Analysis of Globotriaosylceramide (Gb3) in Liquid Urine: A Straightforward Assay Using Tandem Mass Spectrometry
Michel Boutin, Michel Boutin, Bruno Maranda, Bruno Maranda, Paula J. Waters, Paula J. Waters
Abstract
Fabry disease (FD) is a lysosomal storage disorder caused by variants in the GLA gene encoding α-galactosidase A, an enzyme required for catabolism of globotriaosylceramide (Gb3). Accumulation of Gb3 in patients’ cells, tissues, and biological fluids causes clinical manifestations including ventricular hypertrophy, renal insufficiency, and strokes. This protocol describes a methodology to analyze urinary Gb3 and creatinine. Samples are diluted with an internal standard solution containing Gb3(C17:0) and creatinine-D3, centrifuged, and directly analyzed by ultra-high performance liquid chromatography coupled to tandem mass spectrometry (UHPLC-MS/MS) using an 8.7-min method. Eight Gb3 isoforms [C16:0, C18:0, C20:0, C22:1, C22:0, C24:1, C24:0, and (C24:0)OH] are analyzed and the total is normalized to creatinine. Confirmation ions are monitored to detect potential interferences. The Gb3 limit of quantification is 0.023 µg/ml. Its interday coefficients of variation (3 concentrations measured) are ≤15.4%. This method minimizes matrix effects (≤6.5%) and prevents adsorption or precipitation of Gb3. Urine samples are stable (bias <15%) for 2 days at 21°C, 7 days at 4°C, and 4 freeze/thaw cycles, whereas prepared samples are stable for 5 days at 21°C, and 14 days at 4°C. The Gb3/creatinine age-related upper reference limits (mean + 2 standard deviations) are 29 mg/mol creatinine (<7 years) and 14 mg/mol creatinine (≥7 years). This simple, robust protocol has been fully validated (ISO 15189) and provides a valuable tool for diagnosis and monitoring of FD patients. © 2024 The Authors. Current Protocols published by Wiley Periodicals LLC.
Basic Protocol : Analysis of urinary globotriaosylceramide (Gb3) and creatinine by UHPLC-MS/MS
Support Protocol 1 : Preparation of the urinary quality controls
Support Protocol 2 : Preparation of the urine matrix used for the Gb3 calibration curve
Support Protocol 3 : Preparation of the Gb3 calibrators
Support Protocol 4 : Preparation of the working solution containing the internal standards
Support Protocol 5 : Preparation of the creatinine calibrators
Support Protocol 6 : Preparation of the UHPLC solutions and mobile phases
INTRODUCTION
Fabry disease (FD) is an X-linked inborn error of metabolism caused by α-galactosidase A deficiency. This enzyme is required for the catabolism of globotriaosylceramide (Gb3; Fig. 1) (Ortíz et al., 2018). The accumulation of Gb3 and its deacylated form (lyso-Gb3) in biological fluids and tissues causes different clinical manifestations, such as left ventricular hypertrophy, progressive renal failure, ischemic stroke, acroparaesthesias, cornea verticillata, angiokeratomas, and hearing loss (Palaiodimou et al., 2023). In general, FD is more severe for hemizygous males compared to heterozygous females. However, due to X-chromosome inactivation, also called “lyonization”, some women can be as severely affected by FD as men (Izhar et al., 2023). The main FD-specific treatments are enzyme replacement therapy (Oder et al., 2016) and chaperone therapy for patients with amenable mutations (Nowicki et al., 2024). There are also some other FD treatments in development, such as substrate reduction therapy and gene therapy (Palaiodimou et al., 2023; Umer & Karla, 2023). Urinary Gb3 is used as a biomarker for the diagnosis of FD and to monitor the response to treatment (Wanner et al., 2018). In general, the urinary levels of Gb3 are higher for FD patients compared to healthy controls. However, normal levels of urinary Gb3 cannot exclude the diagnosis of FD for heterozygous females or males with a late-onset form of the disease (Carnicer-Cáceres et al., 2021). The diagnosis of FD must be confirmed by α-galactosidase A activity testing and/or by the detection of a disease-causing GLA gene variant (Ortíz et al., 2018).
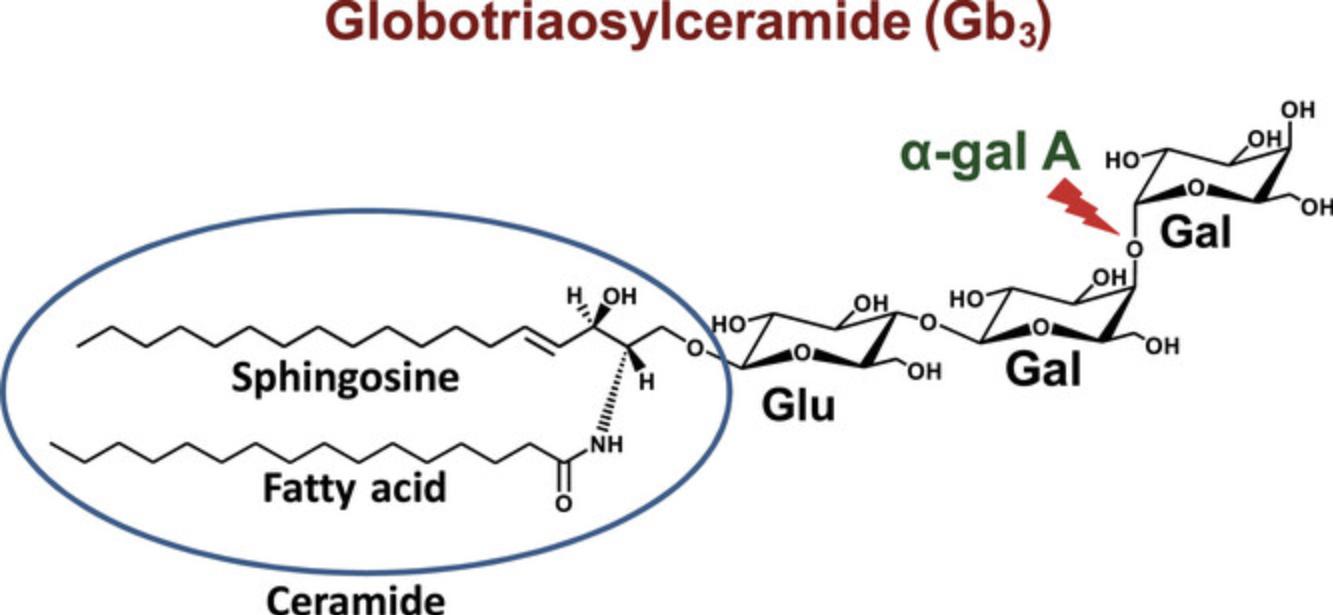
Previously published methods for the analysis of Gb3 in urine from FD patients require time consuming liquid-liquid extractions (Abaoui et al., 2016; Boutin et al., 2017; Heywood et al., 2019; Shiga et al., 2021), solid phase extractions (Gaggl et al., 2015), or the deposition of the urine specimen on filter paper followed by an extraction with methanol (Auray-Blais et al., 2017). Starting with the filter paper method (Auray-Blais et al., 2017) that was previously used in our laboratory for the analysis of urinary Gb3, we modified it to simplify and shorten the sample preparation and to improve the method's robustness. With this new protocol, the urine specimens are only diluted with a solution containing the two internal standards and centrifuged prior to their UHPLC-MS/MS analysis. In contrast, for the previous method, the urine specimens were deposited on filter papers and dried overnight; thereafter, the two internal standards were individually added, then the filters were dried again for four hours and finally extracted with methanol (one hour shaking) prior to sample analysis. Our new method allows the analysis of only 50 µl of urine instead of 1 ml, which is an asset for low volume pediatric specimens.
