An Improved Method for Purification of the Residual Bodies from the Seminiferous Tubules of Mice
Mohd Nazmul Hasan Apu, Mohd Nazmul Hasan Apu, Nikolay E. Shirokikh, Nikolay E. Shirokikh, Saadi Khochbin, Saadi Khochbin, Tatiana A. Soboleva, Tatiana A. Soboleva
infertility
purification
residual bodies
spermatogenesis
spermiogenesis
sucrose gradient ultracentrifugation
Abstract
Human fertility is declining in Western countries, and it is becoming increasingly clear that male infertility plays a pivotal role in the overall fertility decline. To understand the process that drives successful male germ cell maturation, the study of spermatogenesis of model organisms, such as mice, is essential. Residual bodies (RBs) play an important role in the last stages of spermatogenesis. They are formed at the time when post-meiotic spermatids undergo sequential differentiation steps so that the acrosome and flagellum are developed, the nucleus is markedly condensed, and the cytoplasm is lost. The masses of lost cytoplasm become RBs. Our recent work has shown that RB dynamics are highly sensitive to even small fertility defects. It was also noted that the transcriptome and proteome of RBs changes in response to spermatogenic defects. Thus, RBs represent an excellent and highly sensitive entity for studying male fertility. Previously published protocols for RB purification had some major limitations: they produced an RB fraction that was heavily contaminated with spermatozoa and erythrocytes or required tens of grams of starting material. In addition, most of the available protocols were developed for purification of RBs from rat testes. Here, we present a protocol that allows the isolation of 2.5-3 × 106 RBs from mouse testes with a purity of 98% from only 1 g of starting material. The purified material can be used for various downstream applications to study male fertility, such as transcriptome and proteome analyses, super-resolution microscopy, and electron and cryo-electron microscopy, amongst many others. © 2023 The Authors. Current Protocols published by Wiley Periodicals LLC.
Basic Protocol : An improved method for purification of the residual bodies from the seminiferous tubules of mice
INTRODUCTION
Spermatogenesis is a complex and dynamic process that takes place in the seminiferous tubules of the testis. Each day millions of germ cells develop under control of the somatic Sertoli cells. The process of spermatogenesis involves multiple steps that can be divided into three main phases: mitotic, meiotic, and haploid. During the mitotic phase, spermatogonial stem cells produce a pool of self-renewing diploid spermatogonia. Some spermatogonia will differentiate into tetraploid spermatocytes that undergo meiosis and two successive divisions producing haploid spermatids. Maturation of haploid spermatids involves marked condensation of the nucleus mediated by histone-protamine exchange and almost complete loss of cytoplasm, giving rise to spherical membranous structures of 4 to 10 µm in diameter known as residual bodies (RBs) (Stouffs et al., 2016). Concurrently, a flagellum develops to enable motility of mature spermatozoa. Interestingly, it is believed that RBs are formed through an apoptotic mechanism that is restricted to the cytoplasm of the spermatids and does not affect their nuclei (Blanco-Rodríguez & Martínez-García, 1999). It is a current dogma that RBs are engulfed and fully digested by the Sertoli cells, thus providing trophic and energy support for Sertoli cells (Xiong et al., 2009). However, it is possible that not all RBs, or not all their content, undergoes full degradation (Xiao et al., 2017; and our unpublished observations). Thus, it is crucial to continue to study RBs to fully understand their function in spermatogenesis.
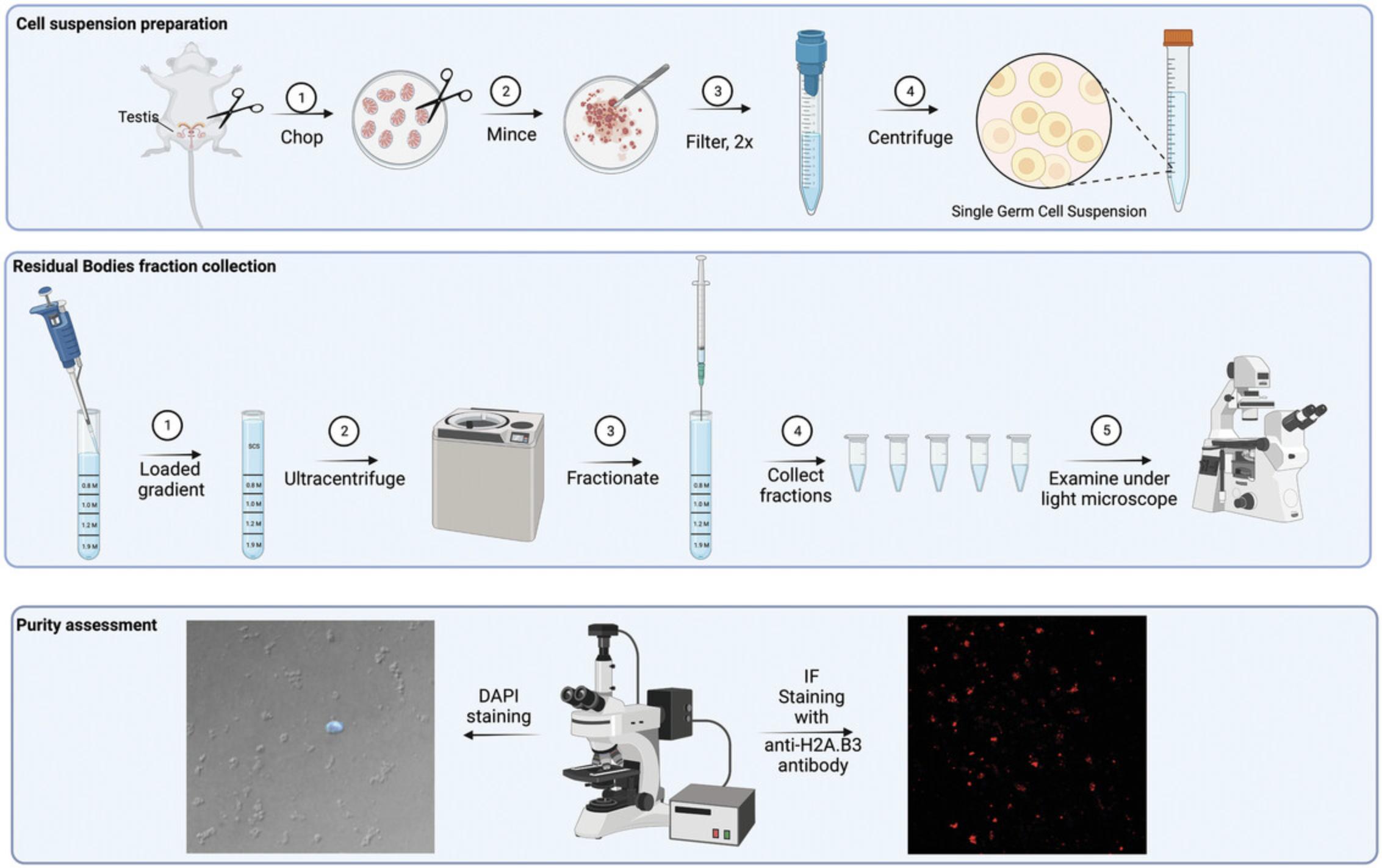
The study of RBs requires a robust, highly reproducible, and cost-effective protocol for purifying RBs from small quantities of mouse tissue, such as the protocol presented here. The method is scalable and can purify 2.5-3 × 106 RBs from four mice or 1 g testis sample, based on ultracentrifugation methodology. This method, based on existing protocols (An Der Lan-Hochbrunn & Lacy, 1974; Nyquist et al., 1973) with significant improvements, isolates RBs to a purity of 95% to 98%. One purification run using this protocol yields 250 µg of protein or 10 µg of total RNA. It does not require mouse perfusion to remove blood cell contamination and relies on a one-step mechanical tubule dissociation, followed by continuous sucrose gradient centrifugation of total cell/RBs suspension in an ultracentrifuge (Fig. 1). We also provide a simple purity assessment method of isolated RBs samples. Purified RBs can be used for demanding downstream applications including, but not limited to, RNA-sequencing, mass spectroscopy, high resolution microscopy, electron microscopy, and others.
