Assembly and sterilization of EcoFAB 2.0 for plant growth experiments
Peter Andeer, Vlastimil Novak
Abstract
The following instructions are for the sterilization, and assembly of EcoFAB 2.0 for studying small plants and their microbiomes during growth.
Steps
Materials
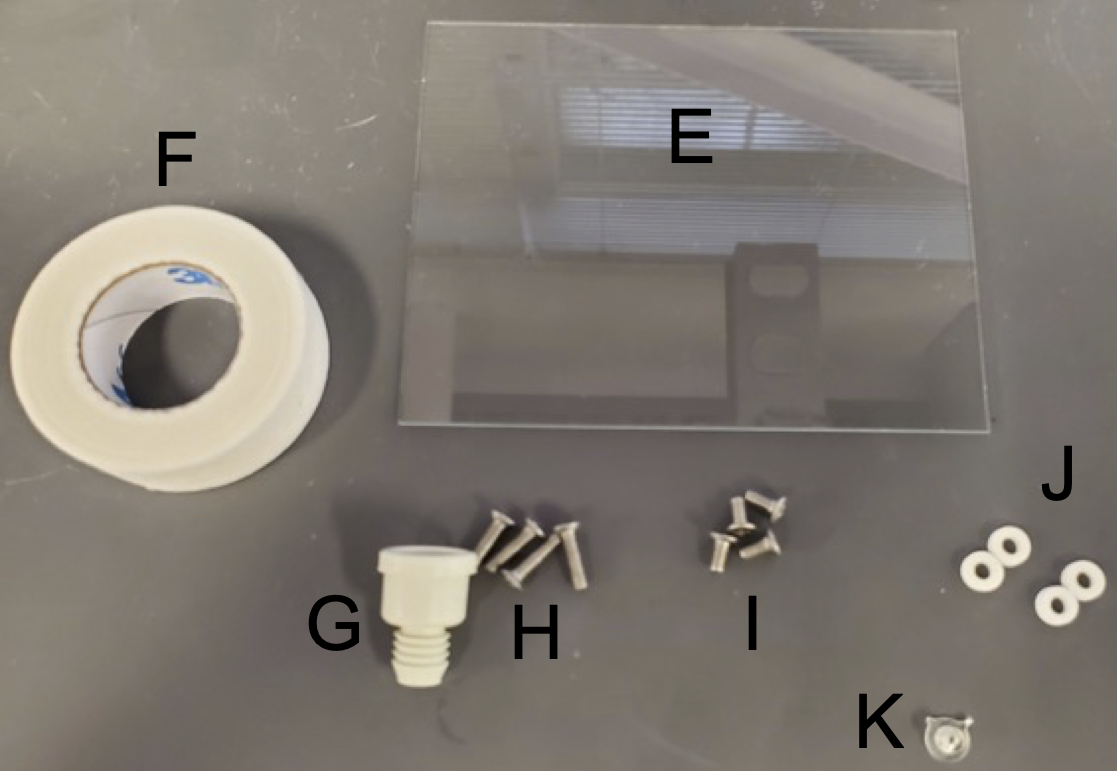
EcoFAB 2.0 parts supplied by LBNL (quantity per device)
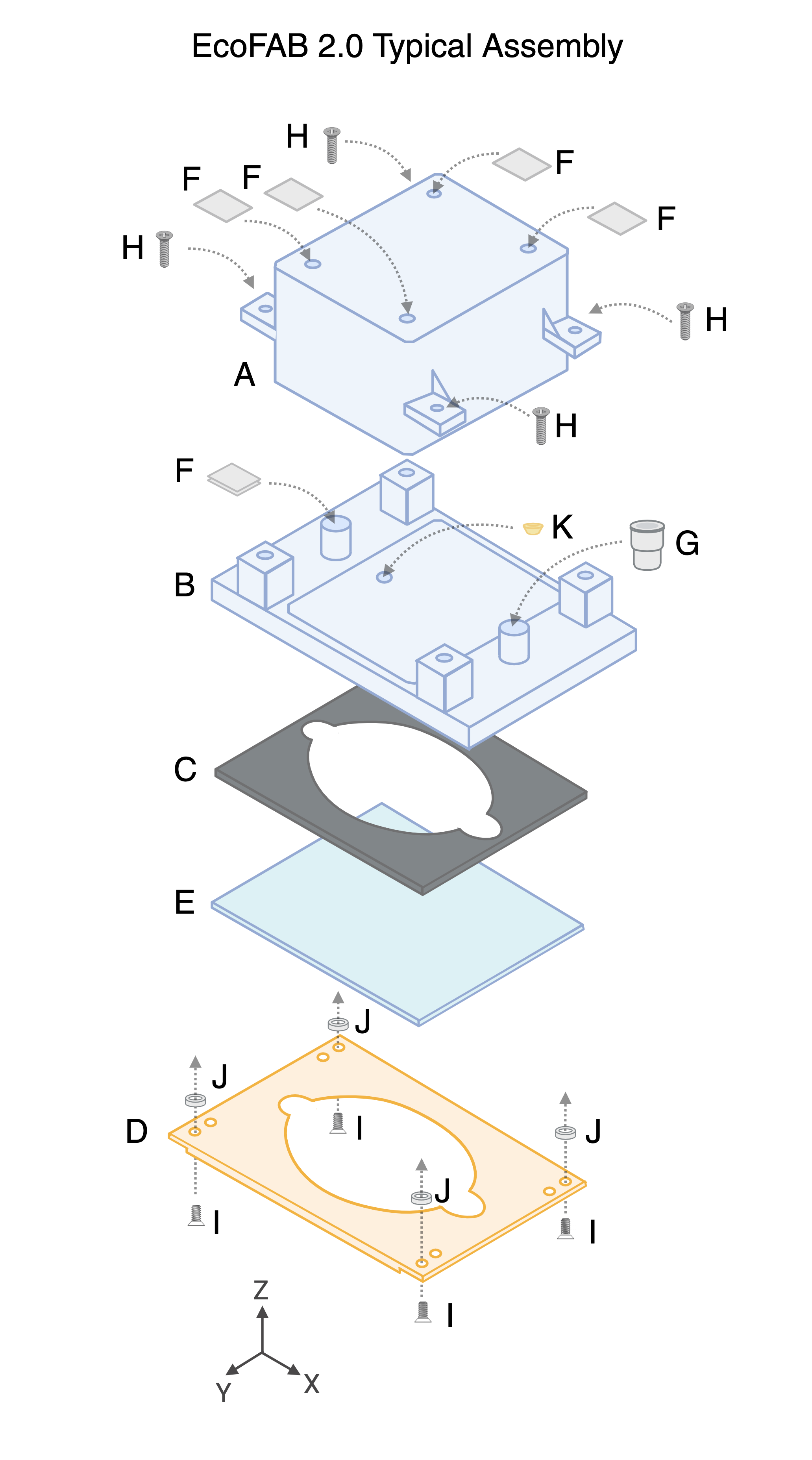
A. EcoFAB Chamber, Clear Polycarbonate (1)
B. EcoFAB Base, Polycarbonate (1)
C. Gasket, Silicone Rubber (1)
D. Backing Plate, Polycarbonate with 20% Glass Fiber (1)
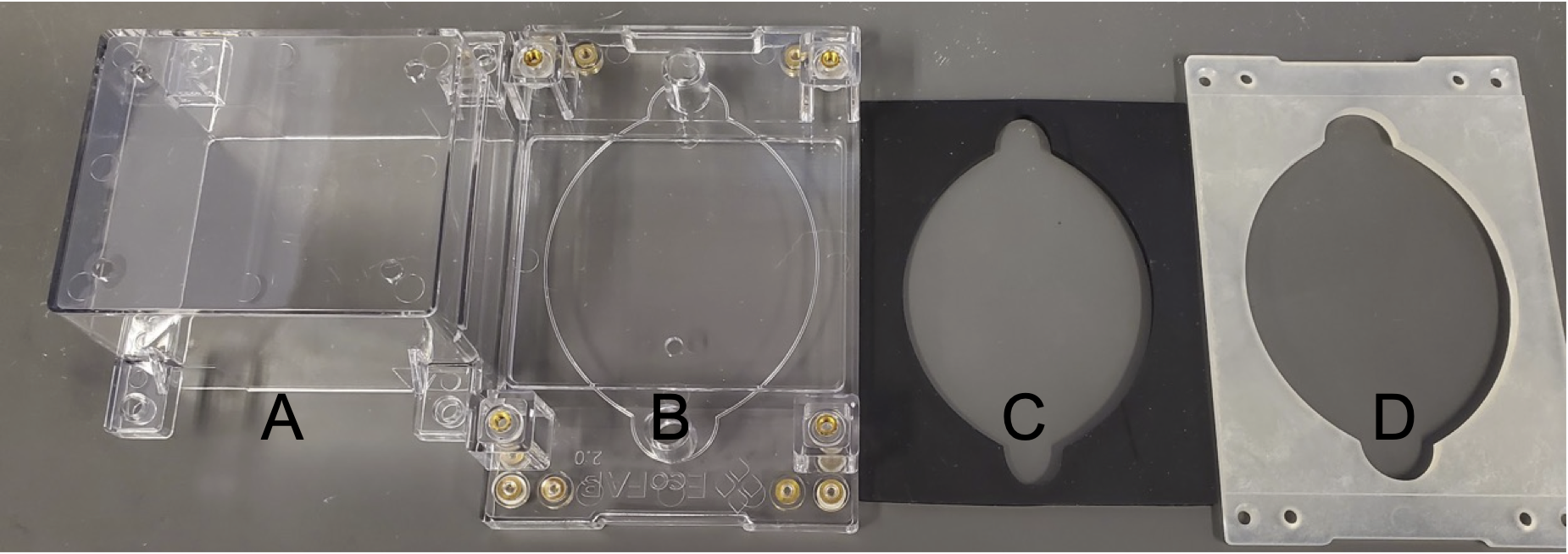
Additional parts that may need to be purchased separately (quantity per device / supplier / supplier ID / quantity per package)
E. 4” x 3” Glass slide (1 / Ted Pella / 260230-50 / 50)
F. 3M Micropore Tape 1530-0 (a few pieces / FischerSci / 19-061655 / 24 rolls)
G. Suba-Seal 8 mm septa (1 / Sigma Aldrich / #Z167231 / 100)
H. 4/40 x 7/16” screws (4 / McMaster Carr / 91099A459 / 100)
I. 4/40 x 1/4” screws (4 / McMaster Carr / 91099A155 / 100)
J. ¼” OD EPDM Rubber Washers ( 4 / McMaster Carr / 99186A111 / 100)
K. 3D Printed Seed Inserts (1 / LBNL - optional based on request)
