Automated Remote Pulse Oximetry System (ARPOS) Methodology and Protocol
Pireh Pirzada, David Morrison, Gayle Doherty, Devesh Dhasmana, David Harris-Birtill
Abstract
Participants took part in the Automated Remote Pulse Oximetry System (ARPOS) study where their vitals including heart rate and blood oxygenation level were measured using a camera-based system. Participants were asked to stand or sit in front of a Kinect V2 camera in resting state and later also retake vitals after doing some light exercise. The study took approximately 35 to 45 minutes per participant. In order to take part in the research study, participants were required to have an XBOX One or a Windows OS computer.
The anonymized data including the regions of interest extracted from the face (forehead, cheeks and lips) collected from the study are available from DOI: https://doi.org/10.5281/zenodo.6522389 (Pireh Pirzada, Automated Remote Pulse Oximetry System (ARPOS), May 2022).
Before start
COVID guideline: Please wash your hands before using the equipment.
Steps
Study Information and Sign Up
Participants were asked to signup for the research study using the Qualtrics consent form. All participants were required to have access to at least a Windows OS PC or an XBOX One to sign up for the study. Participants within the UK provided information regarding the equipment they had access to and details necessary to post equipment and a gift voucher to thank them for their participation. Participants outside the UK (worldwide) were selected on the basis that they had access to the required equipment. In cases where international participants did not have the same commercial pulse oximeter, they were provided the option to use any device that could record vital data or to use the manual method of placing fingers on their pulse to measure heart rate.

Once the consent form was completed, an autogenerated unique participant-id (for example, PI-001) was shown to the participants. An autogenerated email configured using Qualtrics was also sent to the participants with the consent and participation information summary. The email also included their unique participant id number and a link to the study website to guide them further.
The ARPOS study website provided the study description, the application's latest version published on Microsoft Store, requirements for taking part in the research study, step guide for the research study (for Windows and XBOX One users), QR code and links to the content form and contact information of the researcher.


Equipment Postage within the UK
The researcher contacted and confirmed postal information from participants within the UK who required equipment to take part in the research study. International participants were also contacted if they had any questions regarding the study. The researcher activated international participants' unique Id once they confirmed they had access to all the required equipment. The equipment package to UK participants included Kinect V2, Kinect V2 adapter to USB, and Wellue commercial pulse oximeter device. A 10 GBP book voucher to thank them for their participation was also included in this package.
Equipment
| Value | Label |
|---|---|
| Wellue Bluetooth Finger Pulse Oximeter | NAME |
| Pulse Oximeter | TYPE |
| Wellue | BRAND |
| FS20F | SKU |
Equipment
| Value | Label |
|---|---|
| Kinect Adapter for Windows PC and XBOX One | NAME |
| Adapter | TYPE |
| Microsoft | BRAND |
| B07HT4224B | SKU |
Equipment
| Value | Label |
|---|---|
| Kinect V2 | NAME |
| Colour, IR and Depth Camera | TYPE |
| Microsoft | BRAND |
| KinectV2 | SKU |
The researcher packed all the required equipment in the package box, covered it using the black postage bag provided by the University, and labeled it to the participant's address. The researcher also activated participants' unique Id in the database on the server side.
Once the researcher was ready, they informed the University admin team to collect the equipment, which was then arranged via the University post.
The courier collected the package from the researcher and posted it to the participant's address. This would usually take two to three (72h 0m 0s) working days from the time of collection.
The participant then informed the researcher via email that the equipment had been received.
Conducting the research study
Participants were asked to set up the applications and equipment before starting the research study. They were also informed that the study was designed to take approximately 0h 45m 0s minutes.
Download the Wellue application from the smartphone store (Android or Iphone) and connect it to the pulse oximeter device.
Link to Android Application: https://play.google.com/store/apps/details?id=com.viatom.vihealth&hl=en_GB&gl=US
Link to Wellue Application: https://apps.apple.com/us/app/vihealth/id1413644737
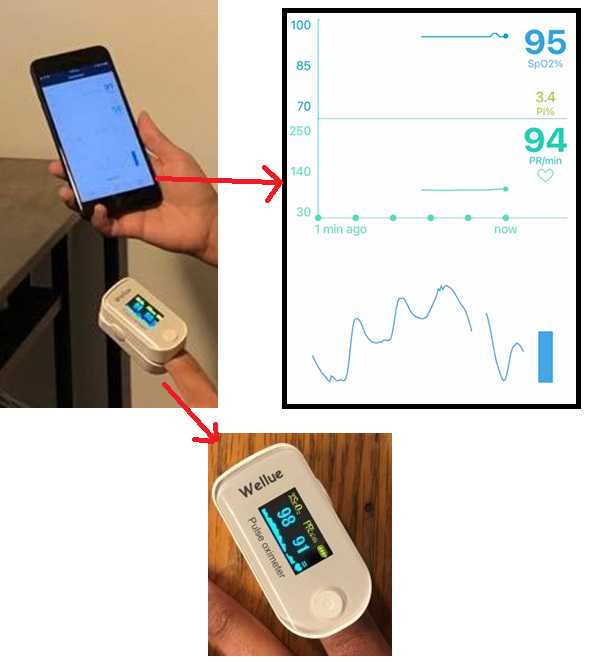
Once the setup mentioned above is complete, the participants can start the screen recording on their smart phone to record vital data from the pulse oximeter device. They can also start the acquisition from the ARPOS system simultaneously while facing the camera. The setup will look like the image presented below:

Executing and navigating ARPOS application
Participants were required to login to the application using their unique PI number provided on the consent form and email.
The application shows data acquisition state which updates automatically after completion of each round.
When ready, click on the 'start acquisition' button to start the data acquisition process from the ARPOS system. Make sure to start screen recording of vital data from the ground truth device and note down the start time at this point.

