10X Multiome TST Nuclei Isolation
Sébastien Vigneau, Yashika_Rustagi
Abstract
This protocol describes the process of nuclei isolation from frozen tissue. The protocol has been slightly modified from the Nature Medicine RNA-Seq toolbox manuscript (Slyper et al., 2020) and applied to frozen melanoma and liver metastases for the Human Tumor Atlas Network (HTAN) single-nuclei RNA-seq Trans-Network Project (snRNAseq TNP).
Steps
PREPARATION
Clean off bench space with RNAse away, then 70% EtOH.
Set swing-bucket centrifuge to 4°C.
Prepare tissue culture hood by turning off UV light, opening the sash, and allowing for the 3-minute ventilation. Clean off work area with 70% EtOH and check for any supplies needed in the hood (i.e. Tips, tubes, serological tips, etc.).
Gather materials.
Clean spring scissors and forceps with 70% EtOH. Leave on Kim wipe to dry.
If working with OCT,prepare ice bucket with enzyme blocks and at least 50 mL PBS.
Take out 10x Reagents. Obtain the 20x Nuclei Buffer and ATAC Buffer from the 10x MO ATAC Kit in -20°. Obtain gel beads and Template Switch Oligo from -80°. Keep enzymes in freezer in kit until it is time to load.
See Materials section, Preparation Materials
Buffer Aliquot Preparation
Obtain aliquots from -20°C. Let thaw for approximately 20-30 minutes. Mix with P1000 once the last reagent is added. DO NOT vortex buffers. Once prepared, keep all buffers on ice. Always add Protective RNAse Inhibitor (PRI) last. DO NOT vortex PRI. See Appendix A for stock buffers. See Appendix B if making buffers the day of nuclei isolation.
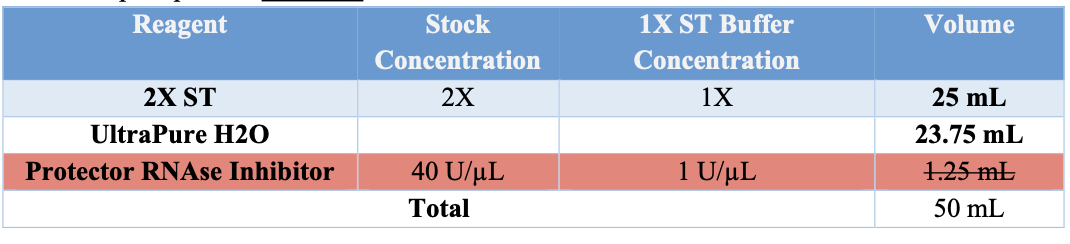
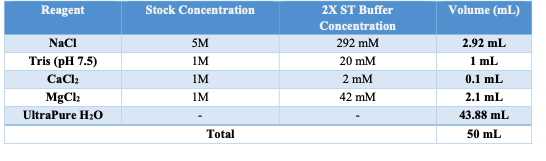
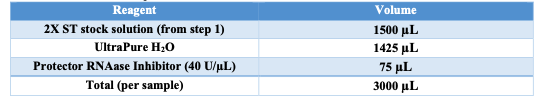
1X ST
- Obtain 1X ST buffer (w/o PRI) aliquot of 2.925mL.
- Prior to use add 75 μL of Protective RNAse Inihibitor.
- Keep on ice.
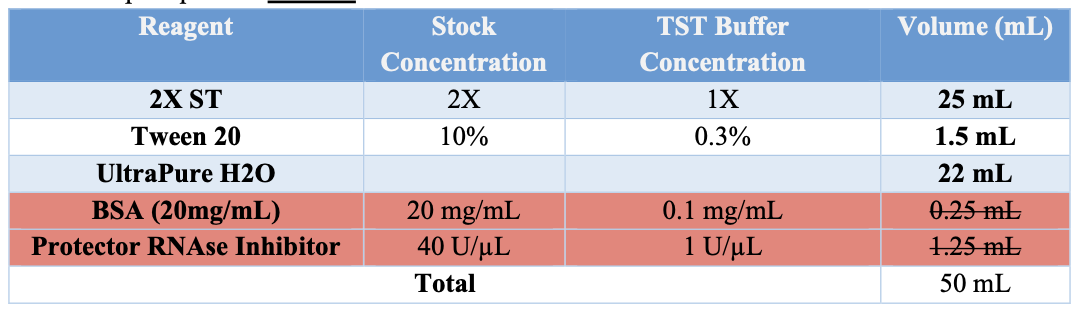
TST
- Obtain TST buffer (w/o BSA, PRI) aliquot of 1.94mL.