The water contained in the urine samples analyzed tends to decrease the reproducibility of the results due to precipitation/adsorption of the hydrophobic Gb3. To overcome this problem, we carefully optimized the water/methanol ratio used during the sample preparation. Similarly, the Gb3 solutions used for the calibration curve were difficult to redissolve after storage in the freezer, which could cause variability in the results. To solve this problem, the Gb3 solubility was enhanced by choosing the appropriate mixture of chloroform and methanol as solvent. For the new method, the Gb3(C17:0) and the creatinine-D3 internal standards were mixed to allow their simultaneous addition in the samples. Compared to the previous method, the samples were more dilute, and the chromatographic separation was optimized to minimize matrix effects (ion suppression/enhancement). Confirmation ions were also added for Gb3, creatinine, and their internal standards to detect the presence of potential interferences. All chromatographic peaks corresponding to the eight Gb3 isoforms analyzed are integrated together to decrease the time required for data analysis. It is also a great advantage to analyze Gb3 and creatinine simultaneously, which is used to normalize the urine concentration. Specimens from a total of 140 healthy controls were also analyzed to establish the normal reference ranges for the Gb3/creatinine levels in urine. No significant differences were observed between males and females, but we found higher levels of Gb3/creatinine for young children (<7 years) compared to older individuals. For this reason, two different age-related reference ranges were established.
The experimental approach presented here is a valuable tool for the diagnosis and monitoring of FD. This protocol describes, step-by-step, a very simple, rapid and robust procedure for the analysis of Gb3 in urine. The preparation of the quality controls, the urine matrix, the working solution containing the internal standards, the working solutions for the calibration curves, and the solutions used for the UHPLC-MS/MS analyses are also described in detail.
STRATEGIC PLANNING
The UHPLC-MS/MS system can be started and conditioned (Basic Protocol, steps 20 to 23) during thawing and preparation of samples.
CAUTION : The usual precautions for processing biological samples must be respected. Manipulate solvents under a chemical fume hood.
NOTE : Appropriate informed consent is necessary for obtaining and use of human study material.
Basic Protocol: ANALYSIS OF URINARY GLOBOTRIAOSYLCERAMIDE (Gb3) AND CREATININE BY UHPLC-MS/MS
Urine samples are diluted with an internal standard (ISTD) solution containing Gb3(C17:0) and creatinine-D3, centrifuged, and analyzed by UHPLC-MS/MS. A Gb3 calibration curve is prepared using control urine as matrix, and a creatinine calibration curve is prepared in water.
Materials
-
Urine quality controls (see Support Protocol 1)
-
Urine matrix used for the Gb3 calibration curve (see Support Protocol 2)
-
Gb3 calibrators (see Support Protocol 3)
-
Working solution containing the internal standards (ISTD) (see Support Protocol 4)
-
Creatinine calibrators (see Support Protocol 5)
-
Methanol (MeOH) Optima LC/MS grade (Fisher Scientific, cat. no. A456-4)
-
99% formic acid (FA) (Thermo Scientific, cat. no. 270480010)
-
H2O, Optima LC/MS grade (Fisher Scientific, cat. no. W6-4)
-
Plastic containers for urine collection (sterility is not required)
-
Freezer, −20°C
-
Refrigerator, 4°C
-
Vortex (Velp Scientifica, Wizard advanced IR vortex mixer)
-
P1000, P200, and P20 pipettes with associated tips
-
2-ml polypropylene tubes with screw cap (Sarstedt, cat. no. 72.693)
-
Benchtop centrifuge capable of 18,000 × g (e.g., Beckman Coulter Microfuge 18)
-
2-ml screw thread glass vials (Chromatographic Specialties, cat. no. C779100W)
-
Pre-slit screw caps with polytetrafluoroethylene (PTFE)/silicone septums for 2-ml vials (Chromatographic Specialties, cat. no. C779200XBB)
-
UHPLC-MS/MS system (Waters, Acquity I-Class/Xevo TQ-S Micro)
-
Analytical guard column, Zorbax Bonus-RP, 4.6-mm × 12.5-mm, 5-micron (Agilent, cat. no. 820950-928)
-
Zorbax guard column holder (Agilent, cat. no. 820999-901)
-
Acquity column in-line filter (Waters, cat. no. 205000343)
-
MassLynx software v4.2 SCN1040 with TargetLynx option (Waters)
Urine sample collection
1.Collect urine samples in hermetic plastic containers that do not need to be sterile.
2.Store the samples at −20°C until their analysis.
Preliminary steps
3.Thaw the following samples/solutions:
- Urine specimens to analyze
- Quality controls (negative—healthy control, heterozygous FD, and hemizygous FD)
- Urine matrix used for the Gb3 calibration curve (creatinine ∼5 mM)
- Gb3 calibrators (P0 to P7)
- Working solution containing the internal standards (ISTD)
- Creatinine calibrators (cP0 to cP5).
4.Prior to their use, leave the Gb3 calibrators and the ISTD at room temperature for 30 min, vortex them for 10 s, wait another 30 min and vortex them again for 10 s.
Sample preparation
Creatinine calibration curve (a)
5.Place 800 µl ISTD working solution in a 2-ml polypropylene tube (screw cap) with a P1000 pipette.
6.Add 150 µl MeOH with a P200 pipette.
7.Add 50 µl creatinine calibrator solution (cP0 to cP5) with a P200 pipette.
Gb3 calibration curve (b)
8.Place 800 µl ISTD working solution in a 2-ml polypropylene tube (screw cap) with a P1000 pipette.