The log is displayed on the user interface of the application. This will alert when the data acquisition process has completed. A button named 'Process without sending data to server' will appear, click on this button to locally process colour, infrared and depth data.
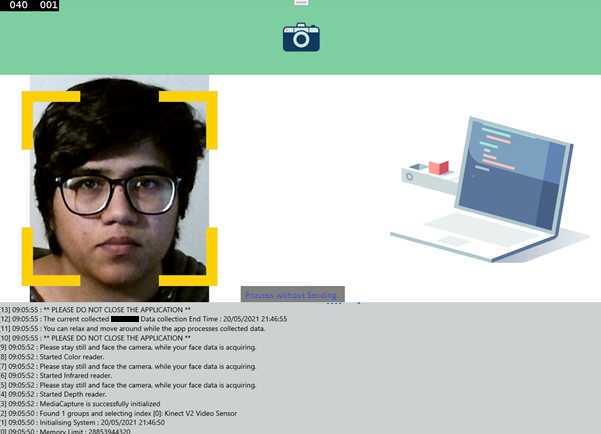
The application will give the option to send data to the server directly. In case the internet connectivity is not stable, the participant can copy the data from the path shown on the user interface and share that with the researcher.

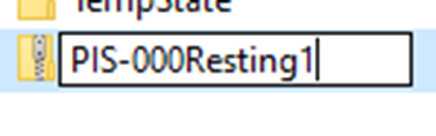
Compress the data to a folder and name it using the participant number id and vital status as shown in figure below and share it using one drive. Participants were also required to include ground truth screen recording of their smart phones or text files if any other device, or manual method was used to obtain ground truth vital data.
Once the researcher confirms that the data has been received, the shared folder is deleted.
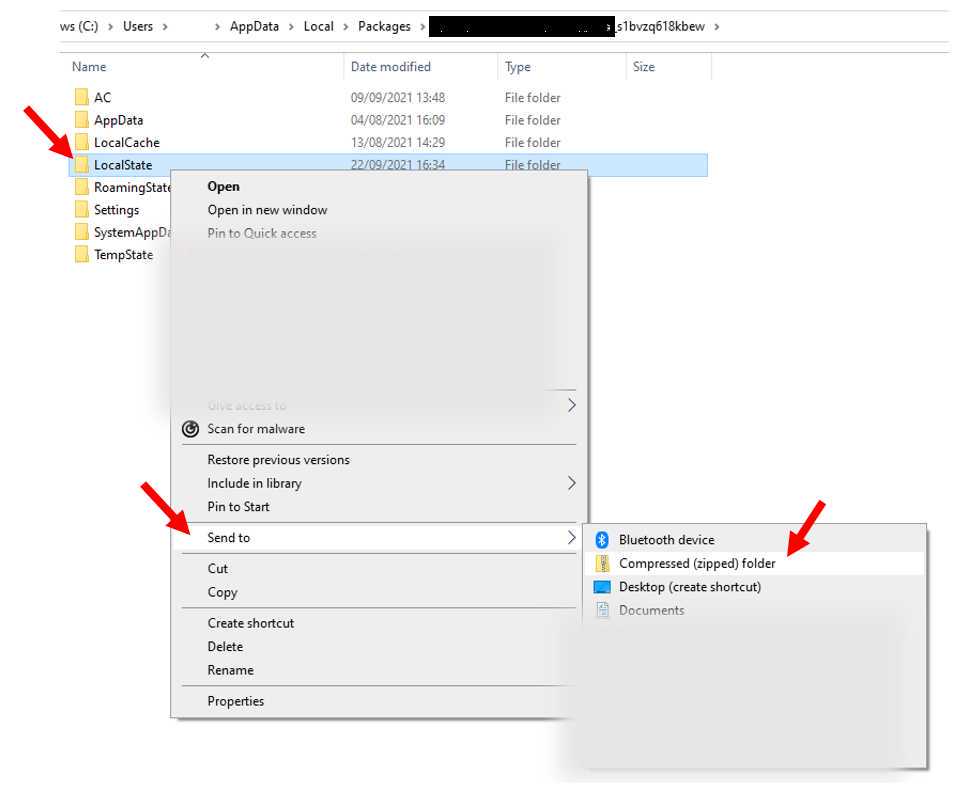
Participants were required to conduct the study for two times at rest e.g. sitting on a chair. They were also required to re-conduct the study immediately after 0h 1m 0s minute of exercise, this is so the system could be stress tested for active state vitals i.e. raised heart rate.
Thank you
Returning Equipment
Once the participant completed the study, they would repack the package according to the instructions provided (COVIDprotocol.pdf) and inform the researcher to arrange collection.
The researcher would then ask the University admin team to arrange collection and delivery of the package via courier from the participant to be delivered back to the researcher's address.