NOTE : All protocols involving animals must be reviewed and approved by the appropriate Animal Care and Use Committee and must follow regulations for the care and use of laboratory animals.
Basic Protocol: AN IMPROVED METHOD FOR PURIFICATION OF THE RESIDUAL BODIES FROM THE SEMINIFEROUS TUBULES OF MICE
This protocol provides detailed step-by-step instructions for the purification of residual bodies (RBs) from four adult mice. It is divided in several sub-protocols: steps 1 to 10 provide information for preparation of a sucrose gradient; steps 11 to 23 describe preparation of single germ cell/RB suspension; steps 24 to 28 explain how to load and centrifuge the suspension through the sucrose gradient cushion; steps 29 to 37 focus on fraction collection; and steps 38 to 60 describe two methods for evaluation of purity of the RB fractions.
Materials
-
Sucrose solutions (see recipe)
-
Four adult male mice (e.g., FVB/NJ strain), aged 8 to 12 weeks
-
70% (v/v) ethanol
-
0.4% Trypan blue stain (Invitrogen, cat. no. T10282)
-
0.05 M phosphate buffer, pH 7.4 (see recipe)
-
16% paraformaldehyde (PFA) (Electron Microscopy Sciences, cat. no. 15710)
-
Triton X-100 (Sigma-Aldrich, cat. no. T8787)
-
Dulbecco′s phosphate buffer saline (DPBS) (Sigma-Aldrich, cat. no. D8537)
-
1 mg/ml DAPI (4′,6-diamidino-2-phenylindole) solution (Thermo Scientific, cat. no. 62248)
-
ddH2O
-
Vectashield Plus antifade mounting medium (Vector Laboratories, cat. no. H-1900-10)
-
Donkey serum (Sigma-Aldrich, cat. no. D9663)
-
Tween 20 (Sigma-Aldrich, cat. no. P2287)
-
Anti-H2A.B3 antibody raised in sheep (custom made by Soboleva group)
-
Cy3 AffiniPure donkey anti-sheep IgG (H+L) (Jackson ImmunoResearch, cat. no. 713-165-003)
-
Dry ice and polystyrene box
-
Stainless-steel test-tube rack for 14 × 89 mm tubes
-
13.2-ml thin wall polypropylene gradient tubes, 14 × 89 mm (Beckman Coulter, cat. no. 331372)
-
Pipette and pipette tips
-
Cling wrap (Glad wrap or other commonly used food wrap)
-
Ultra-low temperature freezer (–80°C)
-
Parafilm
-
Stainless surgical scissors with sharp fine head (F.S.T., cat. no. 14063-09)
-
Small tissue culture dishes, 35 mm × 10 mm (Corning, cat. no. CLS430165)
-
2 tweezers, 1 with thin head and 1 with thick head
-
No. 22 surgical blades (Swann-Morton, cat. no. 0308)
-
15-ml polypropylene tube
-
100-µm MACS SmartStrainers filters (Miltenyi Biotec, cat. no. 130-098-463)
-
1-ml syringes (Becton Dickinson, cat. no. 302100)
-
Benchtop refrigerated centrifuge for 15-ml tubes (e.g., Beckman Allegra X-22R), 4°C
-
Countess cell counter (Invitrogen, cat. no. C10227) and Countess chamber slide (Invitrogen, cat. no. C10312), both optional
-
Retort stand with a clamp
-
200-µl sterile gel loading tips, universal graduated natural (VWR, cat. no. LAC2848)
-
Optima L-90K ultracentrifuge (Beckman Coulter)
-
SW41Ti swinging-bucket rotor (Beckman Coulter, cat. no. 331362)
-
19G × 38 mm Neolus hypodermic needles (Terumo, cat. no. NN65E19380C)
-
2.0-ml microcentrifuge tube (e.g., Axygen, cat. no. MCT-200-C)
-
Phase contrast microscope (e.g., Olympus CK2 with ULWCD 0.30 M condenser)
-
Refrigerated microcentrifuge for 2-ml tubes (e.g., Thermo Scientific Fresc 17), 4°C, or non-refrigerated microcentrifuge that is kept in a cold room
-
Poly-L-lysine coated slides (Sigma-Aldrich, cat. no. PO425)
-
Humidified chamber, made using a plastic container containing paper towels prewet with dH2O
-
20 mm × 50 mm coverslips
-
Nail polish to seal the slides
-
Fluorescence microscope (e.g., Olympus IX 71)
-
50-ml Coplin jar
Preparation of continuous sucrose gradient
1.Place dry ice in the polystyrene box and place a stainless-steel tube rack that tightly fits 13.2-ml thin wall polypropylene gradient tubes (i.e., no movement)
2.Put at least 2 gradient tubes into the tube rack and chill for 5 min.