9.Add 150 µl Gb3 calibrator solution (P0 to P7) with a P200 pipette.
10.Add 50 µl urine matrix used for the Gb3 calibration curve with a P200 pipette.
Urine samples and quality controls (c)
11.Place 800 µl ISTD working solution in a 2-ml polypropylene tube (screw cap) with a P1000 pipette.
12.Add 150 µl MeOH.
13.Add 50 µl urine sample with a P200 pipette.
For (a), (b) and (c)
14.Vortex all the tubes for 5 s.
15.Centrifuge all the tubes 5 min at 18,000 × g , room temperature.
16.Transfer 900 µl supernatant to a 2-ml screw thread glass vial with a P1000 pipette.
17.Cap the vial with a pre-slit screw cap. The samples are ready for their UHPLC-MS/MS analysis.
UHPLC-MS/MS system preparation
The chromatographic separation is performed on an Acquity I-class UHPLC system (Waters) equipped with a flow injector, and the tandem mass spectrometry analysis is achieved with a Xevo TQ-S Micro instrument (Waters).
18.Install the Zorbax ODS 4.6-mm × 12.5-mm guard column into the guard column holder. An Acquity in-line filter is connected to the guard column holder.
19.Install the UHPLC solutions and mobile phases (Support Protocol 6):
-
Line A1 = mobile phase A (MeOH + 0.1% FA).
-
Line B1 = mobile phase B (H2O + 0.1% FA).
-
Purge line = weak needle wash (WNW) (100% MeOH).
-
Wash line = strong needle wash (SNW) (50% MeOH, 50% H2O).
-
Seal wash line = seal wash (SW) (20% MeOH in H2O).
20.Start the system conditioning:
-
Prime lines A1, B1, and SW 3 min.
-
Prime the purge line for 20 cycles.
-
Prime the wash line for 120 s.
-
Wash the column with 100% phase A for 5 min at 0.5 ml/min.
UHPLC-MS/MS analysis
21.Load the UHPLC parameters according to Table 1.
| Parameter | Description |
|---|---|
| Autosampler temperature | 20°C |
| Column temperature | 23°C |
| Autosampler type | Flow through needle |
| Injection volume | 5 µl |
| Post-injection wash | 6 s |
| Weak needle wash solvent | 100% MeOH |
| Strong needle wash solvent | 50% MeOH, 50% H2O |
| Mobile phase A | MeOH + 0.1% FA |
| Mobile phase B | H2O + 0.1% FA |
| Flow rate | 0.5 ml/min |
| Gradient (% mobile phase A) |
0-1.5 min: 50% 1.5-2.0 min: 50%-85% (linear gradient) 2.0-3.0 min: 85%-90% (linear gradient) 3.0-6.5 min: 90%-95% (linear gradient) 6.5-8.0 min: 50% |
22.Load the MS parameters according to Tables 2 and 3.
| Parameter | Description |
|---|---|
| Ionization mode | Positive electrospray |
| Capillary voltage | 3.5 kV |
| Desolvation temperature | 350°C |
| Desolvation gas flow | 600 L/hr |
| Cone gas flow | 0 L/hr |
| Source temperature | 150°C |
| Dwell time | 0.03 s |
| Quantification analysis | Confirmation analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Molecule | Transition (m/z) |
Cone voltage (V) |
Collision energy (V) |
Analytical function |
Transition (m/z) |
Cone voltage (V) |
Collision energy (V) |
Analytical function |
| Creatinine | 114.07 > 44.05 | 20 | 10 | 1 (0-1.5 min) | 114.07 > 86.07 | 20 | 10 | 1 (0-1.5 min) |
| Creatinine-D3 (ISTD) | 117.09 > 47.07 | 20 | 10 | 1 (0-1.5 min) | 117.09 > 89.09 | 20 | 10 | 1 (0-1.5 min) |
| Gb3(C16:0) | 1024.68 > 520.51 | 70 | 30 | 2 (3-7.5 min) | 1024.68 > 264.27 | 70 | 53 | 3 (3-7.5 min) |
| Gb3(C18:0) | 1052.71 > 548.54 | 70 | 30 | 2 (3-7.5 min) | 1052.71 > 264.27 | 70 | 53 | 3 (3-7.5 min) |
| Gb3(C20:0) | 1080.74 > 576.57 | 70 | 30 | 2 (3-7.5 min) | 1080.74 > 264.27 | 70 | 53 | 3 (3-7.5 min) |
| Gb3(C22:1) | 1106.76 > 602.59 | 70 | 30 | 2 (3-7.5 min) | 1106.76 > 264.27 | 70 | 53 | 3 (3-7.5 min) |
| Gb3(C22:0) | 1108.77 > 604.60 | 70 | 30 | 2 (3-7.5 min) | 1108.77 > 264.27 | 70 | 53 | 3 (3-7.5 min) |
| Gb3(C24:1) | 1134.79 > 630.62 | 70 | 30 | 2 (3-7.5 min) | 1134.79 > 264.27 | 70 | 53 | 3 (3-7.5 min) |
| Gb3(C24:0) | 1136.80 > 632.63 | 70 | 30 | 2 (3-7.5 min) | 1136.80 > 264.27 | 70 | 53 | 3 (3-7.5 min) |
| Gb3(C24:0)OH | 1152.80 > 648.63 | 70 | 30 | 2 (3-7.5 min) | 1152.80 > 264.27 | 70 | 53 | 3 (3-7.5 min) |
| Gb3(C17:0) (ISTD) | 1038.69 > 534.53 | 70 | 30 | 4 (3-7.5 min) | 1038.69 > 264.27 | 70 | 53 | 4 (3-7.5 min) |
- a
The Gb3 fragments for the quantification analysis correspond to the dehydrated ceramides, whereas the fragment for the confirmation analysis corresponds to the di-dehydrated sphingosine. The collision energy for creatinine analysis was chosen to decrease its signal and thus prevent saturation of the detector.
23.Analyze the samples according to the following sample list:
-
7 times the hemizygous control as conditioning.
-
A blank sample (5% H2O in MeOH).
-
The creatinine calibration curve (cP0 to cP5).
-
The Gb3calibration curve (P0 to P7).
-
A blank sample (5% H2O in MeOH).
-
Negative (healthy) control.
-
Positive control (heterozygous FD).
-
Positive control (hemizygous FD).
-
A blank sample (5% H2O in MeOH).
-
All the patient samples.
