Sectioning and Counting of Motor Neurons in the L3 to L6 Region of the Adult Mouse Spinal Cord
Adele Austin, Adele Austin, Lynn Beresford, Lynn Beresford, Georgia Price, Georgia Price, Tom Cunningham, Tom Cunningham, Bernadett Kalmar, Bernadett Kalmar, Marianne Yon, Marianne Yon
Abstract
Histology is the study of the microscopic structure of tissues. This protocol permits the generation of frozen transverse sections of lumbar spinal cord regions L3 to L6. It enables counting of murine ventral horn lumbar motor neurons in a reproducible manner. Methods include spinal column dissection, hydraulic extrusion, and histological processing. The preparation for cryo-sectioning includes embedding lumbar spinal cord in optimal cutting temperature (OCT) medium. The correct orientation of the tissue is critical as calculating the amount of tissue to discard saved time overall. Specific details regarding section thickness and mounting are described. These requirements not only allow optimum coverage of specific regions but also ensure that no individual motor neuron was counted twice. The Nissl bodies of the motor neurons were stained using gallocyanin. The sections obtained are all of a comparable area and quality assurance is consistent. The specificity of the staining enables the scientist to identify and reliably quantify lumbar motor neurons. © 2022 The Authors. Current Protocols published by Wiley Periodicals LLC.
Basic Protocol 1 : Euthanasia of mouse and isolation of spinal cord
Basic Protocol 2 : Hydraulic extrusion of the spinal cord
Basic Protocol 3 : Identification of the lumbar region
Basic Protocol 4 : Embedding cord in OCT
Basic Protocol 5 : Collection of frozen sections onto slides
Basic Protocol 6 : Gallocyanin staining
Basic Protocol 7 : Motor neuron counting
INTRODUCTION
Histology is the evaluation of structures within tissues (Maximow & Bloom, 1957). This process allows scientists to assess morphology and structure of organs, tissues, and cells at a macroscopic level (Leeson & Leeson, 1981). The protocol detailed here allows collection of frozen tissue sections of spinal cord within the L3 to L6 region of the cord. The collection of these samples allows for the quantification of motor neurons within the tissues in a reproducible manner. Variations on this protocol have been used to quantify neurodegeneration in mouse models of amyotrophic lateral sclerosis (ALS) and other models of neuromuscular disease (Acevedo-Arozena et al., 2011; Frey et al., 2000; Watson-Scales et al., 2018). ALS is a fatal neurodegenerative condition characterized by motor neuron degeneration and muscle paralysis (Hardiman et al., 2017). Here we provide a detailed and up-to-date protocol for reproducible quantification of lumbar motor neurons, designed to minimize processing time. Although these methods were originally created for use in a mouse, this protocol could easily be adapted for other murine species.
This protocol addresses several problems which have been previously encountered when carrying out histological work of a similar nature. Specific examples include ensuring that no motor neuron is counted twice, reducing the time spent on serial sectioning, and use of appropriate staining techniques. Careful detail was taken with the counting methods described further in Basic Protocol 7 to avoid the overlap of any neuronal body. A trimming method was also introduced whereby the first 3 mm of the sample was discarded prior to collection of serial sections for a further 5 mm with the remaining block stored for any future investigations. This significantly reduced the amount of time spent collecting sections. Previously images were used to match the stage of depth cut, however this mathematical approach was considered more accurate. The staining methods utilized gallocyanin because of its subtle finish and consistency, as this reagent does not overstain or under differentiate.
There are seven basic protocols that make up this process, the first of which is the euthanasia of the mouse and the isolation of the spinal cord. A terminal anesthetic is used to cull the animal before the spinal column is cut from the carcass. Basic Protocol 2 describes the hydraulic extrusion of the spinal cord. This involves the use of a PBS preloaded syringe which utilizes the pressure of the liquid to push out the cord from the surrounding bone column. Basic Protocol 3, identification of the lumbar region, requires identification of the lumbar enlargement and a 1-cm guide to identify the region of interest and dissect this out. Basic Protocol 4 entails embedding the cord in OCT, in which the dissected lumbar spinal cord is submerged into OCT compound and then frozen into a solid block ready for sectioning. Basic Protocol 5, collection of frozen sections onto slides, involves the use of a cryostat to trim and discard waste tissue prior to serial sectioning. Basic Protocol 5, gallocyanin staining, describes how the prepared sections on slides are then treated with a stain in order to identify the motor neurons. The final protocol (Basic Protocol 7) allows for the manual counting of the motor neurons.
NOTE : All protocols using live animals must first be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) and must follow officially approved procedures for the care and use of laboratory animals.
Basic Protocol 1: EUTHANASIA OF MOUSE AND ISOLATION OF SPINAL CORD
The first procedure is to euthanize the mouse so that the spinal cord can be extruded and the lumbar region located.
CAUTION : There is a risk of animal bites and trained and/or licensed personnel must carry out this procedure. Pentobarbital is used for euthanasia rather than cervical dislocation to prevent any physical damage to the brain which is commonly dissected at the same time as the spinal cord. Pentobarbital is an injectable fast acting central nervous system agent which causes loss of consciousness and cardiovascular depression. It must be used in compliance with associated laws and the establishment must have a controlled drug license. There is also a risk from the use of associated sharp needles or related equipment.
Materials
-
C57BL6NTAC mice: 8 weeks old; ALS line Fustm3.1(FUS)Emcf (MGI:6193752) (European Mouse Mutant Archive, EM: 13073), referred to here as hFUS, was used to generate motor neuron count data
-
Diluted pentobarbital solution (Merial Animal Health, cat. no. 0C2287B; see recipe)
-
70% ethanol solution, containing industrial denatured alcohol (IDA; Pioneer Research Chemicals, cat. no. PRC/R/101; see recipe)
-
1-ml syringe (SLS, cat. no. SYR6200) for use with pentobarbital
-
25-gauge needle (Merck, cat. no. 15391547)
-
Scissors for dissection (Interfocus, cat. no. 14007-14)
1.Euthanize mouse with a 0.2-ml overdose of anesthetic via an intra-peritoneal injection of pentobarbital (Fig. 1A).