3.Carefully add 1.5 ml of 1.9 M sucrose solution to the bottom of the tubes.
4.Cover the top of the tubes with cling wrap to avoid the introduction of any foreign material, such as ice from the −80°C freezer.
5.Carefully place the rack into a –80°C freezer and incubate for 30 min to completely freeze the 1.9 M sucrose solution.
6.Take out the rack and put it back on dry ice and remove the cling wrap.
7.Add 1.5 ml of 1.2 M sucrose solution on top of the frozen 1.9 M sucrose, cover it with a new cling wrap, and put it back at –80°C for 30 min.
8.Add 2.5 ml of 1.0 M and 0.8 M of sucrose solutions sequentially following the preceding step 7.
9.Seal the tubes with parafilm and store at –80°C for up to 1 month or overnight if proceeding to step 11.
10.Take out the tubes containing the gradient (from step 9) in the evening before the RB purification experiment and keep them at 4°C, which will allow to thaw and form a continuous gradient.
Preparation of single germ cell/residual body suspension
Four adult mice (8 to 12 weeks old for better yield) of desired strain background should be used. This protocol uses a germ cell suspension from eight testes of adult FVB/NJ strain of mice for loading into two continuous sucrose gradients.
11.Sacrifice mice by cervical dislocation and wet the lower abdomen area with 70% (v/v) ethanol.
12.Open the mouse peritoneal cavity using surgical scissors and expose the testes by pulling out the epididymal fat pads [for steps 12 to 15, follow the instructions provided in the video from Kim et al. (2021)].
13.Cut the ductus deferens while holding the epididymal fat pads and release the testes by cutting the fat pads leaving a small portion still attached to the testes.
14.Transfer the testes to a small sterile tissue culture dish containing 3 ml of cold 0.25 M sucrose solution.
15.Make a small incision on the tunica albuginea while securing the testis by holding the remaining fat pads with tweezers and squeeze the tubules out.
16.Gently spread the tubules with curved tweezers and rinse them in the same solution to release the interstitial and blood cells. Place the rinsed tubules in a new small tissue culture dish containing 2 ml of cold 0.25 M sucrose solution.
17.Cut the tubules with fine end surgical scissors for 5 min (∼150 cuts/min) and then with a surgical blade for 3 to 5 min to achieve the size of tubule fragments of ∼1 mm.
18.Cut ∼2 mm off a 1-ml tip and pipette the cell suspension for 2 to 3 min using the cut tip.
19.Transfer the cell suspension into a 15-ml polypropylene tube, repeatedly wash the culture dish with 0.25 M sucrose solution to collect all the cells and make the volume 8 ml after washing.
20.Filter the suspension twice using 100-μm filters and wash the tube with 2 ml of 0.25 M cold sucrose solution after each filtration step, so the final volume is 12 ml.
21.Spin the filtrate in a benchtop, refrigerated centrifuge 20 min at 1800 × g , 4°C.
22.Decant and discard the supernatant and resuspend the cell pellets in 2 ml of 0.25 M cold sucrose solution via pipetting up and down for 1 min using a cut 1 ml tip.
23.Bring the volume up to 9 ml and count the cells using a trypan blue stain and a countess cell counter.
Loading the gradient with cell suspension and ultracentrifugation
This step is performed in a cold room. Load no more than 1.25 × 108 cells into each sucrose gradient tube in order to prevent overloading.
24.Place one gradient tube at a time into the clamp of the retort stand and tighten it to secure the tube.
25.Cut the end of a 1 ml tip and attach a long 200-µl non-filtered gel loading tip to it to have more control during the gradient loading
26.Pipette 1 ml of cell suspension and load on top of the gradient very slowly without disturbing the gradient; continue the process and load up to 4.2 ml of cell suspension in total (equivalent to 100 to 125 million cells per gradient tube).
27.Weigh each gradient tube and equilibrate the weight with the other gradient tubes using 0.25 M cold sucrose solution in order to balance the centrifuge.
28.Once the gradient tubes have been loaded with cell suspension, ultracentrifuge the tubes using an SW41Ti rotor 45 min at 91,000 × g (23,000 rpm), 4°C.
Fraction collection
29.Number 20 × 2-ml microcentrifuge tubes from 1 to 20 and keep them in the cold room.
30.Remove the bevel of the needle by cutting the 19G needle end using pliers, and then restoring the opening to its original width with the smooth edge of the pliers. Attach the needle to a 1-ml syringe.
31.Once ultracentrifugation is complete, gently transfer all the tubes to the cold room.
32.Gently place one gradient tube into the clamp and start collecting fractions from the top of each gradient.
33.Collect 500 µl per fraction into 2.0-ml microcentrifuge tubes, up to 18 fractions. There is no need to collect any fractions residing in the 1.9 M sucrose solution.
34.Observe each fraction by phase contrast microscopy to visually assess the level of purity of each fraction.
35.Add 1 ml of cold 0.05 M phosphate buffer to dilute the sucrose solution and pellet the desired fractions (i.e., fractions 13 to 15) in a refrigerated microcentrifuge for 30 min at 1800 × g , 4°C.
36.Following centrifugation, snap freeze the pellets and store at –80°C or proceed to step 37.
37.Proceed to perform any downstream analysis i.e., immunofluorescent labeling, protein or RNA extraction.
Evaluation of purity of fractions
DAPI staining of purified residual body fractions
38.At the end of fractionation process, add 1 ml of cold 0.05 M phosphate buffer into each fraction.
39.Pellet the RBs for 15 min at 1800 × g , 4°C, using a refrigerated microcentrifuge.
40.Repeat steps 38 and 39 once more.
41.Resuspend the RBs pellet in 200 µl of cold phosphate buffer.
42.Spot 10 µl RBs on a poly-L-lysine coated glass slide.
43.Fix with 10 µl of a 4% PFA + 0.05% Triton X-100 solution (v/v in DPBS) for 20 min at room temperature in a humidified chamber.
44.Wash with 1× DPBS twice.
45.Stain with DAPI (diluted 1:2500 in ddH2O) for 3 min in the dark at room temperature.
46.Wash with 1× DPBS twice.
47.Mount with Vectashield Plus antifade mounting medium (30 µl per 20 mm × 50 mm coverslip, seal with nail polish).