Consumables that may be required
L. Serological pipette
M. Syringes
N. 21 G needles
O. 0.22 µm syringe filters (PES)
P. Sterilization pouches or aluminum foil to cover during sterilization.
Q. Manual or Willdone 008 electric screwdriver with Philips #1 head (Amazon, B08LPYPTV9)
R. Tweezers
S. Scissors
Notes on materials
The gaskets sometimes have an odor due to the manufacturing process. It is recommended to remove any plastic coatings from the gasket and wash it with alcohol (ethanol or isopropanol) and then purified water and then allow them to sit out in a well-ventilated area (e.g., biological safety cabinet or fume hood) overnight before use.1. The EcoFAB chamber, base, and backing plates can be reused; however, after repeated autoclaving, they may warp somewhat. We have not done extensive testing to determine how many times these parts can be reused. To clean, a laboratory-style dishwasher or soap should be suitable. Alcohol may streak the polycarbonate parts.
- The gasket will often fuse with the glass slide after experiments, and both parts will need to be replaced.
- One type of suba seal has been listed. There are other options available through Sigma Aldrich.
- Seed inserts are not required. However, several have been designed for smaller seeds that are 3D printed out of biologically compatible resin that can be autoclaved.
- Alternatively to glass slides, the design is also compatible with thinner materials like 0.55 mm thick gorilla glass. These can be obtained from specialty manufacturers.
- Instead of covering the holes on top of the chamber with micropore tape. Breathe-Easy or Breathe-Easier membrane material can also be used (Diversified Biotech, #BMTM-1000 or BMTM-2000).
- The EcoFAB Base will pick up fingerprints that are visible under the microscope, so wiping down the surface with a paper towel and wearing gloves is recommended.
Sterilization
Most of the parts for the EcoFAB 2.0 can be sterilized by autoclaving. For most uses it is recommended to partially assemble the EcoFABs, autoclave, and then finish assembly, add media and seedlings within a sterile environment (e.g., biological safety cabinet).1. The one part that can’t be autoclaved is the micropore tape. Misting with 70% ethanol or UV may be used, but typically isn’t necessary if handled carefully.