- Prior to use add 10 μL of 20mg/mL BSA.
- Add 50μL of Protective RNAse Inhibitor.
- Keep on ice.
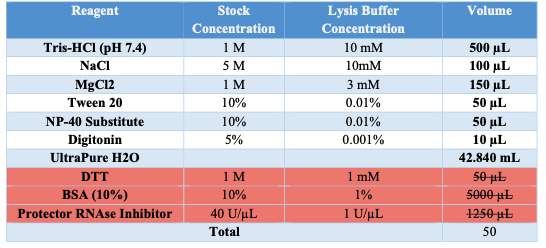
Lysis Buffer
- Obtain Lysis Buffer (w/o DTT, BSA PRI) aliquot of 874μL.
- Prior to use add 1μL of DTT .
- Aliquot 87.5μL into a new tube.
- Add 10μL of 10% BSA to the tube from step 3.
- Add 2.5μL of Protective RNAse Inhibitor to the tube from step 3.
- Keep on ice.
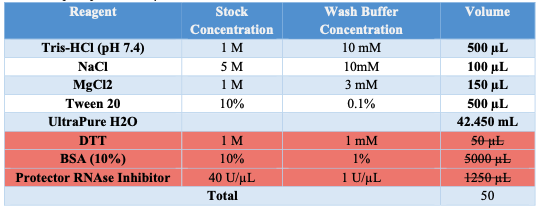
Wash Buffer
- Obtain Wash Buffer (w/o DTT, BSA, PRI) aliquot of 874μL.
- Prior to use add 1μL of DTT
- Add 100μL of 10% BSA
- Add 25μL Protector RNAse Inhibitor.
- Keep on Ice.
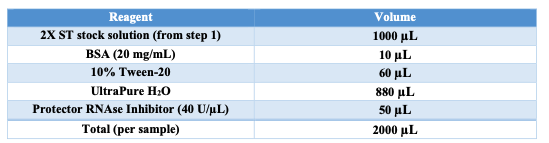
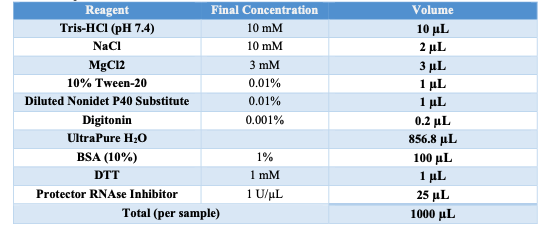
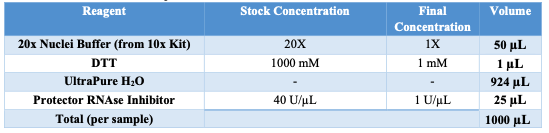
Diluted Nuclei Buffer
-
Obtain 20x Nuclei Buffer from 10x MO ATAC kit. Vortex and spin down the 20x Nuclei Buffer. Prepare the Diluted Nuclei Buffer using the following table: | A | B | | --- | --- | | 20x Nuclei Buffer (10x Kit) | 50 μL | | DTT | 1 μL | | UltraPure H2O | 924 μL | | Protector RNAse Inhibitor | 25 μL |
-
Keep on ice.
MECHANICAL DISSOCIATION
Avoid thawing tissue before dissociation. If tissue is large enough for excess, cut into smaller fragments about the size of a grain of rice on ice. Note percentage of tissue used, and properly store remaining tissue in original storage case (cassette, tube, etc. )
OCT Block:
- Place OCT block on dry petri dish on dry ice. Using a scalpel, scrape away thin pieces of OCT using the “parmesan cheese” method . Stop using the “parmesan cheese” method when the tissue is more visible through the OCT. Be careful not to cut away any tissue.
- Add tissue to petri dish with ~at least 50 mL of cold PBS on ice (enough to submerge sample) and carefully pull away the remaining OCT using two forceps.
- Transfer tissue to 1.5 mL tube on wet ice . Add 1 mL of TST buffer and chop on ice with spring scissors for 10 minutes . Note: After 3-5 minutes, suspension may be homogenized enough to instead pipette with a P1000 for the remainder of the 10 minutes.
Cryoprep - (See COVARIS cryoPREP Protocol)
- Transfer 1mL of TST Buffer with pulverized tissue from tissue pouch into 1.5 mL tube on wet ice. Check pulverization quality to move on to chopping or pipetting.