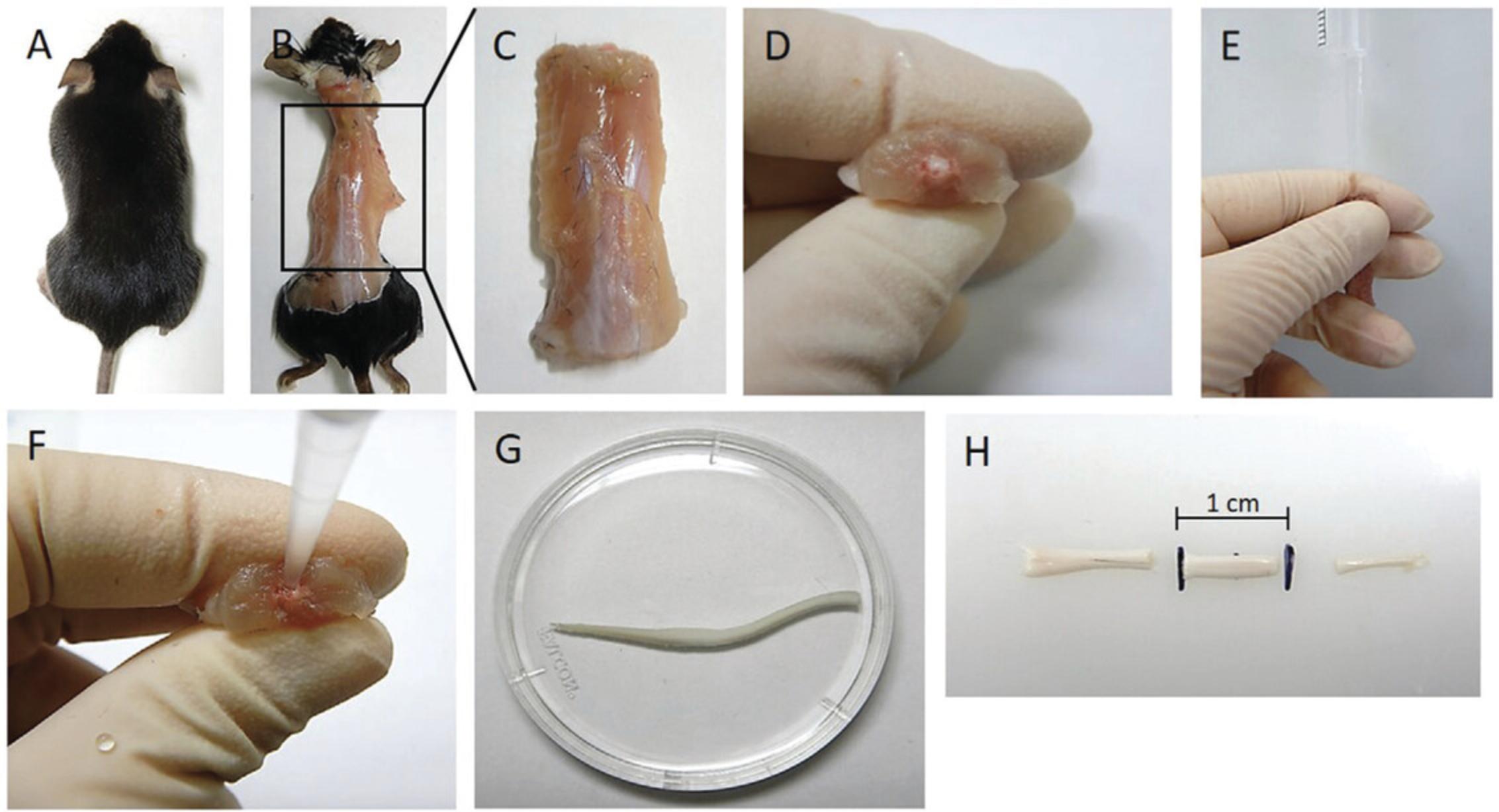
2.Check for the absence of pedal, tail, and orbital reflex using a pair of forceps, then perform the secondary confirmation of death via decapitation by cutting off the head with a pair of scissors.
3.Once the head is removed, position the mouse so that the dorsal surface is uppermost and dampen the coat with 70% alcohol. Make an (dorsal) incision through the skin and expose the spinal column (Fig. 1B).
4.Using scissors trim off all skin, arms, legs, ribcage, and contents of thoracic and abdominal cavity in order to isolate the spinal column (Fig. 1C). Trim spinal column tight to the vertebrae on either side down past the pelvic girdle.
5.Once isolated, trim the ends of the spinal column at the pelvic girdle (distal end) and at the mid-point of the thoracic region (proximal end), ideally through the S-shaped area.
6.Ensure the ends are trimmed straight and the spinal cord is visible at both ends (Fig. 1D).
Basic Protocol 2: HYDRAULIC EXTRUSION OF THE SPINAL CORD
The second procedure is to extrude the spinal cord from within the spinal column.
CAUTION : Ensure that the operator is wearing appropriate personal protective equipment (PPE) that protects eyes from liquids.
Materials
-
Mouse spinal cord (see Basic Portocol 1)
-
Cold sterile PBS (Life Technologies, cat. no. 10010056)
-
200-µl non-filter pipet tip (Sarstedt, cat. no. 70.760.502)
-
5-ml syringe (BD Discardit II; VWR International, cat. no. 309050)
-
Small petri dish (Thermo Fisher Scientific, cat. no. 351)
-
Forceps (Interfocus, cat. no. 11295-00)
1.Trim the large end of a non-filter pipet tip (suitable for 2- to 200-μl pipet) until it fits firmly onto a 5-ml syringe.
2.Pipet cold sterile PBS and leave on ice until needed.
3.Gently grip spinal column with fingers and firmly place pipet tip at the base of the distal end of column, gently pinching at the pipet tip and spinal column to help form a tight seal (Fig. 1E and F).
4.Extrude spinal cord into the petri dish using a short, sharp pulse from the PBS filled syringe, rather than a slower expulsion (Fig. 1G).
Basic Protocol 3: IDENTIFICATION OF THE LUMBAR REGION
The third procedure is to identify the lumbar region of the spinal cord.
CAUTION : Blades must be handled with care.
Materials
-
Mouse spinal cord (see Basic Protocols 1 and 2)
-
Small petri dish (Thermo Fisher Scientific, cat. no. 351)
-
Dissection board (SLS, cat. no. M618)
-
1-cm dissection guide (with the center marked; homemade from cardboard) or ruler or calipers
-
Razor blades (Thermo Fisher Scientific, cat. no. 11904325)
-
Forceps (Interfocus, cat. no. 11295-00)
1.Gently remove spinal cord from the petri dish with forceps and lay flat on the dissection board.
2.Ensure spinal cord is straight and locate the widest section of the lumbar spinal cord.
3.Place the center of a 1-cm guide (with the center marked) at the center of the widest lumbar section of the spinal cord.
4.Cut spinal cord straight at both the proximal and distal ends according to the 1-cm guide (Fig. 1H).
Basic Protocol 4: EMBEDDING CORD IN OCT
The fourth procedure is to transfer and orient the 1-cm piece of lumbar spinal cord into a support medium so it can be quickly frozen with the least amount of damage to the cord. It is essential the cord is dried before placing in the OCT matrix.
CAUTION : Contact with solid carbon dioxide can cause cold burns or frostbite. Solid carbon dioxide can also cause asphyxiation in high concentrations. Use in a well-ventilated area and wear appropriate gloves and safety glasses.