48.Examine via fluorescence microscopy using differential interference contrast function (Fig. 2A to D).
Immunofluorescent labeling of purified residual bodies with anti-H2A.B3 antibody
49.Add 10 µl of 4% PFA + 0.05% Triton X-100 (v/v in DPBS) onto a poly-L-lysine coated slide.
50.Add an equal volume of the RBs suspension and spread the mixture over the slide.
51.Fix for 1 hr at room temperature in a humidified chamber.
52.Remove the fixative and block with 5% donkey serum + 0.01% Tween 20 in 1× DPBS for 1 hr at room temperature.
53.Incubate with anti-H2A.B3 antibody diluted 1:100 in 5% donkey serum + 0.01% Tween 20 in 1× DPBS overnight at 4°C in a humidified chamber.
54.On the following day, wash the slide with 1× DPBS + 0.01% Tween 20 in a 50-ml Coplin jar, three times for 5 min each.
55.Incubate with a secondary antibody (anti-sheep Cy3 diluted 1:200 in 5% donkey serum + 0.01% Tween 20 in 1× DPBS) in a humidified chamber for 45 min at 37°C in the dark.
56.Wash with 1× DPBS + 0.01% Tween 20 in a 50-ml Coplin jar, three times for 5 min each in the dark.
57.Stain with DAPI (diluted 1:2500 in ddH2O) for 3 min at room temperature in the dark.
58.Wash three times with 1× DPBS + 0.01% Tween 20 for 5 min each wash.
59.Mount with Vectashield Plus antifade mounting medium (30 µl per 20 mm × 50 mm coverslip, seal with nail polish).
60.Examine via fluorescence microscopy (Fig. 3).

REAGENTS AND SOLUTIONS
Phosphate buffer, 0.05 M, pH 7.4
- 6.75 g sodium phosphate dibasic dodecahydrate (Na2HPO4.12H2O)
- 738 mg monosodium phosphate (NaH2PO4)
- 450 ml of Milli-Q H2O
- Adjust pH to 7.4 using 3.7% HCl
- Bring volume up to 500 ml with Milli-Q H2O
- Filter using a bottle-top 0.22-µm vacuum filter system (Corning, cat. no. CLS431153)
- Store up to 6 months at 4°C
Sucrose solutions, 0.25 M, 0.8 M, 1.0 M, 1.2 M, and 1.9 M
Prepare 50 ml of 0.25 M, 0.8 M, 1.0 M, 1.2 M, and 1.9 M sucrose solutions by dissolving sucrose in 0.05 M phosphate buffer (see recipe) (see Table 1 for exact details). Use a hotplate and magnetic stirrer to speed up the sucrose dissolution process. Once all the sucrose is dissolved, cool the solution to room temperature, make up the final volume to 50 ml with 0.05 M phosphate buffer. Add 50 µl of 1 M MgCl2 solution (Invitrogen, cat. no. AM9530G; 1 mM final) and filter through a 0.2-µm syringe filter (Sartorius Minisart, cat. no. 16534) and 20-ml Luer lock syringes (Terumo, cat. no. SS+20L). Store up to 3 months at 4°C.
| Final concentration of sucrose solution | Sucrose (g) | 0.05 M phosphate buffer (ml) | 1 M MgCl2 (µl) |
|---|---|---|---|
| 0.25 M | 4.28 | up to 50 ml | 50 |
| 0.8 M | 13.69 | up to 50 ml | 50 |
| 1.0 M | 17.12 | up to 50 ml | 50 |
| 1.2 M | 20.54 | up to 50 ml | 50 |
| 1.9 M | 32.52 | up to 50 ml | 50 |
COMMENTARY
Background Information
Spermatogenesis represents one of the most dramatic cellular reprograming events that is required for proper maturation and formation of fertile sperm. Indeed, spermatozoa are the only cell type that is destined to leave the parental organism and ultimately outlive it. To achieve this, post-meiotic spermatids undergo sequential differentiation steps so that the acrosome and flagellum are formed, the nucleus is markedly condensed, and the cytoplasm is lost. The masses of lost cytoplasm form residual bodies (RBs) that sequentially are cleared away by somatic Sertoli cells. Our recent work (Anuar et al., 2019) has shown that RB dynamics are highly sensitive to even small fertility issues, thus representing an excellent model for studying spermatogenic defects.
Despite being first described more than 80 years ago, RBs are under-explored biological constituents. They were mainly studied by electron microscopy in the 1950s to 1990s, with minimal application of biochemical approaches. As a result, we have very limited knowledge of the RNA, protein, lipid, metabolites, and sugar content of RBs. Moreover, new evidence is emerging that RBs, rather than containing spent material that undergoes disposal within Sertoli cells, may have additional functions delivering important factors to Sertoli cells and outside of the seminiferous tubules (Anuar et al., 2019; Xiao et al., 2017; and our unpublished data).
In addition, evidence from our unpublished research points to RBs as a very sensitive indicator of spermiogenic defects in developing spermatids. Therefore, molecular characterization of RBs can provide novel insights into the processes that control spermatogenesis. Finally, the elusive mechanism that governs the phenomena of uncoupled transcription and translation that haploid spermatids are known for (Braun, 1998; Steger, 2001) is another area in which RBs can provide an excellent study model, as RBs contain large quantities of RNA, ribosomes, and cytoplasmic factors responsible for translation, but not transcriptional machinery.
Over the past five decades several protocols have been developed that aimed at purification of RB fraction. They are either based on: (i) ultracentrifugation in sucrose gradients; (ii) fractionation by gravity sedimentation in BSA gradient (Bellvé, 1993; Yefimova et al., 2008); or (iii) centrifugal elutriation (Barchi et al., 2009). These methods produce different fractions based on physical characteristics of cells, such as size, shape, and density (An Der Lan-Hochbrunn & Lacy, 1974; Nyquist et al., 1973). However, the abovementioned methods have major limitations for isolation of pure RBs from mice: (i) ultracentrifugation in sucrose gradient was developed for use in rats and relied on large quantities of stating material, such as 30 g of rat testes; (ii) sedimentation by gravity yields RBs that are heavily contaminated by erythrocytes and mature spermatozoa; and (iii) the centrifugal elutriation requires specialized and expensive chambers and rotors that rarely comprise the set of common equipment of the modern laboratory (Barchi et al., 2009).