- Chop on ice with spring scissors for 5 minutes. Note: After ~3 minutes, suspension may be homogenized enough to instead pipette with a P1000 for the remainder of the 5 minutes.
FILTRATION
Attach a 30 μm filter to a 15 mL Falcon tube on wet ice . Pass the homogenized ~1 mL nuclei suspension of nuclei through the filter.
Wash the previously used 1.5 mL tube with an additional 1 mL of TST buffer, then pass through the same filter.
Wash the filter with 3 mL of 1X ST buffer. The total volume of the suspension should be about 5 mL. Pull the suspension through the filter using a pipette if necessary.
Centrifuge for 10 minutes at 500 g at 4°C . Set the stop break to 5.
Carefully aspirate the ~5 mL of supernatant to a new 15 mL Falcon tube on wet ice .
NUCLEI PERMEABILIZATION
Resuspend nuclei pellet in 100 μL of Lysis Buffer . Pipette mix.
Incubate for 2 minutes on wet ice .
Add 1 mL of Wash Buffer and pipette mix.
Centrifuge for 10 minutes at 500g at 4°C.
Remove supernatant. Keep supernatant in new tube on wet ice.
Resuspend pellet in 100 - 150 μL of chilled Diluted Nuclei Buffer . Keep on wet ice .
NUCLEI QUALITY ASSESSMENT AND COUNTING
Count nuclei and assess quality using a hemocytometer. Dilute to desired concentration with Diluted Nuclei Buffer for easier counting, if necessary. Make a 1:2 dilution with Trypan Blue (5 μL stock, 5 μL Trypan Blue) to count.
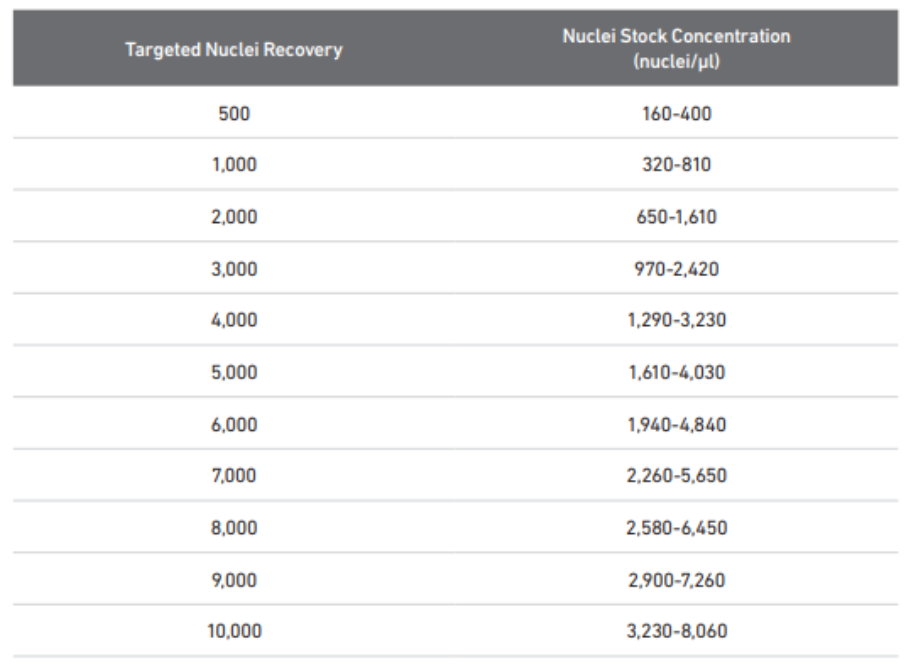
To count nuclei: Count all four corners** and calculate concentration for (nuclei/μL)
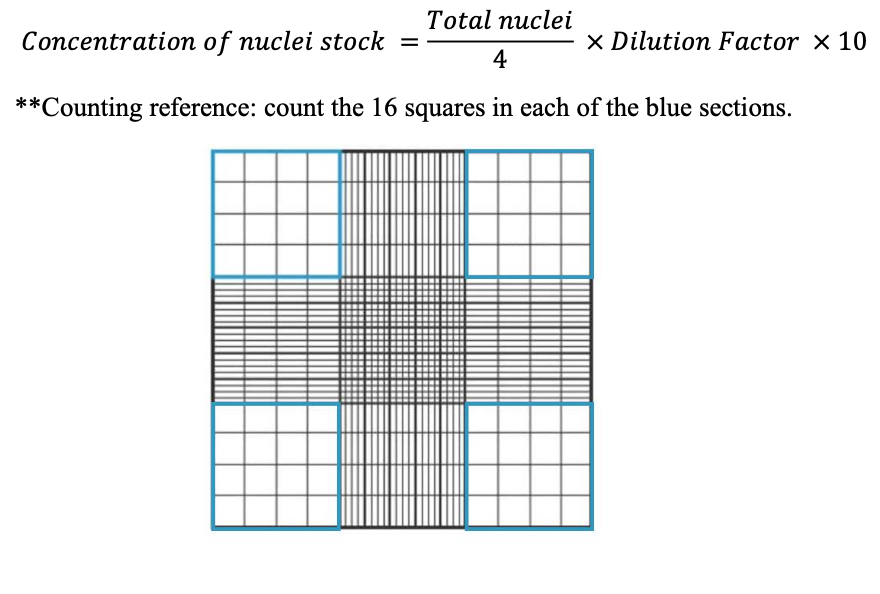
Note: If nuclei concentration is too low for downstream workflow, nuclei can be concentrated by centrifuging for 10 minutes at 500 g at 4°C, removing a fraction of the supernatant and resuspending in the remaining supernatant.
