Panel Optimization for High-Dimensional Immunophenotyping Assays Using Full-Spectrum Flow Cytometry
Joanne Lannigan, Joanne Lannigan, Laura Ferrer-Font, Laura Ferrer-Font, Sam J. Small, Sam J. Small, Brittany Lewer, Brittany Lewer, Katherine R. Pilkington, Katherine R. Pilkington, Laura K. Johnston, Laura K. Johnston, Lily M. Park, Lily M. Park, Maria C. Jaimes, Maria C. Jaimes, Kylie M. Price, Kylie M. Price
assay optimization and troubleshooting
full-spectrum flow cytometry
high-dimensional flow cytometry panel
Abstract
Technological advancements in fluorescence flow cytometry and an ever-expanding understanding of the complexity of the immune system have led to the development of large flow cytometry panels reaching up to 43 colors at the single-cell level. However, as panel size and complexity increase, so too does the detail involved in designing and optimizing successful high-quality panels fit for downstream high-dimensional data analysis. In contrast to conventional flow cytometers, full-spectrum flow cytometers measure the entire emission spectrum of each fluorophore across all lasers. This allows for fluorophores with very similar emission maxima but unique overall spectral fingerprints to be used in conjunction, enabling relatively straightforward design of larger panels. Although a protocol for best practices in full-spectrum flow cytometry panel design has been published, there is still a knowledge gap in going from the theoretically designed panel to the necessary steps required for panel optimization. Here, we aim to guide users through the theory of optimizing a high-dimensional full-spectrum flow cytometry panel for immunophenotyping using comprehensive step-by-step protocols. These protocols can also be used to troubleshoot panels when issues arise. A practical application of this approach is exemplified with a 24-color panel designed for identification of conventional T-cell subsets in human peripheral blood. © 2021 Malaghan Institute of Medical Research, Cytek Biosciences. Current Protocols published by Wiley Periodicals LLC.
Basic Protocol 1 : Preparation and evaluation of optimal spectral reference controls
Support Protocol 1 : Antibody titration
Support Protocol 2 : Changing instrument settings
Basic Protocol 2 : Unmixing evaluation of fully stained sample
Basic Protocol 3 : Evaluation of marker resolution
Support Protocol 3 : Managing heterogeneous autofluorescence
Basic Protocol 4 : Assessment of data quality using expert gating and dimensionality reduction algorithms
INTRODUCTION
Technological advancements in fluorescence flow cytometry and an ever-expanding understanding of the complexity of the immune system have led to the development of large panels reaching 40 fluorophores in the Optimized Multicolor Immunofluorescence Panel (OMIP; Park, Lannigan, & Jaimes, 2020) and 43 colors in a technical note (Sahir, Mateo, Steinhoff, & Siveen, 2020). In contrast to conventional flow cytometry, which primarily measures the peak emission of each fluorophore in a target detector, full-spectrum flow cytometry uses a larger number of detectors with narrow band-pass filters. This allows the entire emission spectrum for every fluorophore to be captured across all laser lines, creating a detailed signature of each fluorophore. This makes it possible to distinguish fluorophores with very similar emission maxima but unique overall spectral fingerprints, increasing the flexibility in fluorophore selection. This feature, coupled with an instrument designed to maximize the detection of emitted light and highly efficient avalanche photodiodes, provides improved detection efficiencies that translate to better detection limits and higher signal resolution (Feher et al., 2016). While these features, unique to full-spectrum flow cytometry, provide the high-quality signals and low noise needed for successful high-dimensional panels, many of the same panel design considerations from conventional flow cytometry still apply. Common characteristics, together with those specific to full-spectrum flow cytometry, have been previously described (Ferrer-Font, Pellefigues, et al., 2020; Park et al., 2020). Now that hardware limitations hindering the use of highly overlapping dyes have been overcome, the main limitations for successful large panel design are the spillover-spreading error inherent to the use of fluorescence and the number of fluorophores available with unique spectral signatures. Many companies have recently begun developing spectrally distinct fluorophores, thus advancing the number of markers available to be analyzed in a single experiment.
Increasing the number of markers in a panel consequently increases the probability of compromising the resolution of the markers and populations of interest. The theoretical approach to panel design aims to avoid issues would that prevent resolution of every marker in the panel; however, in practice it is challenging to perfectly predict the impact of co-expression, variations in marker expression levels, and the performance of each specific reagent. Furthermore, it is difficult to anticipate the accuracy of reference controls for optimal unmixing results. For example, certain fluorophores emit slightly different spectra when bound to compensation beads or in the presence of different buffers.
One obstacle that has always impacted panel design and performance is the unique autofluorescence (AF) signatures of different sample and cell types. Cellular AF levels can vary depending on the type and metabolic state of cells (Mayeno, Hamann, & Gleich, 1992; Roederer, 2016; Shi et al., 2017) as well as sample preparation and staining procedures. This translates into different AF brightness levels and distinct spectral signatures in the samples being analyzed. Full-spectrum flow cytometry can resolve cellular AF signatures and ensure that they are not attributed to any of the fluorophores used. This can improve the signal-to-noise ratio and resolution of markers attached to fluorophores that emit closest to AF maxima (Ferrer-Font, Pellefigues, et al., 2020) in highly autofluorescent tissues such as brain, lung, skin, intestine, and tumor (Schmutz, Valente, Cumano, & Novault, 2016). It is therefore highly recommended to characterize the AF spectrum of an unstained sample from the tissue or cell type of interest prior to panel design. This will provide useful information during fluorophore selection, ensuring that fluorophores are not allocated to areas of the spectrum where AF dominates.