Assembly
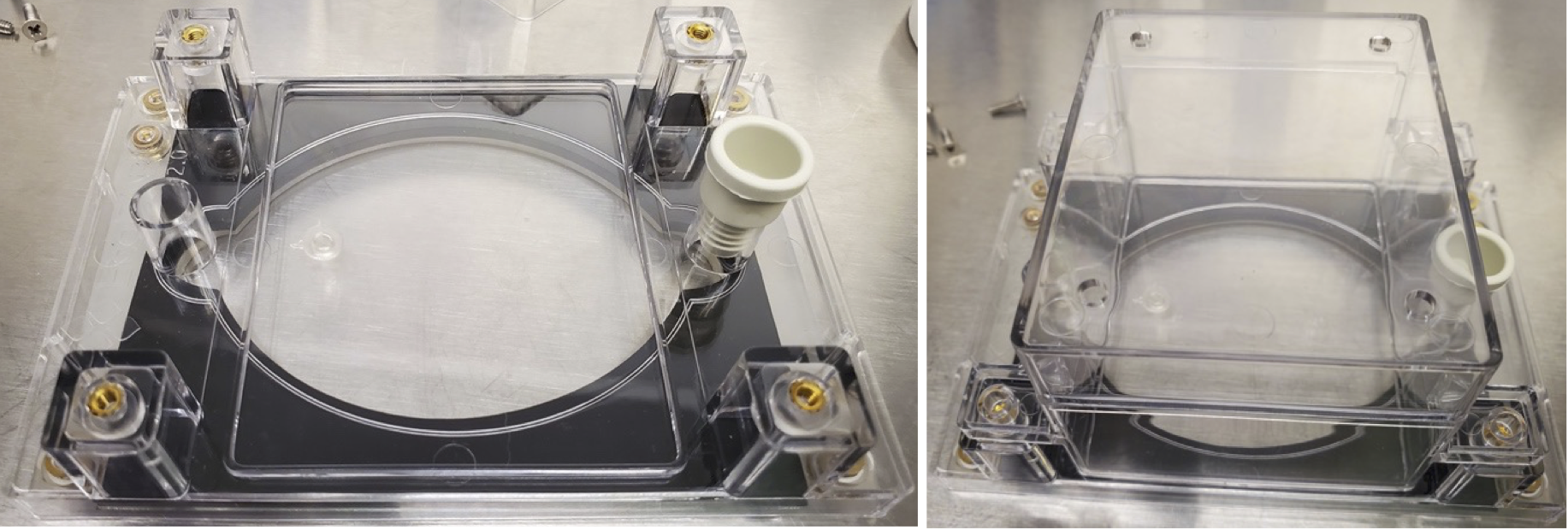
Place the EcoFAB base upside down on a clean surface and lay the gasket down between the threaded inserts. If the gasket has matte and glossy surfaces, place the matte side up and facing the glass so the slide can be adjusted after autoclaving if needed.

Place 4 washers on the four outer threaded inserts. Without the washers screwing in, the backing plate will need to be done very carefully, or the slide will crack, or the device will leak. Afterward, place the backing plate on top of everything with the protruding side facing up. Note: the backing plate is not reversible. Usage in the other direction is for instances where a thinner slide is used. In that case, the 4 internal threaded inserts will be used. You can tell this based on the indents in the backing plate screw taps.
Take the 4 shorter screws (¼” long) and lightly thread them into the four outer ports. If the devices will be sterilized, do not over-tighten the screws enough to distort the washers, or the slide is likely to crack during autoclaving. The screws should be just tight enough so that the backing plate doesn’t fall off when it is turned over.