Materials
-
Mouse spinal cord (Basic Protocol 3)
-
Isopentane (VWR, cat. no. 24872.323)
-
Dry ice (solid carbon dioxide; BOC, cat. no. 576CB)
-
OCT embedding medium (Pioneer Research Chemicals, cat. no. PRC/OCT)
-
100-ml Pyrex crystallizing dish (Thermo Fisher Scientific, cat. no. 12589485)
-
Insulating polystyrene ice box
-
Fiber-free filter paper, 140 × 90 mm (Solmedia, cat. no. PBP140)
-
Peel away embedding plastic molds, 12 × 12 × 20 mm (Thermo Fisher Scientific, cat. no. 1220)
-
Forceps (Interfocus, cat. no. 11295-00)
-
Cryostat (Thermo Shandon, NX70 cryostar)
1.Pour 100 ml isopentane into a Pyrex crystallizing dish and place dish into an insulating ice box containing dry ice. Allow the isopentane to cool. To quicken the cooling process, a few pieces of dry ice can be added into the isopentane.
2.Fill a mold to the top with OCT embedding medium and let stand for a couple of minutes to allow any air bubbles to dissipate.
3.Gently blot the 1-cm section of spinal cord with fiber-free filter paper to remove excess moisture.
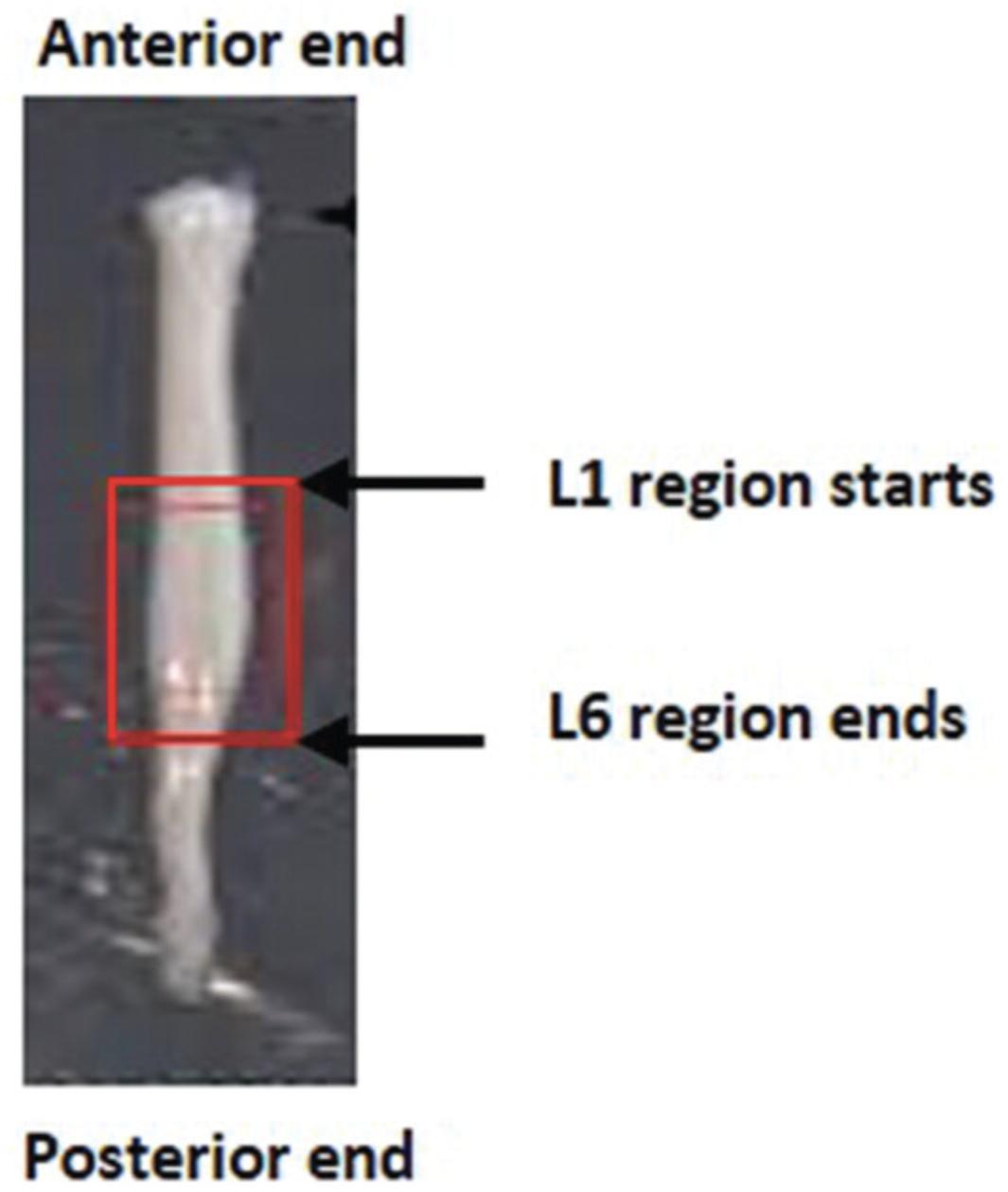
4.Using forceps, orientate spinal cord vertically (L1 end down) and place in the OCT filled mold. Ensure the tissue that requires cutting first goes towards the bottom of the mold, as this is the cutting surface. The largest surface of the block will be connected to the chuck.
5.Completely surround spinal cord with OCT to eliminate the possibility of freezer burn.
6.Place mold into the isopentane and leave to freeze.
7.Embed the cord vertically; it must not be at an angle or it will not be possible to get proper cross sections required for identification of the anatomic level and motor neuron counts.
8.The sample is now at -80°C. It will need to be placed in the cryostat for 10-15 min so it reaches -16°C.
Basic Protocol 5: COLLECTION OF FROZEN SECTIONS ONTO SLIDES
The fifth procedure describes how the block is cut on a cryostat and the sections collected so that the motor neurons are not counted twice.
CAUTION : The cryostat uses extremely sharp blades and must be handled with caution. Prolonged usage of the cryostat may cause repetitive strain and/or carpel tunnel injuries.
Materials
-
Mouse spinal cord sample embedded in OCT (see Basic Protocol 4)
-
Glass charged Superfrost slides (Thermo Fisher Scientific, cat. no. 10149870)
-
Slide printer or Brady labels (Science Warehouse, cat. no. m-97-488)
-
S35 feather blades (VWR 720-1998)
-
Cryostat (Thermo Shandon NX70 cryostar)
-
Plastic box
-
Silicon bag (A5)
-
Electrical tape (Branner, cat. no. 2652808)
1.Section the sample at 20-40 µm thickness; 3 mm of the anterior spinal cord needs to be discarded to reach the L3 region of the lumbar spinal cord, if cutting from anterior to posterior.
2.When at L3, start to collect sections onto slides. Use charged Superfrost slides which feel granular to the touch; the sections go on this side.
