CD34+ isolation from human bone marrow
Mohsen Khosravi-Maharlooei, Markus Holzl, Austin Chen, Megan Sykes
Abstract
This protocol details the steps for isolating CD34+ cells from human bone marrow. The CD34+ cells isolated from this protocol can be used for generating humanized mice through reconstitution of immune cells via IV injection after bone marrow ablation. These cells can also be used for mixed lymphocyte reaction experiments.
Before start
Human bone marrow is a rich source for CD34+ hematopoietic stem cells. CD34+ cells can be easily isolated and further processed.
Steps
Transfer the content of the collection bag into a sterile flask
Add 250mLBM Medium to the bag and rinse it thoroughly
Transfer the content of the collection bag into the sterile flask
Layer 35mLof the suspension over 15mL of Histopaque
Centrifuge the tubes for 30 minutes 500g without brake at RT
Collect the leukocyte ring in 50mL Falcons and fill up with BM medium
Wash once by centrifuging 6 minutes 500g
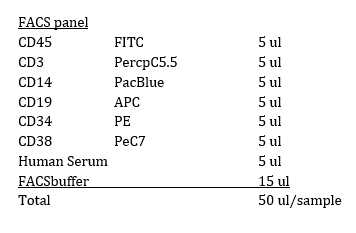
Resuspend the cells in MACS buffer and count ( take for FACS confirmation = PRE 50µLfor FACS confirmation = PRE)
Wash down again and resuspend the pellet according to the protocol (130-046-702 MACS Human CD34+ kit)
Add 300µL of MACS buffer per 10^8 cells
Add 100µL of FcR-B reagent per 10^8 cells, mix it and incubate in fridge for 15 minutes
Add 100µLof CD34 beads per 10^8 cells to the suspension and mix and let it sit for 30 min (fridge)
Fill up with 50mL MACS buffer and strain through a blue strainer (40 uM)
Wash cells (500g6 min) and resuspend in MACS buffer 500 uL/200,000,000 cells. If you have more cells, increase volume accordingly. E.g 3 *10^9 cells = 7,5mL. Aliquot this volume to more than one (with 3mL prerinsed) MACS column.
Wash with buffer 3mL 3 times and keep negative fraction ( Take 50µL for FCM = POST neg )
Put the column out of the magnet and push out positive fraction with 5mL Buffer and the plunger
Collect the positive fractions. (Take 50µL for FCM = Post pos)
Process the cells as desired (injection in mouse or cryopreservation)
Check the puritiy with FACS