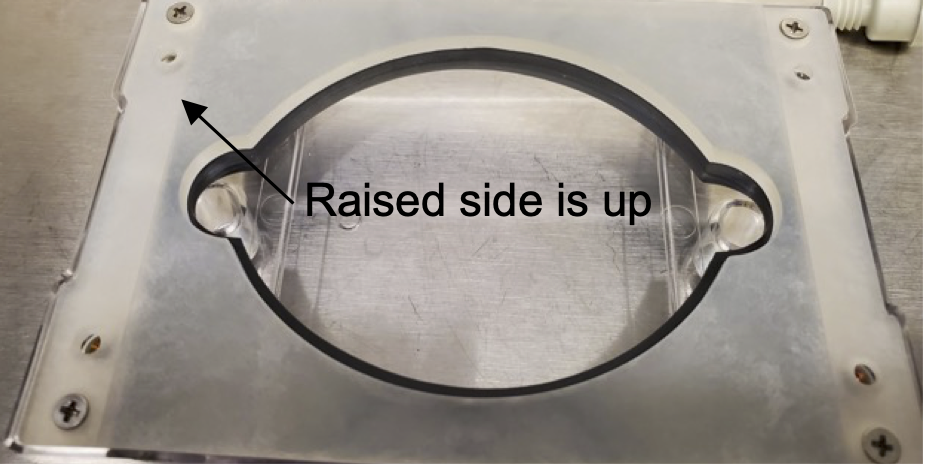
After sterilization, unpack the EcoFABs inside a biological safety cabinet or another sterile environment. Remove the chamber and turn the EcoFAB upside down to tighten the bottom screws. If any screws fall out during sterilization, make sure the washers are aligned with the screws and the slide and gasket with the ports. The screws should be tightened until the washers distort and begin to flatten.

Place micropore tape pieces over the 4 top chamber vents (or other breathable covering like Breathe-Easy membranes). Place two pieces of micropore tape over the rear sampling port (near the seed insert) to allow air exchange during filling.

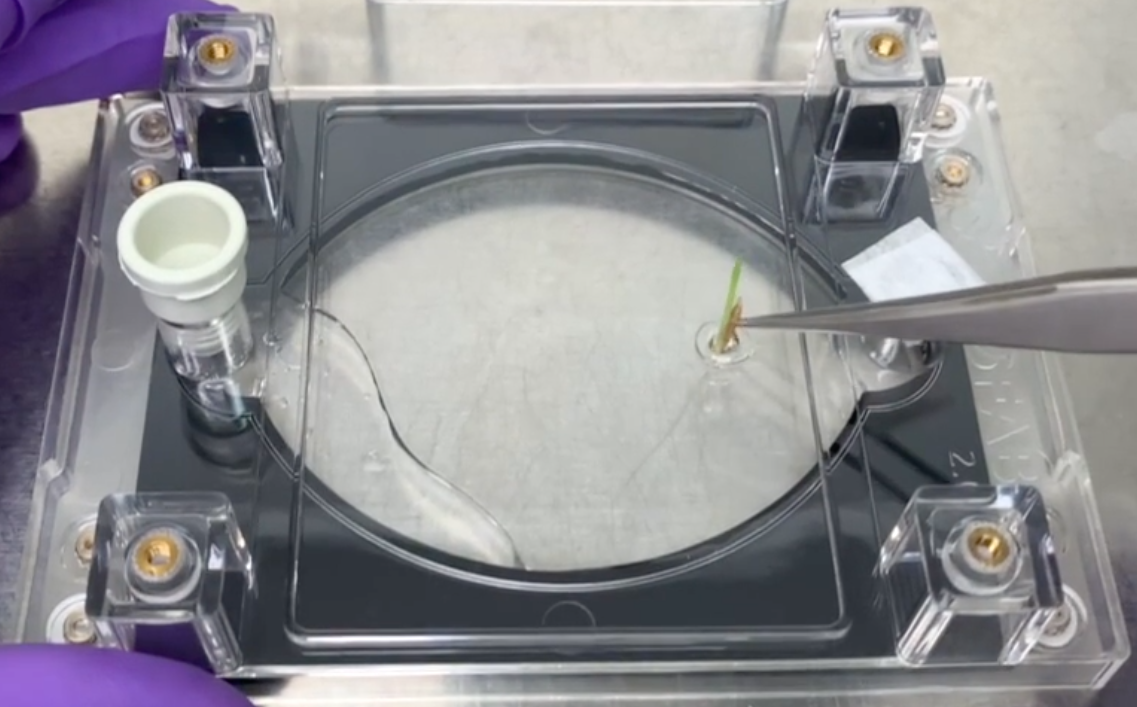
To fill the EcoFABs, prop the EcoFABs on an angle and fill from the bottom port either with a serological pipet with the septa removed or through the septa with a syringe and needle. Approximately 10 ml of liquid will fill the root chamber. Using sterile tweezers, place a seedling in the seed port/holder.
see