In addition, all the abovementioned approaches rely on generation of a single germ cell suspension before fractionation. Historically, enzymatic and mechanical dissociation methods were used to produce a single-cell suspension from testes (An Der Lan-Hochbrunn & Lacy, 1974; Bellvé, 1993; Nyquist et al., 1973). The enzymatic methods rely on treatment of seminiferous tubules with non-selective proteolytic and nucleolytic enzymes, such as trypsin, collagenase, and DNase I, thus often result in the degradation of specific macromolecules, consequently hampering downstream analyses. Another downside of the enzymatic digestion of cellular material is the generation of cytosolic remnants surrounded by cytoplasmic membrane or “artificial RBs” because of the over-digestion phenomenon (Rodríguez-Casuriaga et al., 2013).
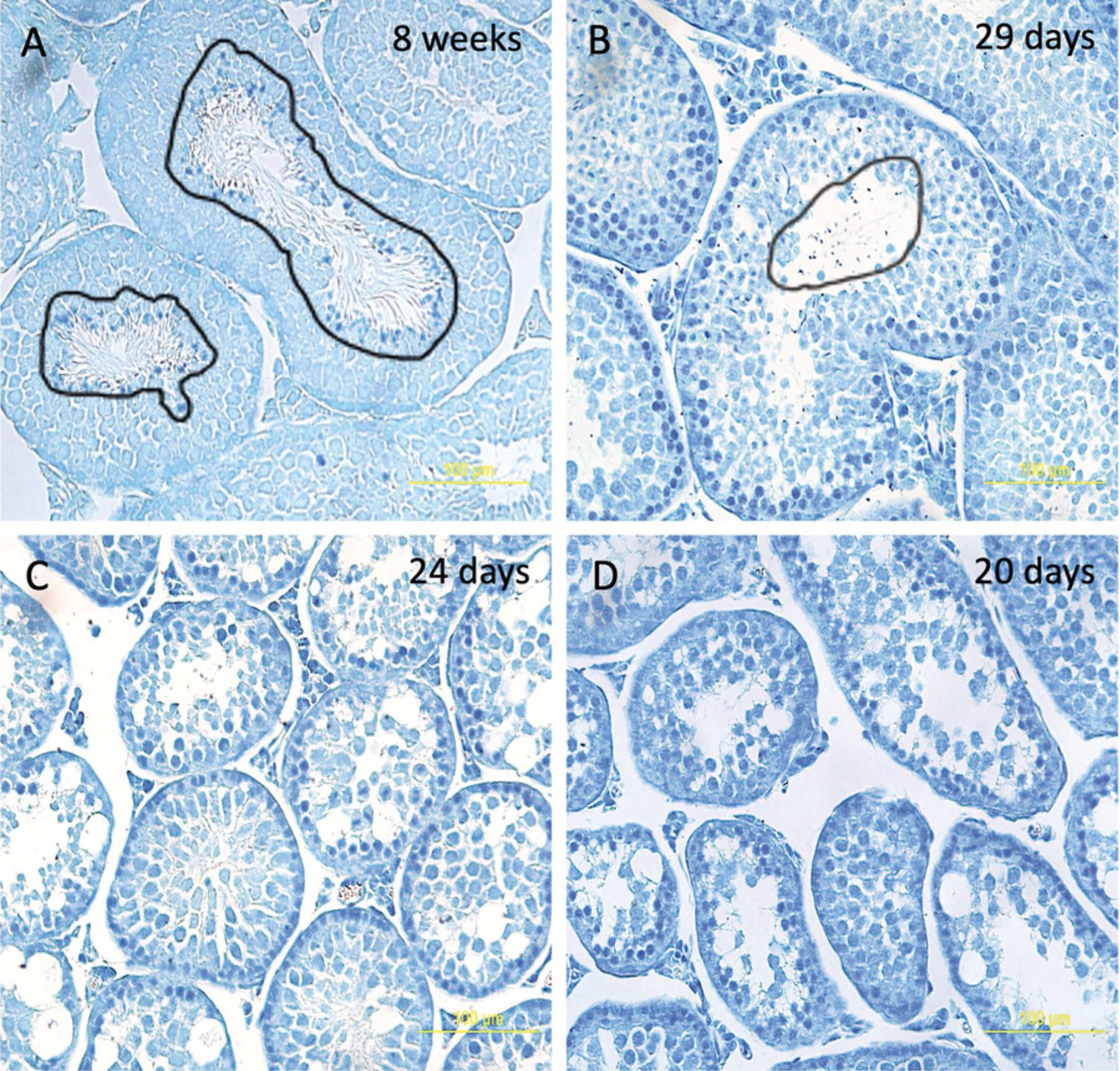
In contrast, mechanical dissociation does not generate cellular debris or artificial RBs. To confirm this, we performed purification of RBs from testes of immature mice that lack RBs. To identify the correct age of mice that have produced spermatocytes and spermatids but have not yet shed the cytoplasm in the form of RBs, we performed EMA (Eosin Y/Methylene blue/Azure II) staining as previously described (Xiao et al., 2017) of seminiferous tubule sections of mice of various ages (mature 8-week-old, and immature 29-, 24-, and 20-day-old mice). Figure 4 shows that the testes of 8-week-old and 29-day-old mice already produce RBs (Fig. 4A and 4B), while testes of younger 20- and 24-day-old mice do not contain RBs (Fig. 4C and 4D). Thus, we performed the procedure using material from the testes of 24-day-old mice, when RBs have not formed (Fig. 5). We did not detect any cellular debris reminiscent of RBs (Fig. 5, in contrast to purification of RBs from testes of mature mice in Fig. 2). This experiment confirmed that mechanical disruption was superior to enzymatic dissociation as it did not generate cellular debris, although cell types, such as spermatocytes and spermatids that normally generate these artifacts during enzymatic preparation, are present in the 24-day-old immature testes.
