Recombinant protein expression and purification of codon-optimized Pfu-Sso7d
Maira Rivera, Javiera Reyes, Cesar A Ramirez-Sarmiento, Paula Blazquez-Sanchez
Abstract
This protocol has been optimized for the recombinant expression of a codon-optizimed Pfu-Sso7d DNA polymerase. This is a fusion protein composed of the Pfu enzyme from Pyrococcus furiosus for DNA amplification by PCR fused to a small 7 kDa protein from Sulfobulus solfataricus that binds to double-stranded DNA without any preference for specific sequences, thus enhancing polymerization processivity without affecting the catalytic activity or thermal stability of the enzyme.
The goal of this protocol was to eliminate the use of large volumes for dyalisis and potential issues with the protein crashing out of the solution due to the use of concentrators for buffer exchange of this enzyme into storage conditions. We also eliminated the use of DTT, which is often found in other similar protocols.
The sequence plasmid encoding the codon-optimized Pfu-Sso7d enzyme used here can be found at https://benchling.com/s/seq-2TcUPjO2uMbDG5ufTQN4
Steps
DAY 1 – Plasmid transformation
Transform 100ng of plasmid containing codon-optimized into E. coli C41 competent cells using either heat shock or electroporation.
Spread transformed cells in LB Agar plates supplemented with 0.05mg/mLKan. Grow plate overnight at 37°C
DAY 2 – Preinoculum
Select a single colony from the LB agar plate to prepare a preinoculum in 10mL LB media supplemented with 0.05mg/mLKan. Grow overnight at 250rpm
DAY 3 – Protein Overexpression
Use the full volume of the preinoculum to inoculate 1L of LB (or TB) media supplemented with 0.05mg/mLKan (1% inoculation). Grow at 160rpm until reaching an optical density at 600 nm (OD600) = 0.8
Upon reaching OD600= 0.8, add IPTG to a final concentration of 0.5millimolar (mM) and incubate overnight at 160rpm
DAY 4A – Protein Purification by IMAC
Centrifuge the cell culture 4000x g,4°C.Then, resuspend the cell pellet in 50mL of Buffer A freshly supplemented with 0.5millimolar (mM) PMSF and 0.2mg/mL lysozyme.
Incubate the resuspended cells at 80rpm
Sonicate on ice for 0h 4m 0s using cycles of 0h 0m 1s ON and 0h 0m 4s OFF at 40% amplitude (Qsonica Q125, 125W).
On an ultracentrifugation tube, Incubate the unclarified lysate at 70°C for 0h 30m 0s to precipitate most of E. coli proteins, and then place on ice for 0h 5m 0s. Centrifuge 20000x g,4°C and collect the supernatant. You might want to collect a small sample for SDS-PAGE afterwards.
On a 1 mL HisTrap column preequilibrated with 10 column volumes (c.v.) (here, 10 mL) of Buffer A , load the supernant.
Wash with 10-20 c.v. of Buffer A.
Then, elute with 5 c.v. of Buffer B , collecting the eluted fractions every 0.5mLin 1.5 ml tubes.
To quickly pool the fractions containing the protein of interest, prepare a 96-well plate or 1.5 mL tubes with 40µLof Bradford reagent and 150µLof distilled water. Then, add 10µLof each protein fraction and compare against a blank reference sample corresponding to 10µLof Buffer B . You can determine your protein-containing fractions either by absorbance at 595 nm on a plate reader or visually by comparing the blue coloration of each fraction against the blank reference. Pool your fractions and collect a 10µL sample for SDS-PAGE
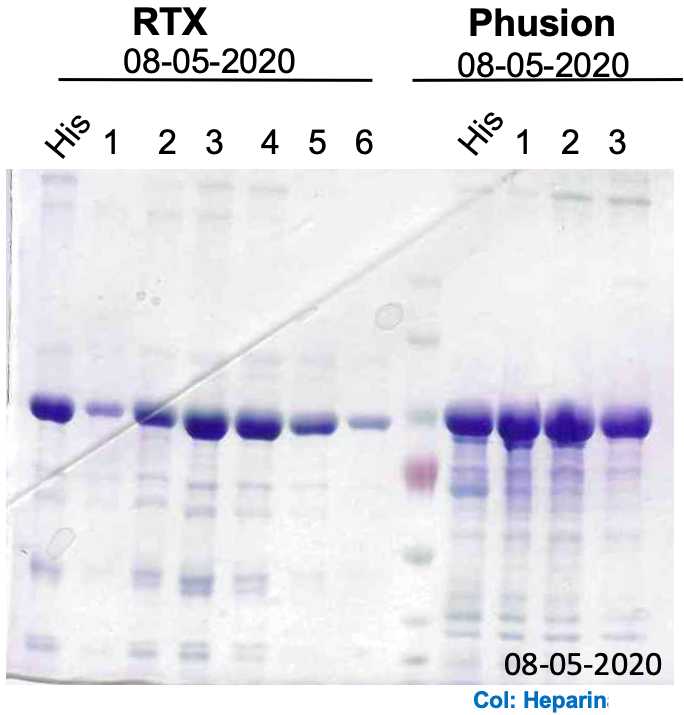
DAY 4B – Second purification and buffer exchange by Heparin
This method was preferred over protein dyalisis or Amicon protein concentration to avoid using large buffer volumes and proteins crashing out of the solution.
Dilute the pooled fractions 3X in 50 mM Tris-HCl pH 8.0, such that the final concentration of NaCl is 100 mM.
Next, load the diluted sample onto a 1 ml HiTrap Heparin column previously equilibrated with 10 c.v. (here, 10 mL) of Buffer HA.
Then, elute the protein using a 10 c.v. linear gradient against Buffer HB , collecting the eluted fractions every 1mL in 1.5 mL tubes. The protein will elute at high concentrations between 300 and 600 mM NaCl
This linear gradient can be achieved by connecting two containers, one with 5 c.v. Buffer HA and the other with 5 c.v. Buffer HB , with a syphon or a tube, and withdrawing solution from Buffer HA container to the column using a cheap peristaltic pump or by gravity.
Again, determine your protein-containing fractions using the Bradford assay. Pool your fractions and determine its protein concentration using the same method and collect a 10µL sample for SDS-PAGE.
For storage, supplement your pooled fraction with 0.2% volume Nonidet P-40 and 0.2millimolar (mM) EDTA. Then, dilute the sample by adding glycerol up to 50% volume to reach final storage conditions: 25 mM Tris-HCl pH 8.0, ~250 mM NaCl, 0.1 mM EDTA, 0.1% Nonidet P-40, 50% glycerol.
Generate200µL aliquots of the enzyme and store it at -20°C until required. Usual final protein concentrations for storage are between 0.2mg/mL and 0.6mg/mL