Data analysis
The quantification of Gb3 and creatinine must be performed independently (two TargetLynx files) since two different calibration curves are used. This is necessary for two reasons: (1) a urine matrix is required to stabilize Gb3 in the calibration curve and to decrease the matrix effect during the mass spectrometry analysis; and (2) the creatinine calibration curve cannot be prepared with a urine matrix, since it would contain creatinine.
For Gb3
24a. Analyze the results with TargetLynx XS software according to the following parameters:
1.The ratio between the total ion chromatogram (TIC) corresponding to the signal of the 8 Gb3 isoforms analyzed (Table 3, Function 2) and the ISTD [Gb3(C17:0)] (Table 3, Function 4) is analyzed for the quantification. 2.The calibration curve is 2nd order. 3.The origin is excluded. 4.A 1/x weighting is used for the calibration curve points. 5.For Gb3, the ratio between the TIC of the quantification ions (Table 3, Function 2) and the TIC of the confirmation ions (Table 3, Function 3) is analyzed to detect interferences. The target ratio is 0.47 ± 0.12. 6.For the ISTD [Gb3(C17:0)], the target ratio between the quantification and the confirmation ions (Table 3, Function 4) is 0.50 ± 0.10.
Ratios not in the target range are often observed for healthy controls and for the P1 point of the calibration curve, due to a low Gb3 signal.
For Creatinine
24b. Analyze the results with TargetLynx XS software according to the following parameters:
- The ratio between creatinine and its ISTD (creatinine-D3) is used for the quantification. Both transitions are in Function 1 of Table 3.
- The calibration curve is linear.
- The origin is excluded.
- A 1/x weighting is used for the calibration curve points.
- For creatinine and its ISTD, the target ratio between the quantification and confirmation ions (Table 3, Function 1) is 3.0 ± 0.3.
Support Protocol 1: PREPARATION OF THE URINARY QUALITY CONTROLS
Urine samples at three different Gb3 levels [negative control, positive control (heterozygous FD), and positive control (hemizygous FD)] are aliquoted and used as quality controls for each analysis batch. For the negative control, urine from a healthy control with a relatively high creatinine level (∼10 mM) is chosen. The urine used for the negative control must be sufficiently concentrated to be able to detect and quantify Gb3. For the first positive quality control, urine is chosen from a woman heterozygous for FD with a Gb3/creatinine level slightly over the upper limit of the reference range (14.0 mg Gb3/mol creatinine, age > 7 years). For the second positive control, urine is selected from a man hemizygous for FD with a high Gb3/creatinine level (>300 mg Gb3/mol creatinine).
Additional Materials (also see Basic Protocol)
- 10 ml of each urine specimen used as quality control
1.Vortex the urine sample to disperse the sediment well just before the pipetting of each aliquot.
2.Transfer 75 µl urine to a 2-ml polypropylene tube.
3.Cap the tube.
4.Repeat steps 1 and 3 for all urine aliquots to be prepared.
5.Store the tubes in the freezer (−20°C) until their use (stable for at least 1 year).
Support Protocol 2: PREPARATION OF THE URINE MATRIX USED FOR THE Gb3 CALIBRATION CURVE
The Gb3 calibration curve is prepared with a urine matrix to stabilize the Gb3 isoforms and to partially compensate for the matrix effect observed in the samples. Urine with a creatinine concentration of ∼5 mM, presenting no visible sediment particles, is used as matrix for the Gb3 calibration curve.
Additional Materials (also see Basic Protocol)
- 10 ml of the urine used as matrix
1.Vortex the urine sample.
2.Transfer 600 µl urine to a 2-ml polypropylene tube with a P1000 pipette.
3.Cap the tube.
4.Repeat steps 1 to 3 for all the aliquots to be prepared.
5.Store the tubes in the freezer (−20°C) until their use (stable for at least 1 year).
Support Protocol 3: PREPARATION OF THE Gb3 CALIBRATORS
The quantification of Gb3 is performed with a calibration curve prepared from a commercial mixture of Gb3 isoforms purified from porcine red blood cells.
Additional Materials (also see Basic Protocol)
-
Chloroform (CHCl3), ≥99.8% ACS grade (VWR, cat. no. BDH1109-4LG)
-
Gb3 (mixture of isoforms), ≥98%, 1 mg pre-weighed vial (Cayman Chemical, cat. no. 24870)
-
Pyrex 20-mm × 125-mm culture tubes with screw caps containing PTFE liners (Corning, cat. no. 9826-20)
-
Sonic bath (Cole Parmer, model 8845-3)
-
10-ml glass serological pipettes (Fisher Scientific, cat. no. 13-678-27F)
-
50-ml glass volumetric flask
-
Pasteur pipettes
-
Parafilm M (VWR, cat. no. 291-1211)
-
P5000 pipette with associated tips
-
4-ml glass sample vials with PTFE faced rubber lined caps (Millipore Sigma, cat. no. DWKW224604)
CHCl3:MeOH (2:1) solution
1.Add 14 ml CHCl3 and 7 ml MeOH to a 20-mm × 125-mm glass culture tube using glass serological pipettes.
2.Mix well.
Gb3 stock solution, 1 mg/50 ml
3.Add 1 ml CHCl3:MeOH (2:1) to the glass vial containing 1 mg Gb3 using a P1000 pipette.
4.Vortex 3 s and sonicate 1 min.
5.Transfer the solution quantitatively to a 50-ml glass volumetric flask using a Pasteur pipette.
6.Repeat steps 3 to 5 four times to be sure to retrieve all the Gb3 from the glass vial.
7.Add ∼25 ml MeOH to the volumetric flask.
8.Vortex and sonicate the volumetric flask alternately for as long as needed to dissolve all the Gb3 powder and to obtain a clear solution.
9.When the powder is dissolved, leave the volumetric flask on the bench 30 min to allow it to come back to room temperature.
10.Bring the volume in the flask up to 50 ml with MeOH and mix well.
11.Use the Gb3 stock solution to prepare the Gb3 calibrators and store the remaining part of the solution in 20-mm × 125-mm culture tubes with PFTE liner caps (stable at −20°C for at least 1 year).