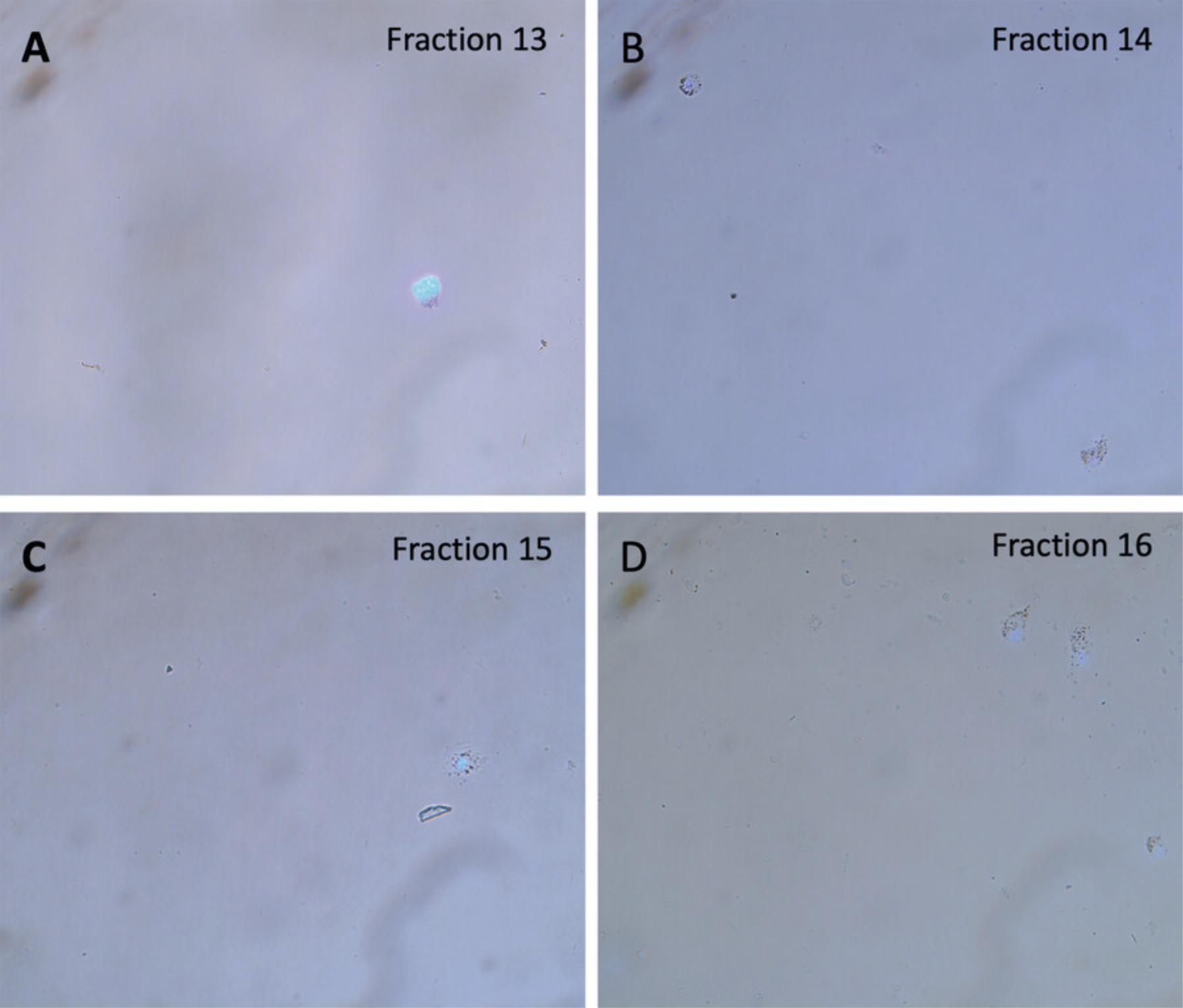
The method described in this protocol enables the production of RBs of exceptional purity. It relies on mechanical disruption of the testis, thus minimizing generation of artificial RBs and cellular debris. Mechanical disruption also producers a larger yield of cells with better viability compared to an enzymatic approach (Table 2). Finally, the protocol represents a modernized and optimized ultracentrifugation method (An Der Lan-Hochbrunn & Lacy, 1974; Nyquist et al., 1973) that allows the use of modern centrifuges and rotors; is scaled down for the use of 1 g starting material (as opposed to ∼30 g of tissue from 20 rat testes); and is optimized for the use in mice.
| Method of dissociation | No. of cells per 4 mice | Viability (%) |
|---|---|---|
| Collagenase, trypsin, DNase I | ∼180 × 106 | 78.0 +/− 3.5 |
| Mechanical (scissors, scalpel blade) | ∼240 × 106 | 86.8 +/− 1.2 |
Critical Parameters
The success of the RB preparation method described in this protocol depends largely on the following factors: (i) the number of mice used; (ii) preparation of a single germ cell suspension of good quality i.e., high viability of single cells; (iii) the quantity of cells loaded into each gradient; and (iv) slow and steady collection of fractions after centrifugation.
The use of four mice aged 8 to 12 weeks for a single experiment produces the best single-cell suspension with highest viability when all steps have been performed very gently, i.e., recommended chopping rate, no vigorous pipetting, all procedures performed in a cold room or on ice. Features like high cell viability (>80%) and absence of cellular debris and aggregates indicate a good single cell suspension. Cellular debris and aggregates can quickly be assessed using a light microscope.
The SW41Ti rotor accommodates six tubes at once; however, we find that working with only two gradients/tubes simultaneously produces better results as preparation of total germ cell suspension from four mice results in obtaining 200 to 250 million cells, an optimal cell number to load into two gradient tubes (100 to 125 million cells each).
Overloading the gradient tubes with cells results in contamination of RBs with germ cells.
Precise unloading of the sucrose gradient fractions after ultracentrifugation is crucial for obtaining highly pure RB fractions. Too much pressure on the syringe plunger will disturb the gradient resulting in germ cell-contaminated RB fractions.