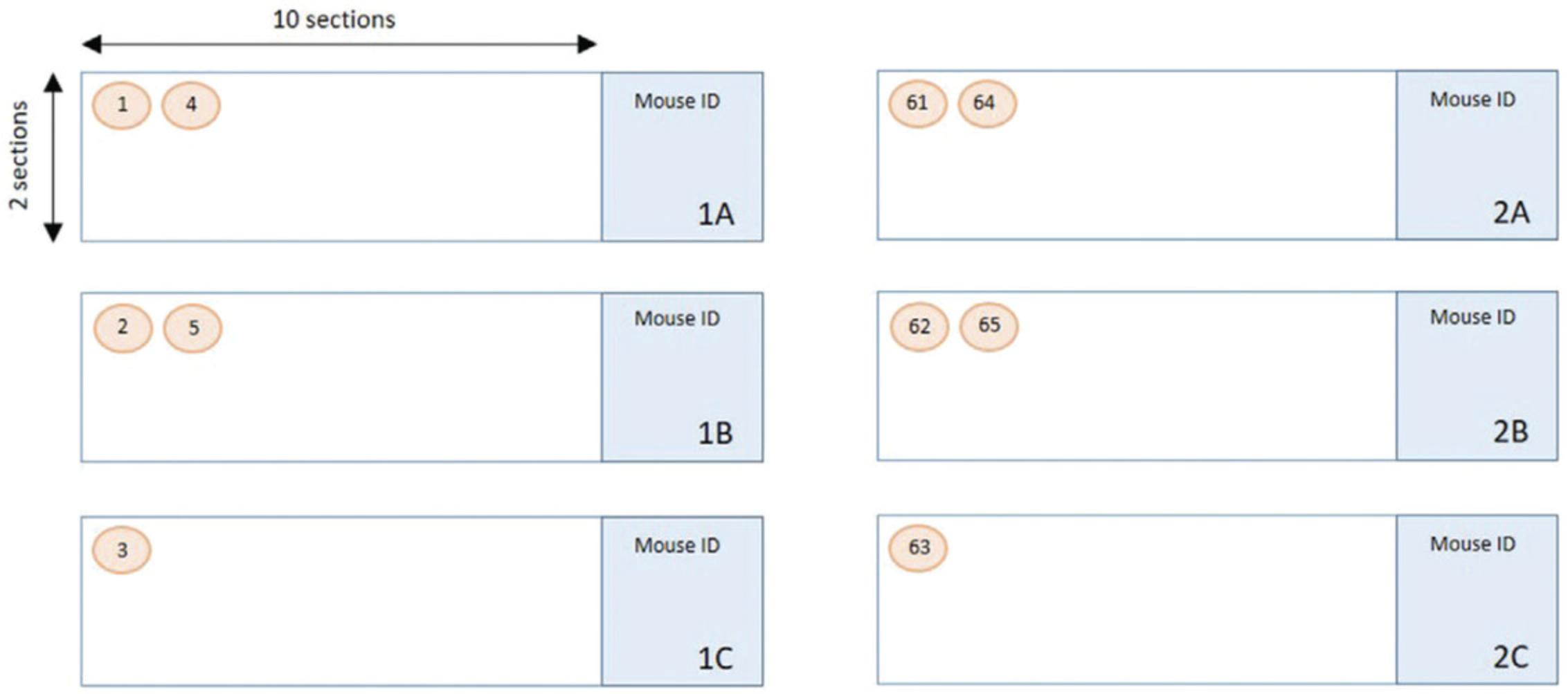
3.Label three slides with 1A, 1B, and 1C (slide A to C in set 1). To cover the lumbar area, four sets are required, i.e., 240 sections in total (4,800 μm).
4.Collect the first section onto 1A, the second onto 1B, and the third onto 1C and then repeat until there are twenty sections per slide.
When you have twenty sections on each of the slides in the set, start again with 2A, 2B, 2C (Fig. 3).
5.The remainder of the block can be put into the -80oC freezer in case any further sections are required.
6.Air dry slides at room temperature for at least 1 hr. Once dry, store in a plastic box sealed with electrical tape and place inside a silicon bag. Store the labeled box in a -20oC freezer in case any further experiments are required at a later date.
7.The first slide of each set, i.e., 1A, 2A, 3A, and 4A, should be stained with gallocyanin (see Basic Protocol 6) and the motor neurons counted.
Basic Protocol 6: GALLOCYANIN STAINING
The sixth procedure explains how to prepare and carry out the gallocyanin staining; this is an ideal staining method for numerous slides that need to be stained serially, equivalently, and reproducibly (Shah, 1991). The main advantages over most other Nissl stains (such as toluidine blue, cresyl violet, and neutral red) are that there is no overstaining and the color resists most subsequently applied chemicals such as alcohol-water mixtures and acidic solutions of anionic dyes. It stains the Nissl bodies pale blue (Kellett, 1963).
CAUTION : When applying the stain, you should wear adequate personal protection and work in a well-ventilated space.
Materials
-
Slide sets with mouse spinal cord samples (see Basic Protocol 5)
-
Gallocyanin solution (see recipe)
-
Ethanol (EtOH; Thermo Fisher Scientific, cat. no. 10437341)
-
70% ethanol solution, containing industrial denatured alcohol (IDA; Pioneer Research Chemicals, cat. no. PRC/R/101; see recipe)
-
90% ethanol solution, containing industrial denatured alcohol (IDA; Pioneer Research Chemicals, cat. no. PRC/R/101; see recipe)
-
Xylene, sulfur free (Pioneer Research Chemicals, cat. no. PRC/R/201)
-
D.P.X., neutral mounting medium (MilliporeSigma, cat. no. 1.0079)
-
Industrial denatured alcohol (IDA; Pioneer Research Chemicals, cat. no. PRC/R/101)
-
Staining jars or Coplin jars (SLS, cat. no. MIC6000)
-
Coverslips, 24 × 50 mm (SLS, cat. no. MIC3234)
-
Paster pipets (Alpha Laboratories, cat. no. LW4212)
-
Mountant needle (SLS, cat. no. INS4460)
-
Cardboard holder (SLS, cat. no. MIC4200)
1.Use an automatic stainer running the dewax program. Alternately, perform staining manually in a fume hood using 50-ml Coplin jars or 250-ml staining jars, depending on how many slides there are to stain.
Process slides through three changes of xylene. The first change is for 15 min, the second for 4 min, and the final change for 2 min. After the xylene treatment, slides are placed into three changes of 100% IDA as follows: 2 min, then 1 min, and finally 30 s. Finally, a 70% IDA wash is used for 30 s. Place slides into distilled water to wash off alcohol.
2.Filter gallocyanin solution into a Coplin jar or staining jar. Place slides in the gallocyanin solution and ensure sections are completely covered with the solution and leave in the solution for 30 min at room temperature.