Gb3 calibrators (P0 to P7)
12.According to Table 4, for each calibrator (P0 to P7), add the indicated volumes of MeOH, CHCl3:MeOH (2:1), and Gb3 stock solution (1 mg/50 ml) to a 4-ml glass vial or a 20 × 100 glass culture tube. P5000, P1000, P200, and P20 pipettes are used to prepare the solutions.
|
Calibrator |
MeOH (ml) | CHCl3:MeOH (2:1) (ml) | 1 mg/50 ml Gb3 (µl) | Total volume (ml) | Standard concentration (µg/ml) | Concentration in urine (µg/ml)a |
|---|---|---|---|---|---|---|
| P0 | 3.600 | 0.4 | 0.0 | 4 | 0.0000 | 0.0 |
| P1 | 22.490 | 2.5 | 10.4 | 25 | 0.0083 | 0.025 |
| P2 | 17.967 | 2.0 | 33.3 | 20 | 0.0333 | 0.1 |
| P3 | 3.573 | 0.4 | 26.7 | 4 | 0.1333 | 0.4 |
| P4 | 3.533 | 0.4 | 66.7 | 4 | 0.3333 | 1.0 |
| P5 | 3.400 | 0.4 | 200.0 | 4 | 1.0000 | 3.0 |
| P6 | 3.133 | 0.4 | 466.7 | 4 | 2.3333 | 7.0 |
| P7 | 2.800 | 0.4 | 800.0 | 4 | 4.0000 | 12.0 |
- a
For 150 µl of calibrator added and 50 µl of urine analyzed.
13.Mix the calibrators well and store them at −20°C (stable for at least 1 year).
Support Protocol 4: PREPARATION OF THE WORKING SOLUTION CONTAINING THE INTERNAL STANDARDS
A constant concentration of both internal standards [Gb3(C17:0) and creatinine-D3] is added to the samples and calibrators to compensate for losses during the sample preparation, matrix effects, and signal fluctuations during the mass spectrometry analyses.
Additional Materials (also see Basic Protocol)
-
CHCl3:MeOH (2:1) (see Support Protocol 3)
-
Gb3(C17:0), ≥98%, 0.5 mg pre-weighed vial (Cayman Chemical, cat. no. 24876)
-
Creatinine-D3 (methyl-D3), 99 atom % D, (CDN Isotopes, cat. no. D-3689)
-
Chloroform (CHCl3), ≥99.8% ACS grade (VWR, cat. no. BDH1109-4LG)
-
Pyrex 20-mm × 125-mm culture tubes with screw caps containing PTFE liners (Corning, cat. no. 9826-20)
-
Sonic bath (Cole Parmer, model 8845-3)
-
Pasteur pipettes
-
15-ml polypropylene centrifuge tube with cap (Corning, cat. no. 430766)
-
P5000 pipette with associated tips
-
250-ml glass bottle
Gb3(C17:0) stock solution, 0.5 mg/10 ml
1.Add 1 ml CHCl3:MeOH (2:1) (see Support Protocol 3) to the glass vial containing 0.5 mg Gb3(C17:0) using a P1000 pipette.
2.Vortex 3 s and sonicate 1 min.
3.Transfer the solution quantitatively to a 20-mm × 125-mm glass culture tube with screw cap using a Pasteur pipette.
4.Repeat steps 1 to 3 four times to be sure to retrieve all the Gb3(C17:0) from the glass vial.
5.Add 5 ml MeOH to the tube.
6.Vortex and sonicate the tube alternately for as long as needed to dissolve all the Gb3(C17:0) powder and to obtain a clear solution.
7.Use the Gb3(C17:0) stock solution to prepare the internal standard (ISTD) working solution and store the remaining part of the solution at −20°C (stable for at least 1 year).
Creatinine-D3 stock solution, 20 mg/10 ml
8.Accurately weigh 20 mg creatinine-D3 and quantitatively transfer it to a 15-ml polypropylene centrifuge tube.
9.Add 10 ml H2O with a P5000 pipette.
10.Store at 4°C (stable for at least 1 year).
Internal standard (ISTD) working solution, 0.0125 µg/ml Gb3(C17:0) and 2.5 µg/ml creatinine-D3
11.To a 250-ml glass bottle, add 200 ml MeOH.
12.Add 50 µl Gb3(C17:0) stock solution (0.5 mg/10 ml) with a P200 pipette.
13.Add 250 µl creatinine-D3 (20 mg/10 ml) with a P1000 pipette.
14.Mix well.
15.Store at −20°C (stable for at least 1 year).
Support Protocol 5: PREPARATION OF THE CREATININE CALIBRATORS
The quantification of creatinine is performed with a calibration curve prepared from a commercial creatinine standard.
Additional Materials (also see Basic Protocol)
-
Creatinine, ≥98% (Sigma Aldrich, cat. no. C4255-10G)
-
50-ml polypropylene centrifuge tube with cap (Progene, cat. no. 71-5000-B)
-
P5000 pipette with associated tips
-
15-ml polypropylene centrifuge tube with cap (Corning, cat. no. 430766)
Creatinine stock solution, 30 mM
1.Accurately weigh 101.82 mg creatinine and quantitatively transfer it to a 50-ml polypropylene centrifuge tube.
2.Add 30 ml H2O with a P5000 pipette.
3.Mix well.
4.Store at 4°C (stable for at least 1 year).
Creatinine calibrators (cP0 to cP5)
5.According to Table 5, for each calibrator (cP0 to cP5), add the indicated volumes of water and creatinine stock solution (30 mM) to a 15-ml polypropylene centrifuge tube. P5000, P1000, P200, and P20 pipettes are used to prepare the solutions.
| Calibrator | H2O (µl) | 30 mmol/L creatinine (µl) | Total volume (µl) | Standard concentration (mmol/L) | Concentration in urine (mmol/L)a |
|---|---|---|---|---|---|
| cP0 | 3000 | 0 | 3000 | 0.0 | 0.0 |
| cP1 | 2990 | 10 | 3000 | 0.1 | 0.1 |
| cP2 | 2900 | 100 | 3000 | 1.0 | 1.0 |
| cP3 | 2300 | 700 | 3000 | 7.0 | 7.0 |
| cP4 | 1500 | 1500 | 3000 | 15.0 | 15.0 |
| cP5 | 0 | 3000 | 3000 | 30.0 | 30.0 |
- a
For 50 µl of calibrator added and 50 µl of urine analyzed.
6.Mix the calibrators well and store them at 4°C (stable for at least 1 year).
Support Protocol 6: PREPARATION OF THE UHPLC SOLUTIONS AND MOBILE PHASES
Solutions and mobile phases are prepared with high purity solvents suitable for the UHPLC system.
Additional Materials (also see Basic Protocol)
- 1-L glass bottle for UHPLC system (VWR, cat. No. 89000-240)
- 1-L glass graduated cylinder
- 2-ml glass serological pipette (VWR, cat. no. 93000-742)
- 500-ml glass bottles for UHPLC system (VWR, cat. No. 10754-818)
Mobile phase A: MeOH + 0.1% FA
1.To a 1-L glass bottle, add 1 L MeOH with a graduated cylinder.
2.Add 1 ml FA with a 2-ml glass serological pipette.
3.Mix well (stable for at least 2 months at room temperature).