Understanding Results
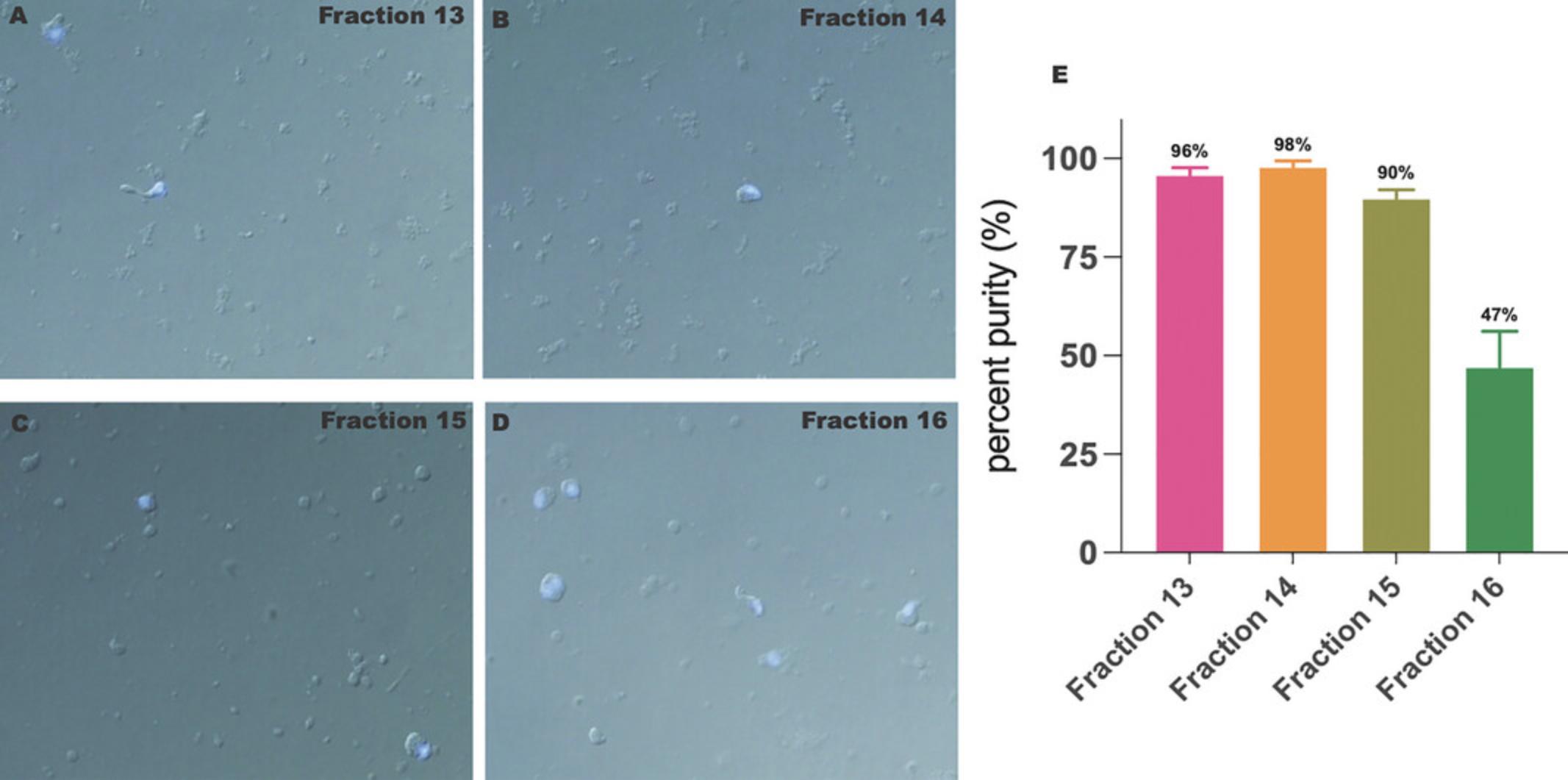
The successful application of the protocol yields high quality and purity RBs predominantly in fractions 13 to 15 (fraction 13, ∼4.8 × 105 RBs, 96% pure; fraction 14, ∼7.9 × 105 RBs, 98% pure; fraction 15, ∼1.5 × 106 RBs, 90% pure) (Fig. 2A). Fractions 1 to 12 contain no material (not shown), while fractions 16-18 combine all cell types (Fig. 2D). On average, 2.5-3 × 106 RBs are obtained from combined fractions 13 to 15 in a single purification experiment, which is sufficient to perform downstream protein or RNA extraction analyses. Typically, 250 µg of protein or 10 µg of total RNA can be extracted when all three fractions are pooled together. In addition, fraction 16 also contains a significant number of RBs (∼47%); however, it is generally heavily contaminated with round spermatids and pachytene spermatocytes. To enable a quick assessment of the purity of the isolated RBs, DAPI-stained fractions can be examined under brightfield in combination with fluorescence microscopy. This will reveal any nuclear impurities within the RB fraction. As shown in Figure 2A-C, fractions 13 to 15 contain DAPI-negative RBs with a negligible number of DAPI-positive cells, clearly indicating a significant enrichment/separation of RBs.
Time Considerations
The main protocol (steps 1 to 36) takes ∼7 hr. This includes: the preparation of the sucrose gradient (∼3 hr, including preparation of different molarities of sucrose solutions); obtaining mouse testis tissue and the preparation of single cell suspension (∼2 hr); loading the gradient tubes with cell suspension and ultracentrifugation (∼1.5 hr); collecting fractions from two tubes (∼30 min), followed by a 15-min assessment of RB fractions under brightfield microscope. Evaluation of purity using the DAPI-staining procedure (steps 38 to 48) takes ∼1 hr. The staining of RBs with anti-H2A.B3 antibody (steps 49 to 60) requires 4 to 4.5 hr plus an overnight (∼16 hr) incubation with primary antibodies.
Troubleshooting
Table 3 provides a troubleshooting guide with detailed solutions to problems that may arise during the purification process.