3.Rinse sections by transferring the slides into a Coplin jar containing distilled water.
4.To dehydrate the sections, transfer slides through a series of jars containing different percentages of ethanol (made up with distilled water): 70% EtOH, 90% EtOH, 100% EtOH. Treat for 2 min with each solution.
5.To clear the sections, transfer slides into xylene: three changes, 3 min for each change; this step removes the alcohol and the slides should not be cloudy. Carry out this step in the fume hood.
6.To mount the sections, use a Pasteur pipet to add a thin line of D.P.X. to a coverslip and gently lower slide with the sections facing down onto the coverslip. Turn slide over and allow the weight of coverslip to press the D.P.X. to the edges of the coverslip.
7.Take care to avoid creating bubbles as these get worse as the medium dries. Gently tease out any air bubbles using a mountant needle.
8.Place slides in a cardboard holder and leave to dry overnight at room temperature.
Basic Protocol 7: MOTOR NEURON COUNTING
In the context of motor neuron counting in ALS research, the motor neurons of the sciatic motor pool are the neurons of interest in mouse models of ALS. Sciatic motor neurons innervate fast fatigable muscles of the mouse's hind limbs, which are predominantly affected in disease (Frey et al., 2000) and thus a reduction in motor neurons of the sciatic pool can be a readout of disease progression in mouse models.
Identifying the region of the lumbar spinal cord
In the spinal cord, motor neurons are located in the ventral horn of the gray matter. With the dorsal spinal cord located upwards, the gray matter (butterfly-like structure in the center of the spinal cord, will be stained a darker purple/blue than surrounding white matter) extends towards the dorsal edges of the spinal cord. At the ventral side, the gray matter does not extend as far towards the white matter. There is a U/V shape in the gray matter at the dorsal side, and an inverted U in the gray matter, below the central cord, on the ventral side (Fig. 4A).
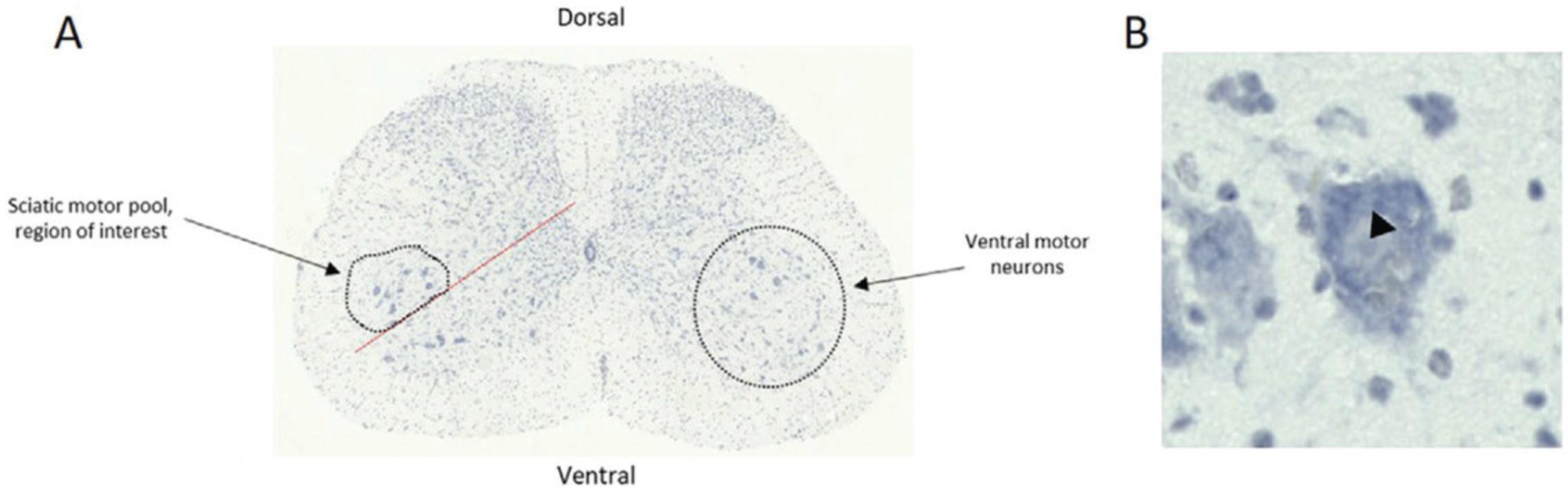
Motor neurons of the sciatic motor pool are large, pyramidal neurons which coordinate conscious movement of hind limb skeletal muscles. The sciatic motor pool is clustered laterally in the ventral horn and so a line is drawn at 45° on the ventral gray matter so the more medial motor neurons are not mistakenly included in the count (Fig. 4A). Motor neurons in the region above the 45° line are counted.
Identifiable features of a motor neuron include:
- Large size and darkest staining.
- The cytoplasm stains more darkly than the nucleus. Within the nucleus, the nucleolus can be seen as a dark dot (Fig. 4B, arrow head).
- Usually, three or more dendrites extend from the motor neuron.
In order to correctly count the sciatic motor neurons, most abundantly found in regions L4-L6, you must first identify the region of the lumbar spinal cord you are looking at. L1-L6 regions have subtle differences in appearance (Fig. 5).
- L1 (Fig. 5B): Cord is oval in shape. Central cord is rounded. Wide U/V in dorsal gray matter. Dorsal gray matter has bulges (red circle). Motor neurons are spread throughout ventral horn.
- L2 (Fig. 5C): Central cord is becoming more oval in shape. U/V in dorsal gray matter is becoming narrower. Dorsal gray matter bulges still present. Motor neurons are still dispersed in ventral horn.
- L3 (Fig. 5D): U/V in dorsal gray matter is becoming narrower still. Dorsal gray matter bulges are becoming triangular, rather than bulging out. Motor neurons are starting to cluster laterally.
- L4 (Fig. 5E): Dorsal groove is becoming narrower and more V in shape. Dorsal bulges are becoming smoother and less pronounced. Motor neurons are clustering laterally and becoming easier to distinguish from surrounding interneurons.
- L5 (Fig. 5F): Cord is becoming less oval and more circular in shape. Dorsal groove is still V shaped and narrow. Motor neurons are clustered laterally. Gray matter is extending towards the ventral edge of the cord.
- L6 (Fig. 5G): Cord is more circular shaped still. Dorsal groove is less deep than before, extending back towards the dorsal side of the cord, is more U in shape, and the sides are wider. Ventral gray matter is extending towards the ventral side of the cord.