Mobile phase B: H2O + 0.1% FA
4.To a 500-ml glass bottle, add 500 ml MeOH with a graduated cylinder.
5.Add 0.5 ml FA with a 2-ml glass serological pipette.
6.Mix well (stable for at least 1 month at room temperature).
Strong needle wash (SNW) (50% MeOH, 50% H2O)
7.To a 500-ml glass bottle, add 250 ml MeOH and 250 ml H2O with a graduated cylinder.
8.Mix well (stable for at least 2 months at room temperature).
Seal wash (20% MeOH, 80% H2O)
9.To a 500-ml glass bottle, add 100 ml MeOH and 400 ml H2O with a graduated cylinder.
10.Mix well (stable for at least 1 year at room temperature).
COMMENTARY
Method Validation
The method validation results are summarized in the supporting information file (see Supporting Information).
Critical Parameters
Due to their amphiphilic properties, Gb3 and Gb3(C17:0) are difficult to dissolve and their solutions can become non-homogeneous, which can significantly affect the analytical results. Thus, it is very important to follow the detailed recommendations outlined in this protocol concerning the preparation and use of the solutions containing these two molecules.
Troubleshooting
For a list of problems, possible causes, and solutions, see Table 6.
| Problem | Possible cause | Solution |
|---|---|---|
| Difficulty to dissolve the Gb3 or Gb3(C17:0) standards | Solvents not added in the right order | Dissolve the standard with the CHCl3:MeOH (2:1) mixture before adding MeOH; submit the solution to a sonic bath |
| Sample contamination by the pellet after centrifugation | Pipetting speed too rapid; pipette tip too close to the pellet during pipetting | Centrifuge the sample again before repeating the process |
| Poor linearity of the Gb3 calibration curve | Heterogeneity of the Gb3 calibrator solutions | Vortex the solutions for a longer time before their use and if needed submit them to the sonic bath |
| Poor reproducibility of the urine QCs | Heterogeneity of the QC aliquots | Vortex frequently the urine pool to disperse uniformly the sediment particles during the preparation QC aliquots |
| The ratio between the quantification ions and the confirmation ions is not in the target range | Sample with a low Gb3 level (healthy control or P1 point of the calibration curve) or sample with a low creatinine level (<0.1 mM) | No action is required, it is due to the variability of the low detected signal |
| Presence of interference | Repeat the analysis with a new specimen | |
| Low pressure of the UHPLC system | Column leakage | Screw the column fittings slightly harder |
| High pressure of the UHPLC system | Clogging of the guard column in-line filter | Replace the column in-line filter |
| Clogging of the UHPLC transfer lines, valves, or needle seat | Replace or unblock the lines, valves and/or needle seat (assistance from the instrument supplier might be required) | |
| Poor UHPLC peak shape | Contaminated or damaged guard column | Wash the guard column with 100% MeOH and then with 100% acetonitrile; if the problem is not solved, replace the guard column |
| Dead volumes in the UHPLC system | Check all the connections of the UHPLC system to remove dead volumes | |
| Low signal | Poor mass calibration of the mass spectrometer | Calibrate the instrument |
| The mass spectrometer internal components are dirty | Clean the ion block, stepwave and/or quadripoles (assistance from the instrument supplier might be required) |
Statistical Analysis
A total of 140 urine specimens from healthy controls was analyzed to establish normal reference ranges. According to the results obtained, we decided to establish two age-related reference ranges (<7 years and ≥7 years). The mean + 2 standard deviations was chosen as upper reference limit for both groups. For the <7 years group, the normal reference range was set to 0 to 29 mg Gb3/mol creatinine (n = 82), and for the ≥7 years group it was set to 0 to 14 mg Gb3/mol creatinine (n = 58).
Understanding Results
The proposed method is semi-quantitative since the relative abundance of the Gb3 isoforms contained in the commercial standard used for the calibration curve is not exactly the same as that observed in urine samples. The Gb3 commercial standard is purified from porcine red blood cells.
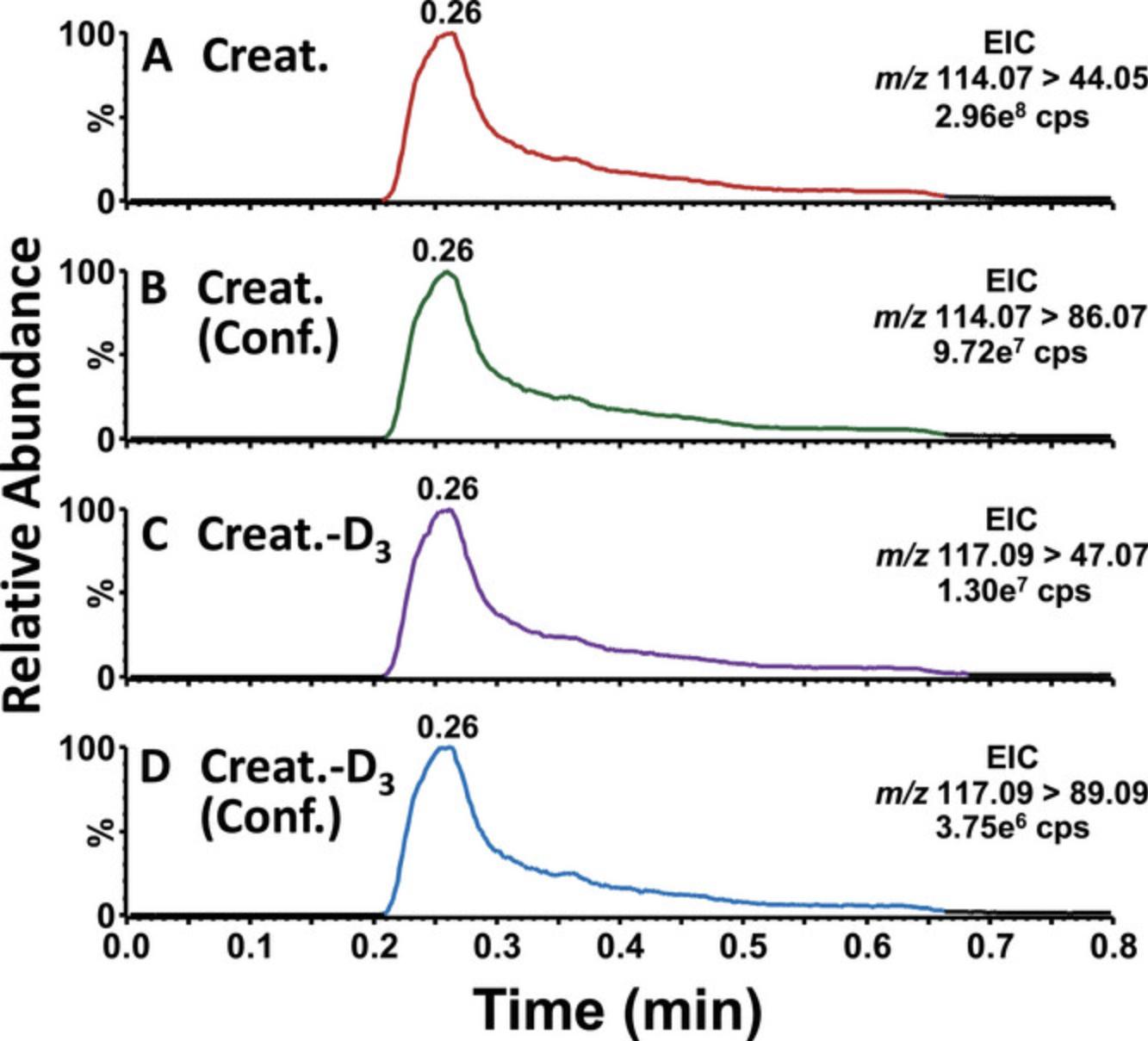