| Problem | Possible cause | Solution |
|---|---|---|
| Low RB purity with germ cell contamination | Desired RB fractions are contaminated with germ cells due to overloading of the gradient with more than the recommended quantity of total germ cells; the presence of cellular aggregates, or factors related to inappropriate loading and unloading (fractionation) of gradients | Load fewer cells per gradient; examine the sample under microscope before loading to ensure that cells have not aggregated; if aggregates are observed, filter cells through 100-μm filter again and do not skip step 20 (double filtering through 100-μm filter); load cells slowly without disturbing the gradient; unload fractions after centrifugation very carefully ensuring that fractions do not mix |
| Gradient loading with >125 million cells interferes with the process of density-based separation of different cell types during ultracentrifugation | Load 100-125 million total germ cells per gradient tube | |
| Preparation of germ single-cell suspension is one of the key factors in the success of this purification method; insufficient chopping of testes followed by a harsh filtration process or vigorous pipetting results in cell lysis and release of chromatin from lysed cells that could lead to the formation of cellular aggregates | Chopping rate per minute should be maintained close to the recommended number (150 cuts per minute); over or under chopping could cause RB-like artefacts or insufficient release of single cells; use of a 1-ml syringe plunger during filtration should be very gentle | |
| Any disturbance due to vibration, sudden movement, or quick pipetting during loading or unloading of the gradient tube mixes up the RB population with other cell types; use of a narrow-end 200-µl gel loading tip during cell loading is essential to minimize potential disturbance of the sucrose gradient; fractionation must be a very slow process | Use of a narrow-end 200 µl gel loading tip during cell loading is essential to minimize potential disturbance of the sucrose gradient; try to maintain a steady hand and posture. | |
| Collecting fractions with a needle still having the bevel could lead to the drawing of material from a wider space and result in contamination with material from the next fraction below; as the bevel part has an angled opening, it might also create the possibility of drawing air bubbles, leading to an inaccurate fraction volume | Cut off the bevel from the end of the needle and widen the opening afterwards to draw accurate and purer fractions; the needle can be washed and re-used | |
| Low germ cell number and viability | Insufficient chopping of seminiferous tubules leaves cells in aggregates, which will ultimately be lost during the double filtration step; handling cell suspension at room temperature as well as vigorous pipetting with an uncut tip end and harsh filtration can reduce cellular viability | It is recommended to maintain the chopping rate at ∼150 cuts per minute; in addition, thorough washing of the tissue culture dish and 100-µm filters with 0.25 M sucrose buffer maximizes the yield of single cells in the resulting filtrate |
| Low RB yield | Loading less than the minimum recommended number of total germ cells in each gradient | Each sucrose gradient tube should be loaded with at least 100 million cells in a volume of 4.0-4.2 ml |
| Putting too much pressure on the syringe plunger can suck up cells from the fraction below | Fractionation of RBs from the gradient tube should be performed gently and maintain a steady hand and posture; it is also recommended to use swing bucket rotor-based centrifugation during RB washing step to preserve intact RB pellet at the bottom |
Acknowledgments
The authors would like to thank Prof David Tremethick for many fruitful discussions. This protocol was supported by NHMRC Ideas Grant fund to Soboleva and Khochbin #GNT2012453.
Open access publishing facilitated by Australian National University, as part of the Wiley - Australian National University agreement via the Council of Australian University Librarians.
Author Contributions
Mohd Nazmul Hasan Apu : Data curation; formal analysis; methodology; validation; visualization; writing original draft; writing review and editing. Nikolay E. Shirokikh : Methodology; supervision. Saadi Khochbin : Conceptualization; funding acquisition; writing review and editing. Tatiana A. Soboleva : Conceptualization; funding acquisition; investigation; supervision; writing review and editing.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
All the data related to the manuscript were added to the manuscript main file, figures, and tables. The corresponding author will provide additional information on a valid request if required.
Literature Cited
- An Der Lan-Hochbrunn, B. C., & Lacy, D. (1974). Isolation of residual bodies and spermatid cytoplasm from the testis of the adult rat. Micron , 5(1969), 359–375.
- Anuar, N. D., Kurscheid, S., Field, M., Zhang, L., Rebar, E., Gregory, P., Buchou, T., Bowles, J., Koopman, P., Tremethick, D. J., & Soboleva, T. A. (2019). Gene editing of the multi-copy H2A.B gene and its importance for fertility. Genome Biology , 20, 23. https://doi.org/10.1186/s13059-019-1633-3
- Barchi, M., Geremia, R., Magliozzi, R., & Bianchi, E. (2009). Isolation and analyses of enriched populations of male mouse germ cells by sedimentation velocity: The centrifugal elutriation. Methods in Molecular Biology , 558, 299–321. https://doi.org/10.1007/978-1-60761-103-5_18
- Bellvé, A. R. (1993). Purification, culture, and fractionation of spermatogenic cells. Methods in Enzymology , 225, 84–113. https://doi.org/10.1016/0076-6879(93)25009-Q
- Blanco-Rodríguez, J., & Martínez-García, C. (1999). Apoptosis is physiologically restricted to a specialized cytoplasmic compartment in rat spermatids. Biology of Reproduction , 61, 1541–1547. https://doi.org/10.1095/biolreprod61.6.1541
- Braun, R. E. (1998). Post-transcriptional control of gene expression during spermatogenesis. Seminars in Cell & Developmental Biology, 9, 483–489. https://doi.org/10.1006/scdb.1998.0226
- Kim, C. R., Noda, T., Okada, Y., Ikawa, M., & Baek, S. H. (2021). Protocol for isolation of spermatids from mouse testes. STAR Protocols , 2, 100254. https://doi.org/10.1016/j.xpro.2020.100254
- Nyquist, S. E., Acuff, K., & Mollenhauer, H. H. (1973). Residual bodies and their components. I. Isolatin methods. Biology of Reproduction , 8, 119–124. https://doi.org/10.1093/biolreprod/8.1.119
- Rodríguez-Casuriaga, R., Folle, G. A., Santiñaque, F., López-Carro, B., & Geisinger, A. (2013). Simple and efficient technique for the preparation of testicular cell suspensions. Journal of Visualized Experiments : JoVE , (78), 50102. https://doi.org/10.3791/50102
- Steger, K. (2001). Haploid spermatids exhibit translationally repressed mRNAs. Anatomy and Embryology , 203, 323–334. https://doi.org/10.1007/s004290100176
- Stouffs, K., Gheldof, A., Tournaye, H., Vandermaelen, D., Bonduelle, M., Lissens, W., & Seneca, S. (2016). Sertoli cell-only syndrome: Behind the genetic scenes. BioMed Research International , 2016, 6191307. https://doi.org/10.1155/2016/6191307
- Xiao, C.-Y., Wang, Y.-Q., Li, J.-H., Tang, G.-C., & Xiao, S.-S. (2017). Transformation, migration and outcome of residual bodies in the seminiferous tubules of the rat testis. Andrologia , 49, e12786. https://doi.org/10.1111/and.12786
- Xiong, W., Wang, H., Wu, H., Chen, Y., & Han, D. (2009). Apoptotic spermatogenic cells can be energy sources for Sertoli cells. Reproduction , 137, 469–479. https://doi.org/10.1530/REP-08-0343
- Yefimova, M. G., Sow, A., Fontaine, I., Guilleminot, V., Martinat, N., Crepieux, P., Canepa, S., Maurel, M.-C., Fouchécourt, S., Reiter, E., Benzakour, O., & Guillou, F. (2008). Dimeric transferrin inhibits phagocytosis of residual bodies by testicular rat Sertoli cells. Biology of Reproduction , 78, 697–704. https://doi.org/10.1095/biolreprod.107.063107