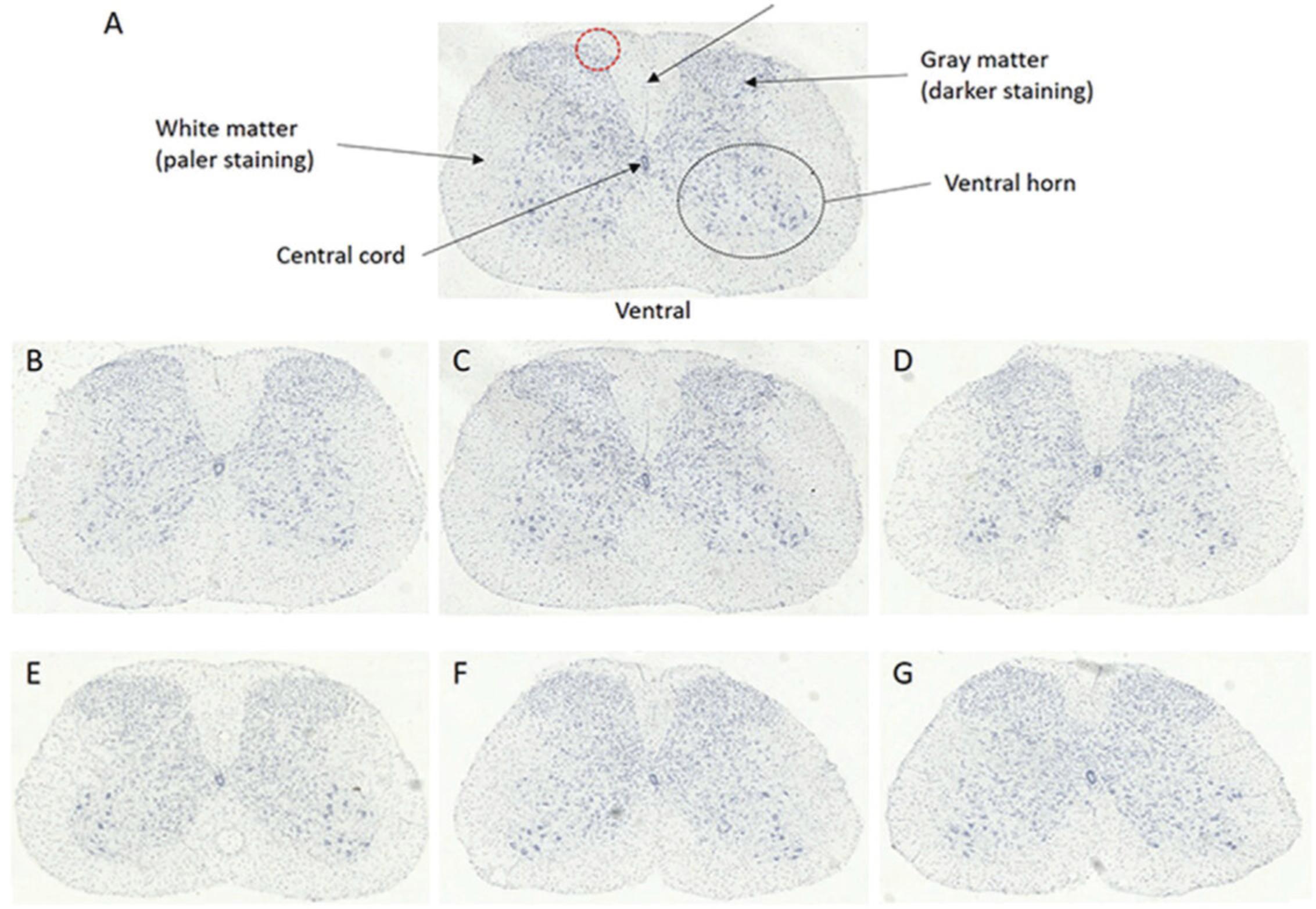
Below L6, the lumbar spinal cord starts to look like L1-L3 again, hence the need to be consistent when preparing, cutting, and analyzing the sections.
Collecting results
The most important factor when counting the motor neurons is to be consistent. Some interneurons will be mistakenly included in the count because they cannot be distinguished from the motor neurons. This is not an issue as they will be included in counts from both control and experimental group sections. If a motor neuron dissects the 45° line, include it in your count and be consistent between sections. As is expected, not all sections will come out perfectly. Sections may break when cut, fold over when annealing to the slide, or be obscured by air bubbles for example. If a section is folded, broken, and/or motor neurons cannot be counted, then make a note in the table and move on to the next section. The fewer broken sections the better to ensure reliability and validity of the counting.
You should expect around six to seven motor neurons per side, per spinal cord section in L5 in a wild-type mouse, however this varies between sections. Some sections will have very few motor neurons per side, with others having more than ten. Aged mice or mice with mutations affecting motor neurons may have fewer motor neurons, hence the need for a robust control group of sufficient size for you to be confident in the results.
When counting motor neurons, collect the information as shown in Tables 1 and 2. Numbering each section in the table makes it easier to refer back when looking at the slide image.
| Number of motor neurons | ||||
|---|---|---|---|---|
| Slide ID (includes mouse ID) | Section number | Region of spinal cord | Left | Right |
| Slide 1A, mouse A | 1 | L4 | 7 | 7 |
| 2 | L4 | 8 | 5 | |
| 3 | L4 | 4 | 9 | |
| 4 | L4 | 5 | 6 | |
| Problem | Possible cause | Solution |
|---|---|---|
| Split cord during extraction | Not cut close enough | More training and experience |
| Too much pressure with the syringe | More training and experience | |
| Rough handling during orientation | More training and experience | |
| Ice artifact | Isopentane not cold enough | Leave on dry ice longer |
| Poor staining | Stain solution has gone off | Make new |
| Precipitate over sections | Unfiltered stain | Filter stain before use |
| Wrong area | Spinal cord embedded the wrong way round | More training and experience |
| Crunching up of sections | Anti-roll plate not lined up properly or is dirty | Clean roll plate and move it slightly backwards |
| Sections going behind blade | Anti-roll plate too far back | Move roll plate slightly forward |
| Section damage | Scored or blunt knife | New blade |
REAGENTS AND SOLUTIONS
Diluted pentobarbital solution
- Pentobarbital (Merial Animal Health, cat. no. 0C2287B)
- Physiological saline (Merck, cat. no. S8776-100ML)
- In a safety cabinet using a sterile 10-ml syringe, draw up 5 ml of sodium pentobarbital (200 mg/ml stock concentration; commercial names: Pentoject or Euthatal) and expel contents into a clean universal flask.
- Using a new sterile 10-ml syringe, draw up and expel 5 ml of physiological saline into the same universal flask. Invert to mix. Place an adhesive label around the universal flask which indicates the following information: 5 ml physiological saline; 5 ml Pentoject or Euthatal. Return to drugs cabinet when empty.
- Store at room temperature for 1 month.
All drugs are kept in lockable cabinets or fridge.
Ethanol solution, 70%,
- 70 ml of industrial denatured alcohol (IDA) in 100 ml distilled water depending on percentage required.