Figure 2 shows examples of ion chromatograms obtained for Gb3 and its internal standard Gb3(C17:0) in urine from the hemizygous FD quality control. The profiles of the quantification and confirmation ions, as well as the ratio of their areas, are compared to detect the presence of potential interferences. For the quantification analysis, the Gb3 fragment corresponds to the dehydrated ceramide and is different for each Gb3 isoform. For the confirmation analysis, the Gb3 fragment corresponds to the di-dehydrated sphingosine and is the same for all the Gb3 isoforms. Figure 3 shows examples of ion chromatograms obtained for creatinine and creatinine-D3. All the Gb3 results are normalized to creatinine as correction for the urine concentration. Extremely low creatinine levels can lead to an overestimation of the Gb3/creatinine ratio. For this reason, we usually recommend collecting a new specimen when the creatinine concentration is <1.0 mmol/L.
![Details are in the caption following the image Gb<sub>3</sub> analysis in urine from a hemizygous Fabry disease patient (creatinine = 9.2 mmol/L). (A) Total ion chromatogram (TIC) of the quantification ions corresponding to the multiple reaction monitoring (MRM) transitions of the 8 Gb<sub>3</sub> isoforms analyzed [C16:0. C18:0, C20:0, C22:1, C22:0, C24:1, C24:0, (C24:0)OH]. (B) TIC of the confirmation (conf.) ions corresponding to the MRM transitions of the 8 Gb<sub>3</sub> isoforms analyzed. (C) Extracted ion chromatogram (EIC) of the quantification ion of the Gb<sub>3</sub> internal standard (ISTD) [Gb<sub>3</sub>(C17:0)]. (D) EIC of the conf. ion of the ISTD. Cps = count per second.](https://static.yanyin.tech/literature_test/cpz11087-fig-0002-m.jpg)
Concerning the interpretation of the results, it is important to note that some heterozygous females (Wanner et al., 2018) and males with a “cardiac variant” in the GLA gene (Hwu, 2023) can have normal levels of Gb3. Moreover, different health conditions, such as heart diseases (Schiffmann et al., 2014), chronic kidney diseases (Gaggl et al., 2015), and high loads of urinary leukocytes and bacteria (Gaggl et al., 2015), may increase urinary Gb3 levels to some degree. In our experience, the impact of such factors is rarely sufficient to cause diagnostic confusion, but it is nonetheless important to be aware of their potential influence when interpreting Gb3 results in the context of diagnostic investigations for possible or suspected FD. For patients with high levels of urinary Gb3, the diagnosis of FD is confirmed by the measurement of α-galactosidase A activity (usually conclusive for male patients with the “classic” form of the disease) and/or by the analysis of GLA gene variants (Ortíz et al., 2018).
Urinary Gb3 is a good biomarker to monitor response to treatment for FD patients. Even if the enzyme replacement therapy is administered every second week to FD patients, no cyclic variation of their urinary Gb3 levels was observed (Boutin et al., 2020).
Time Considerations
Basic Protocol (for a batch of 10 urine samples): 1 hr to thaw the samples and working solutions; 45 min to prepare the samples; 15 min to set up the UHPLC system; 1 hr to condition the guard column (7 injections of 8.7 min; the chromatography is 8 min long, but the injection to injection running time is 8.7 min); 4.4 hr for the UHPLC-MS/MS analyses (30 injections of 8.7 min); and 30 min for data analysis.
Support Protocol 1: 2 hr in total to prepare 100 aliquots for each of the 3 QCs.
Support Protocol 2: 30 min to prepare 15 aliquots.
Support Protocol 3: 2 hr to prepare the Gb3 stock solution and the Gb3 calibrators.
Support Protocol 4: 1.5 hr to prepare the Gb3(C17:0) and the creatinine-D3 stock solutions, and the ISTD working solution.
Support Protocol 5: 1 hr to prepare the creatinine stock solution and the creatinine calibrators.
Support Protocol 6: 30 min to prepare the UHPLC solutions and mobile phases.
Acknowledgments
The authors acknowledge Marie-Eve Tétreault-Garneau and Mélanie Lafrance for technical assistance, as well as Denis Cyr, Tommy Gagnon, and Patrick Bherer for helpful scientific discussions.
Author Contributions
Michel Boutin : Conceptualization; data curation; formal analysis; methodology; supervision; validation; writing—original draft; writing—review and editing. Bruno Maranda : Conceptualization; project administration; supervision; writing—review and editing. Paula J. Waters : Conceptualization; methodology; project administration; supervision; writing—review and editing.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data, tools, and material (or their source) that support the protocol are available from the corresponding author upon reasonable request.