LOADING THE CONTROLLER
Double check with Group Lead and sample sheet to determine how many nuclei to load.
If stock concentration is higher, create a dilution using Diluted Nuclei Buffer for optimal loading concentration.
If stock concentration is lower , assess volume needed to load optimal number/minimal number of nuclei. If needed, stock can be spun down and resuspended in Diluted Nuclei Buffer at a lower volume to create higher concentration for loading.
When creating dilution, always use at least 5 μL of nuclei stock to ensure accurate pipetting. If dilution factor is higher than 25, create a serial dilution.
Example of dilution calculation:
Concentration of stock: 60,000 nuclei/μL
Need a 1:12 dilution to get to 5,000 nuclei/μL
Create a 1:3 dilution from stock
5 μL of stock with 10 μL of Diluted Nuclei Buffer
Left with 20,000 nuclei/μL
From the 1:4 dilution, create a 1:4 dilution
5 μL of 1:3 dilution with 15 μL of Diluted Nuclei Buffer
Left with 5,000 nuclei/μL
Once sample is prepared to be loaded, perform another quality assessment . Using a new 1.5 mL tube, add 5μL of loading sample to 5 μL of Trypan blue. Using a new hemocytometer, count the Trypan-stained sample. This calculation is to make sure the loading sample is the correct concentration. Use the calculation below to determine the concentration of the loading sample prior to loading it on the controller:

Proceed to transposition steps as outlined in “Chromium Next GEM Single Cell Multiome ATAC + Gene Expression” User Guide (CG000338).
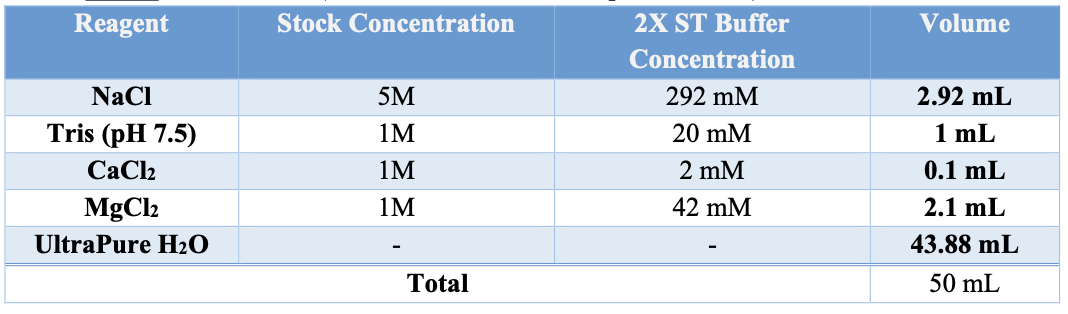
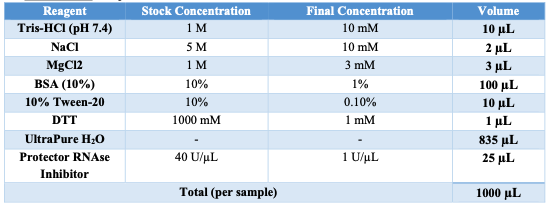
Appendix A: BUFFER STOCK PREPARATION
Prepare the necessary buffers and solutions as outlined below for both nuclei isolation and permeabilization steps:
Appendix B: BUFFER PREPARATION
If no stock buffers are available, please use the following tables.