- Store at room temperature indefinitely.
Ethanol solution, 90%
- 90 ml of IDA in 100 ml distilled water depending on percentage required.
- Store at room temperature indefinitely.
Gallocyanin solution
- 0.3g gallocyanin (MilliporeSigma, cat. no. 124508-10G)
- Chrome alum, 10 g (Merck Life Science, cat. no. 243361-5G)
- Distilled water up to 100 ml
- 270-mm folded filter paper (SLS, cat. no. 1213-270)
- Dissolve 10 g of chrome alum in hot distilled water, then at the boiling point, add 0.3 g of gallocyanin and leave for 20 min. Once cooled to room temperature, add distilled water to a final volume of 100 ml. Filter through 270-mm filter paper.
- Store at room temperature for up to 6 months.
The solution can be reused several times.
COMMENTARY
Background Information
The protocol provided here is an up-to-date method that scientists can follow to consistently assess the number of motor neurons in the lumbar region of murine spinal cords. This is particularly useful in mouse models of neuromuscular diseases, such as amyotrophic lateral sclerosis (ALS), where motor neuron loss can be used as a read out of disease progression. It is critical to be accurate and consistent when dissecting the lumbar region of the spinal cord as different axial levels harbor variable numbers of motor neurons, with a concentrated pool in the lumbar region innervating the hind limbs.
In this protocol, fresh, unfixed, frozen spinal cord tissue is used as opposed to the historical perfusion fixed tissue. This is advantageous as it allows other tissues in the mouse, which are also unfixed, to be used for various downstream techniques and experiments such as gene and protein expression, RNA sequencing, and proteomics. This ultimately reduces the number of mice used overall. There is also less handling of the cord when it is fresh frozen so it is less likely to get damaged. Perfusion is also time consuming whereas fresh freezing is significantly quicker.
Motor neurons can be counted using perfused tissue that is processed into paraffin wax embedded blocks but the chemicals and the heat of the wax can destroy proteins that can otherwise be demonstrated. For this reason, fresh frozen sections were used as these are more compatible with several downstream staining techniques. Additionally, sections cut on a microtome are much thinner than sections cut on a cryostat so it would take a lot more sections and slides to cover the same distance of cord, wasting time and resources.
Immunohistochemistry can be used to demonstrate the motor neurons but the gallocyanin stain is easier, cheaper, and more consistent. Gallocyanin binds to the granular structures of nucleic acid. Its method of binding and specificity are still not completely known. However, it is thought that gallocyanin-Cr(H2O)4 selectively binds to nucleic acid phosphate groups, particularly within a pH range of 1.5 to 1.75 (Bourne, 1976). The basophilic compounds take on a bluish color, which are very prominent and make the cell boundaries easy to define and identify (Shah, 1991).
Critical Parameters
The extraction of the lumbar spinal cord undamaged and identification of the correct anatomical region are essential as this will affect the downstream task of counting motor neurons from each lumbar region. The orientation of the spinal cord is also critical because 3 mm of the anterior cord is discarded before serial cutting of sections begins. If it is embedded the wrong way up, the sections will be taken from the incorrect region. It is also essential that care is taken when cutting serial sections because if too many sections are lost, damaged, or misshapen, large areas of the lumbar regions cannot be considered in the final motor neuron counts. This can be prevented by using a sharp, fresh blade in the cryostat. Ensuring sufficient numbers of mice used is also important to ensure results are reliable and valid. When counting motor neurons, it is most important to be consistent when drawing the 45° line and deciding if the neuron observed is a motor neuron. By the nature of the manual counting protocol, some interneurons will be included in the count as they are a similar size to motor neurons, or some motor neurons will be missed from the count. As long as the counter is consistent and blind to the genotypes of the mice, this shouldn't affect the results as it will occur when counting motor neurons in both the experimental and control group. Ensure the correct statistical test is selected depending on how many experimental groups are being compared.
Troubleshooting
While carrying out the steps detailed in this protocol, detailed troubleshooting was carried out at each documented step. For example, during dissection of the cord, there may be damage if the protocol is not carried out carefully. This type of damage can make the counting of motor neurons difficult resulting in an inability to draw any scientific conclusions. There can also be potential issues when delineating the correct region of the spinal cord during sectioning. Initially views down a microscope were used to compare images of spinal cord, however this was extremely difficult as the sections were unstained at this point and the pattern of cells unclear. This method was extremely time consuming and led to numerous discrepancies. Ultimately, this process was eliminated by dissecting a set length of spinal cord and calculating how much tissue could be discarded before sectioning of the correct region of the lumbar spinal cord commenced. If a different region of the spinal cord is required then the calculation must be reconfigured. See Table 2 for reference.
Understanding Results
At least 40 histological sections should be counted for motor neurons to ensure valid conclusions can be drawn. Motor neuron counts can only be compared between the same regions, for example, L6 compared to L6, so an equivalent number of sections from each lumbar region must be included in the 40 section count. If a section is broken, folded, and/or motor neurons cannot be counted, discard the data for this section, even if only one side was affected as otherwise this will affect counts if normalizing to number of sections counted in total.
If data is collected in this way, total motor neuron numbers can be calculated, along with motor neurons per section by dividing total motor neurons (from both sides) by the number of sections counted.
