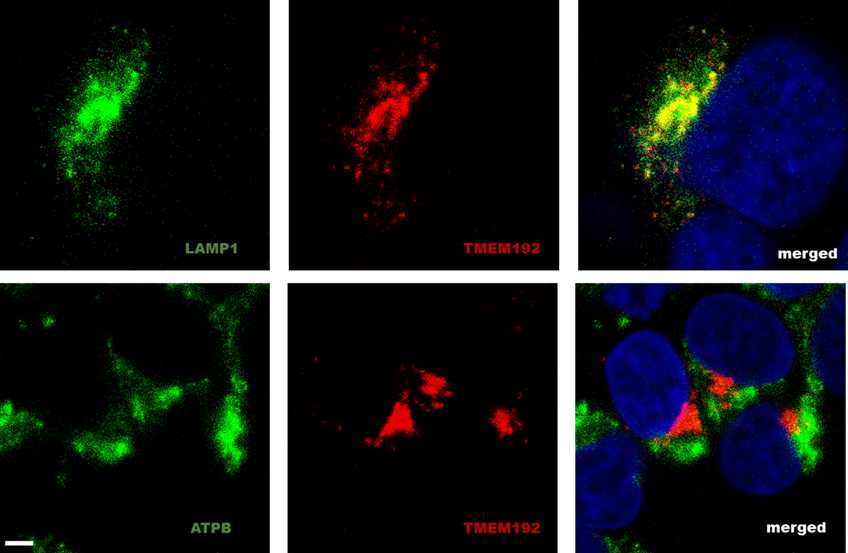
Colocalisation imaging of endogenous TMEM192 with lysosomal and mitochondria markers
Dario R. Alessi, Rotimi Y. Fasimoye
Disclaimer
DISCLAIMER – FOR INFORMATIONAL PURPOSES ONLY; USE AT YOUR OWN RISK
The protocol content here is for informational purposes only and does not constitute legal, medical, clinical, or safety advice, or otherwise; content added to protocols.io is not peer reviewed and may not have undergone a formal approval of any kind. Information presented in this protocol should not substitute for independent professional judgment, advice, diagnosis, or treatment. Any action you take or refrain from taking using or relying upon the information presented here is strictly at your own risk. You agree that neither the Company nor any of the authors, contributors, administrators, or anyone else associated with protocols.io, can be held responsible for your use of the information contained in or linked to this protocol or any of our Sites/Apps and Services.
Abstract
Immunofluorescent (IF) microscopy is a powerful tool used in cellular and molecular biology to monitor the subcellular localisation of proteins. By combining the advantages of immunostaining and confocal light microscope, IF microscopy can be used to assess the colocalization of two or more proteins within the cell. Here, we describe a method that can be used to verify the correct localisation of endogenously expressed TMEM192, by assessing their colocalization with LAMP1 (a lysosomal marker) and ATPB1 (a mitochondrial marker). Furthermore, our data showed that the anti-TMEM192 antibody is compatible for immunofluorescence assay.
Attachments
Steps
Seeding cells for immunofluorescence microscopy
Coat coverslips (sterilised in 100% ethanol prior to use) with poly-L-lysine by immersing the coverslips in poly-L-lysine solution for 1h 0m 0s.
Rinse the coated coverslips in media and place in a 6-well plate (one coverslip in each well).
Seed cells to 50-60% confluency in growth media on coated coverslips from step 2.
Incubate .
Preparing cells for Immunofluorescence imaging
Remove media completely using an aspirator and wash cells 3 times with 3mL PBS added with 0.2% (w/v) BSA and 0.02% (w/v) sodium azide (0h 5m 0s per wash on a see-saw rocker).
Fix cells by adding 4% (w/v) PFA in PBS and Incubate at Room temperature for 0h 10m 0s.
Permeabilise cells with 1% (v/v) NP-40 in PBS + 0.2% (w/v) BSA + 0.02% (w/v) sodium azide.
Block with 3% (w/v) BSA in PBS at Room temperature for 0h 30m 0s.
Prepare the primary antibody dilutions in 0.2% BSA (w/v) in PBS + 0.02% (w/v) sodium azide (See Table 1 for a list of antibodies and their working dilution).
| A | B | C | D | E |
|---|---|---|---|---|
| Antibody | Company | Cat. number | Host Species | dilution |
| TMEM192 | Abcam | Ab185545 | Rabbit | 1:1000 |
| LAMP1 | Santa Cruz | Sc-20011 | Mouse | 1:1000 |
| ATPB | Abcam | Ab14730 | Mouse | 1:1000 |
Table 1: List of primary antibodies
Incubate cells at Room temperaturewith diluted primary antibodies for 1h 0m 0s.
Wash the coverslips 3 times with 0.2% (w/v) BSA in PBS + 0.02% sodium azide. (0h 5m 0s per wash).
Prepare a combination of Secondary antibodies as described below (see Table 2 for more information about the secondary antibodies). Antibodies are diluted in PBS +0.2%BSA+0.02% sodium azide.
- anti-Mouse Alexa 488 (1:500) and anti-Rabbit Alexa 594 (1:500).
| A | B | C | D | E |
|---|---|---|---|---|
| Antibody | Conjugated Fluorophore | Company | Cat. number | Host Species |
| anti-Mouse | Alexa 488 | Invitrogen | A21206 | Donkey |
| anti-Rabbit | Alexa 594 | Invitrogen | A11012 | Goat |
Table 2: List of fluorophore-conjugated secondary antibodies
Add 0.5µL Hoechst 33342 solution for nuclear staining.
Incubate cells at Room temperature with diluted secondary antibodies for 1h 0m 0s. Do this in a humid chamber on a piece of Parafilm. Put a 60µL drop of diluted antibodies on the parafilm. Carefully place coverslip on the droplet, with the side containing attached cells, facing inward, making contact with the droplet.
Wash cells, 3 times, with 3mL PBS +0.2%BSA+0.02% sodium azide.
Rinse cells by dipping briefly in MilliQ water and gently dry on Kleenex wipes.
Label microscope glass slides (preferably the one with frosted side) according to the primary antibody used. Take note of the emission wavelength of the probe on the secondary antibodies.
Add a drop of VECTASHIELD antifading Mounting media.
Mount cover slip (containing cells) on the glass slide, ensuring that the side containing the cells is facing inward, making contact with the oil. Allow to dry for 0h 30m 0s, ensuring slides are prevented from direct light.