Supporting Information
| Filename | Description |
|---|---|
| cpz11087-sup-0001-SuppMat.pdf240.3 KB | The file contains the results concerning the following elements of the method validation (Matrix effect, Limit of detection (LOD) and limit of quantification (LOQ), Intra- and interday precisions, Interday accuracy, Linearity of the calibration curves, Analytical measurement range, Sample stability, Adsorption of samples to glassware and plasticware, Carryover evaluation). |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
Literature Cited
- Abaoui, M., Boutin, M., Lavoie, P., & Auray-Blais, C. (2016). High-risk screening of Fabry disease: Analysis of fifteen urinary methylated and non-methylated GB3 isoforms using tandem mass spectrometry. Current Protocols in Human Genetics , 91(1), 24.1–17.24.11. https://doi.org/10.1002/cphg.24
- Auray-Blais, C., Lavoie, P., Boutin, M., & Abaoui, M. (2017). High-risk screening for Fabry disease: Analysis by tandem mass spectrometry of globotriaosylceramide (GB3) in urine collected on filter paper. Current Protocols in Human Genetics , 93(1), 17.26.1–17.26.12. https://doi.org/10.1002/cphg.34
- Boutin, M., Menkovic, I., Martineau, T., Vaillancourt-Lavigueur, V., Toupin, A., & Auray-Blais, C. (2017). Separation and analysis of lactosylceramide, galabiosylceramide, and globotriaosylceramide by LC-MS/MS in urine of Fabry disease patients. Analytical Chemistry , 89(24), 13382–13390. https://doi.org/10.1021/acs.analchem.7b03609
- Boutin, M., Lavoie, P., Menkovic, I., Toupin, A., Abaoui, M., Elidrissi-Elawad, M., Arthus, M., Fortier, C., Ménard, C., Maranda, B., Bichet, D. G., & Auray-Blais, C. (2020). Diurnal variation of urinary Fabry disease biomarkers during enzyme replacement therapy cycles. International Journal of Molecular Sciences , 21(17), 6114. https://doi.org/10.3390/ijms21176114
- Carnicer-Cáceres, C., Arranz-Amo, J. A., Cea-Arestin, C., Camprodón-Gómez, M., Moreno-Martínez, D., Lucas-Del-Pozo, S., Moltó-Abad, M., Tigri-Santiña, A., Agraz, I., Rodríguez-Palomares, J. F., Hernández-Vara, J., Armengol-Bellapart, M., Del-Toro-Riera, M., & Pintos-Morell, G. (2021). Biomarkers in Fabry Disease. Implications for clinical diagnosis and follow-up. Journal of Clinical Medicine , 10(8), 1664. https://doi.org/10.3390/jcm1008166
- Gaggl, M., Hofer, M., Weidner, S., Kleinert, J., Fauler, G., Wallner, M., Kotanko, P., Paschke, E., & Sunder-Plassmann, G. (2015). Interfering parameters in the determination of urinary globotriaosylceramide (Gb3) in patients with chronic kidney disease. Journal Of Nephrology , 28(6), 679689. https://doi.org/10.1007/s40620-015-0193-1
- Heywood, W., Doykov, I., Śpiewak, J., Hällqvist, J., Mills, K., & Nowak, A. (2019). Global glycosphingolipid analysis in urine and plasma of female Fabry disease patients. Biochimica Et Biophysica Acta (BBA) - Molecular Basis of Disease , 1865(10), 2726–2735. https://doi.org/10.1016/j.bbadis.2019.07.005
- Hwu, W. (2023). Deciphering the diagnostic dilemma: A comprehensive review of the Taiwanese cardiac variant in Fabry disease. Journal Of The Formosan Medical Association , https://doi.org/10.1016/j.jfma.2023.10.004
- Izhar, R., Borriello, M., La Russa, A., Di Paola, R., De, A., Capasso, G., Ingrosso, D., Perna, A. F., & Simeoni, M. (2023). Fabry Disease in women: Genetic basis, available biomarkers, and clinical manifestations. Genes , 15(1), 37. https://doi.org/10.3390/genes15010037
- Nowicki, M., Bazan-Socha, S., Błażejewska-Hyżorek, B., Kłopotowski, M., Komar, M., Kusztal, M., Liberek, T., Małyszko, J., Mizia−Stec, K., Oko−Sarnowska, Z., Pawlaczyk, K., Podolec, P., & Sławek, J. (2024). A review and recommendations for oral chaperone therapy in adult patients with Fabry disease. Orphanet Journal of Rare Diseases , 19(1), 16. https://doi.org/10.1186/s13023-024-03028-w
- Oder, D., Nordbeck, P., & Wanner, C. (2016). Long term treatment with enzyme replacement therapy in patients with Fabry disease. Nephron , 134(1), 30–36. https://doi.org/10.1159/000448968
- Ortíz, A., Germain, D. P., Desnick, R. J., Politei, J., Mauer, M., Burlina, A. P., Eng, C. M., Hopkin, R. J., Laney, D. A., Linhart, A., Waldek, S., Wallace, E., Weidemann, F., & Wilcox, W. R. (2018). Fabry disease revisited: Management and treatment recommendations for adult patients. Molecular Genetics And Metabolism , 123(4), 416427. https://doi.org/10.1016/j.ymgme.2018.02.014
- Palaiodimou, L., Kokotis, P., Zompola, C., Papagiannopoulou, G., Bakola, E., Papadopoulou, Μ., Zouvelou, V., Petras, D., Vlachopoulos, C., & Tsivgoulis, G. (2023). Fabry disease: current and novel therapeutic strategies. A narrative review. Current Neuropharmacology , 21(3), 440456. https://doi.org/10.2174/1570159x20666220601124117
- Schiffmann, R., Forni, S., Swift, C., Brignol, N., Wu, X., Lockhart, D. J., Blankenship, D., Wang, X., Grayburn, P. A., Taylor, M. R., Lowes, B. D., Fuller, M., Benjamin, E. R., & Sweetman, L. (2014). Risk of death in heart disease is associated with elevated urinary globotriaosylceramide. Journal Of The American Heart Association , 3(1), e000394. https://doi.org/10.1161/jaha.113.000394
- Shiga, T., Tsukimura, T., Namai, Y., Togawa, T., & Sakuraba, H. (2021). Comparative urinary globotriaosylceramide analysis by thin-layer chromatography-immunostaining and liquid chromatography-tandem mass spectrometry in patients with Fabry disease. Molecular Genetics and Metabolism Reports , 29, 100804. https://doi.org/10.1016/j.ymgmr.2021.10080
- Umer, M., & Kalra, D. (2023). Treatment of Fabry disease: Established and emerging therapies. Pharmaceuticals , 16(2), 320. https://doi.org/10.3390/ph16020320
- Wanner, C., Arad, M., Baron, R., Burlina, A. P., Elliott, P., Feldt-Rasmussen, U., Фомин, В. В., Germain, D. P., Hughes, D., Jovanović, A., Kantola, I., Linhart, A., Mignani, R., Monserrat, L., Namdar, M., Nowak, A., Oliveira, J. P., Ortíz, A., Pieroni, M., … Hilz, M. J. (2018). European expert consensus statement on therapeutic goals in Fabry disease. Molecular Genetics And Metabolism , 124(3), 189203. https://doi.org/10.1016/j.ymgme.2018.06.00
Key References
- Auray-Blais et al. (2017). See above.
This method was used as basis for the development of the new method presented in this article.