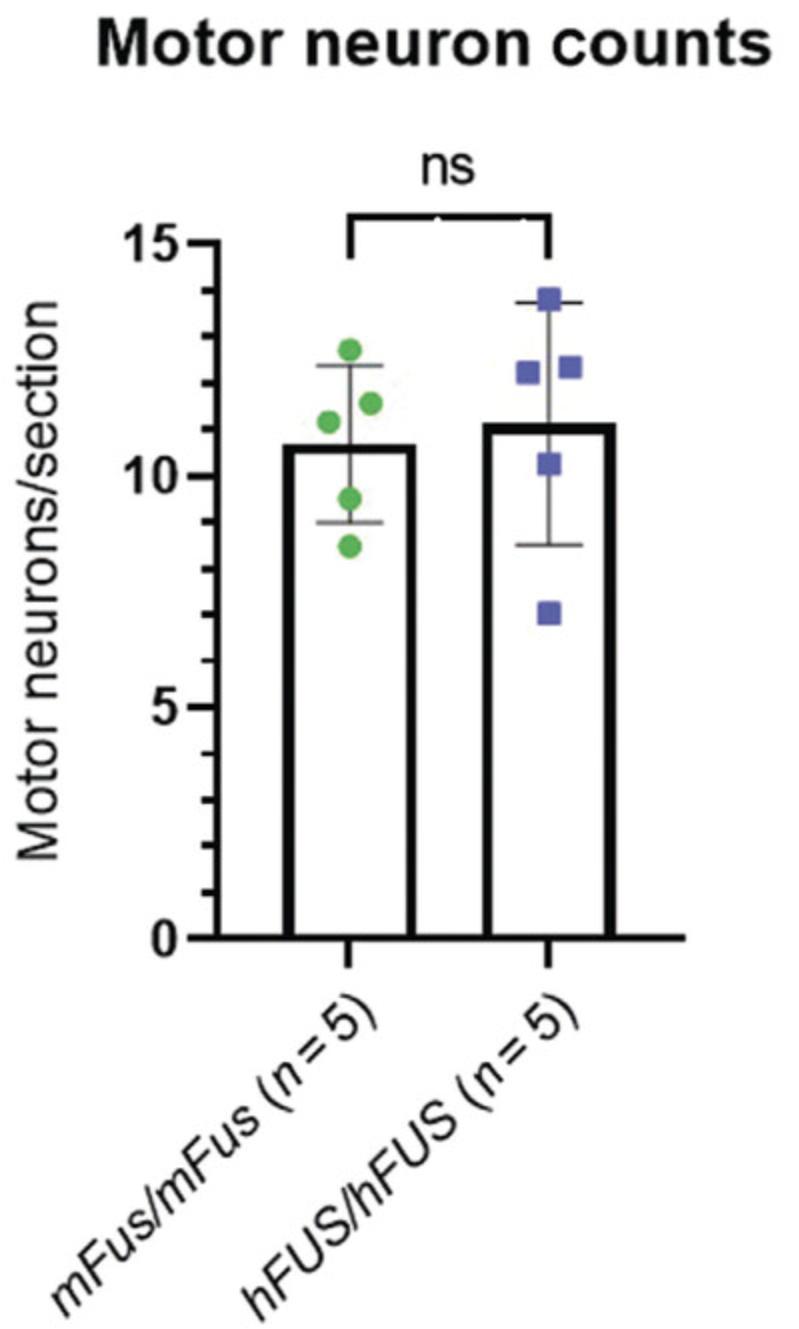
In this example, n = 5 mice per genotype of one sex (female) were used to calculate motor neuron counts. The quantity of motor neurons in the lumbar region of a wild-type mouse were counted and compared to those of mice in the experimental group (Fig. 6). A single sex was used due to the extensive time and labor it takes to prepare and section the spinal cord and count the motor neurons. Both sexes should be considered if mice exhibit a sex-specific phenotype. Total motor neurons divided by the number of sections counted (∼40 per animal) was plotted and an unpaired, two-tailed Student's t -test calculated to assess if there was any significant difference between genotypes (Fig. 6). If several genotypes are being compared, or the change in motor neuron numbers over time, a different statistical test must be used.
Time Considerations
Basic Protocol 1: Euthanasia of mouse: 5 min.
Basic Protocol 2: Hydraulic extrusion of the spinal cord: 10 min.
Basic Protocol 3: Identification of the lumbar region: 2 min.
Basic Protocol 4: Embedding cord in OCT: 15 min.
Basic Protocol 5: Collection of frozen sections onto slide: 2 min.
Basic Protocol 6: Gallocyanin staining: 6 min.
Basic Protocol 7: Motor neuron counting: 1 hr.
Acknowledgments
The authors would like to thank the staff of the Mary Lyon Centre with specific reference to Alex Rodrigues who took the photographs featured in Figure 1. The authors received no financial support for the research, authorship, and/or publication of this article.
Author Contributions
Adele Austin : Methodology, resources, validation, writing original draft; Lynn Beresford : Methodology, validation, writing review and editing; Georgia Price : Methodology, writing review and editing; Tom Cunningham : Validation, writing review and editing; Bernadett Kalmar : Data curation; Marianne Yon : Supervision, validation, writing review and editing.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed during the writing of this protocol.
Literature Cited
- Acevedo-Arozena, A., Kalmar, B., Essa, S., Ricketts, T., Joyce, P., Kent, R., … Fisher, E. M. C. (2011). A comprehensive assessment of the SOD1 G93A low-copy transgenic mouse, which models human amyotrophic lateral sclerosis. Disease Models & Mechanisms, 4(5), 686–700. doi: 10.1242/dmm.007237.
- Bourne, G. H. (1976). International review of cytology (Vol. 44). Amsterdam, Netherlands: Elsevier.
- Devoy, A., Price, G., De Giorgio, F., Bunton-Stasyshyn, R., Thompson, D., Gasco, S., … Cunningham, T. J. (2021). Generation and analysis of innovative genomically humanized knockin SOD1 , TARDBP (TDP-43), and FUS mouse models. iScience , 24, 103463. doi: 10.1016/j.isci.2021.103463.
- Frey, D., Schneider, C., Xu, L., Borg, J., Spooren, W., & Caroni, P. (2000). Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. Journal of Neuroscience , 20(7), 2534–2542. doi: 10.1523/JNEUROSCI.20-07-02534.2000.
- Hardiman, O., Al-Chalabi, A., Chio, A., Corr, E. M., Logroscino, G., Robberecht, W., … van den Berg, L. H. (2017). Amyotrophic lateral sclerosis. Nature Reviews Disease Primers , 3, 17071. doi: 10.1038/nrdp.2017.71.
- Kellett, . S. (1963). Gallocyanin-chrom alum: A routine stain for Nissl substance in paraffin sections. Journal of Medical Laboratory Technology , 20, 196–198.
- Leeson, C. R., & Leeson, T. S. (1981). Histology ( 4th ed., p. 600). Philadelphia: W. B. Saunders Company.
- Maximow, A. A., & Bloom, W. (1957). A textbook of histology ( 7th ed.). Philadelphia: W. B. Saunders Company.
- Richner, M., Jager, S. B., Siupka, P., & Vaegter, C. B. (2017). Hydraulic extrusion of the spinal cord and isolation of dorsal root ganglia in rodents. Journal of Visualized Experiments , 119, e55226. doi: 10.3791/55226.
- Shah, C. K. (1991). Modern dictionary cyto and histo chemistry. Daryaganj, New Delhi: Discovery Publishing House.
- Watson-Scales, S., Kalmar, B., Lana-Elola, E., Gibbins, D., La Russa, F., Wiseman, F., & Tybulewicz, V. L. J. (2018). Analysis of motor dysfunction in Down Syndrome reveals motor neuron degeneration. PLoS Genetics , 14(5), e1007383. doi: 10.1371/journal.pgen.1007383.
Citing Literature
Number of times cited according to CrossRef: 1
- Amira A. Kamel, Ahmed Y. Nassar, Fatma Y. Meligy, Yomna A. Omar, Gamal A. Y. Nassar, Ghada M. Ezzat, Acetylated oligopeptide and N‐acetylcysteine protect against iron overload‐induced dentate gyrus hippocampal degeneration through upregulation of Nestin and Nrf2/HO‐1 and downregulation of MMP‐9/TIMP‐1 and GFAP, Cell Biochemistry and Function, 10.1002/cbf.3958, 42 , 2, (2024).