STRATEGIC PLANNING
Once a high-dimensional full-spectrum flow cytometry panel has been optimally designed (Ferrer-Font, Pellefigues, et al., 2020), the step-by-step protocols presented here provide a series of practical steps for full and successful optimization. As outlined in Figure 1, the main procedures include evaluation of spectral reference controls (SRCs; see Basic Protocol 1), evaluation of unmixing of the fully stained (FS) sample (see Basic Protocol 2), evaluation of marker resolution (see Basic Protocol 3), and assessment of data quality (see Basic Protocol 4). Before any of these methods are performed, it is essential to titrate the antibodies used as well as any viability dye used. This is described in Support Protocol 1. An additional protocol describes changes that can be made to the instrument settings (see Support Protocol 2). Methods for evaluating and mitigating autofluorescence are included in Basic Protocol 3 and Support Protocol 3. Together, these protocols can also be used when troubleshooting a panel to identify sources of problems and provide insights into fixing them.
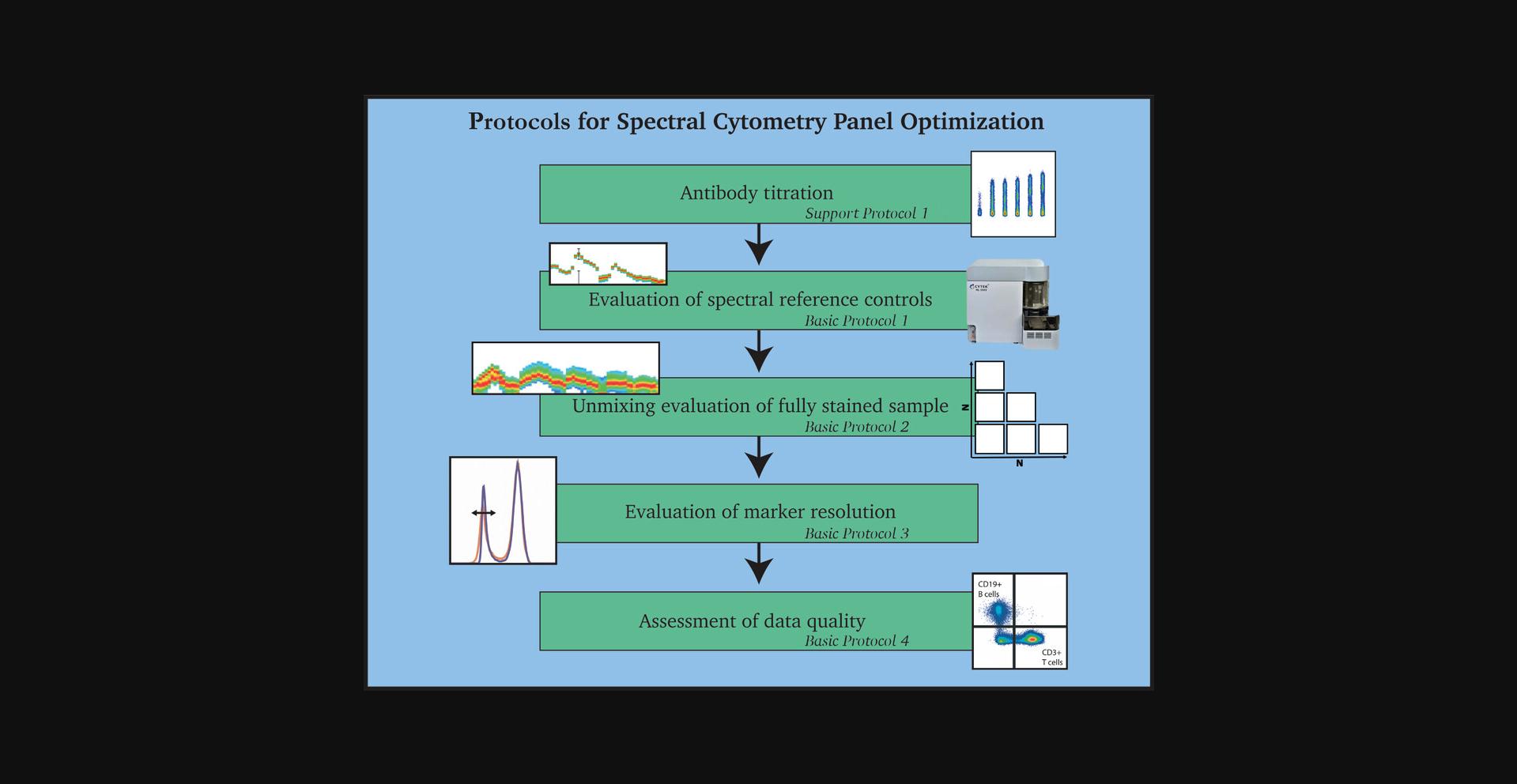
A 24-color panel optimization for identification of conventional T-cell subsets in human peripheral blood is provided to illustrate these procedures. The protocols were developed using the five-laser (5L) Cytek Aurora (Cytek Biosciences), but should be adaptable to any spectral flow cytometer. The protocols were designed for new full-spectrum flow cytometry users. Once familiarity and experience with specific tissue types is achieved, it is possible to modify the steps and order of the protocols to reduce the overall time spent evaluating the panel. If a modified approach is taken, it is recommended that all the overall goals of the protocols (as outlined in Fig. 1) still be carried out.
Basic Protocol 1: PREPARATION AND EVALUATION OF OPTIMAL SPECTRAL REFERENCE CONTROLS
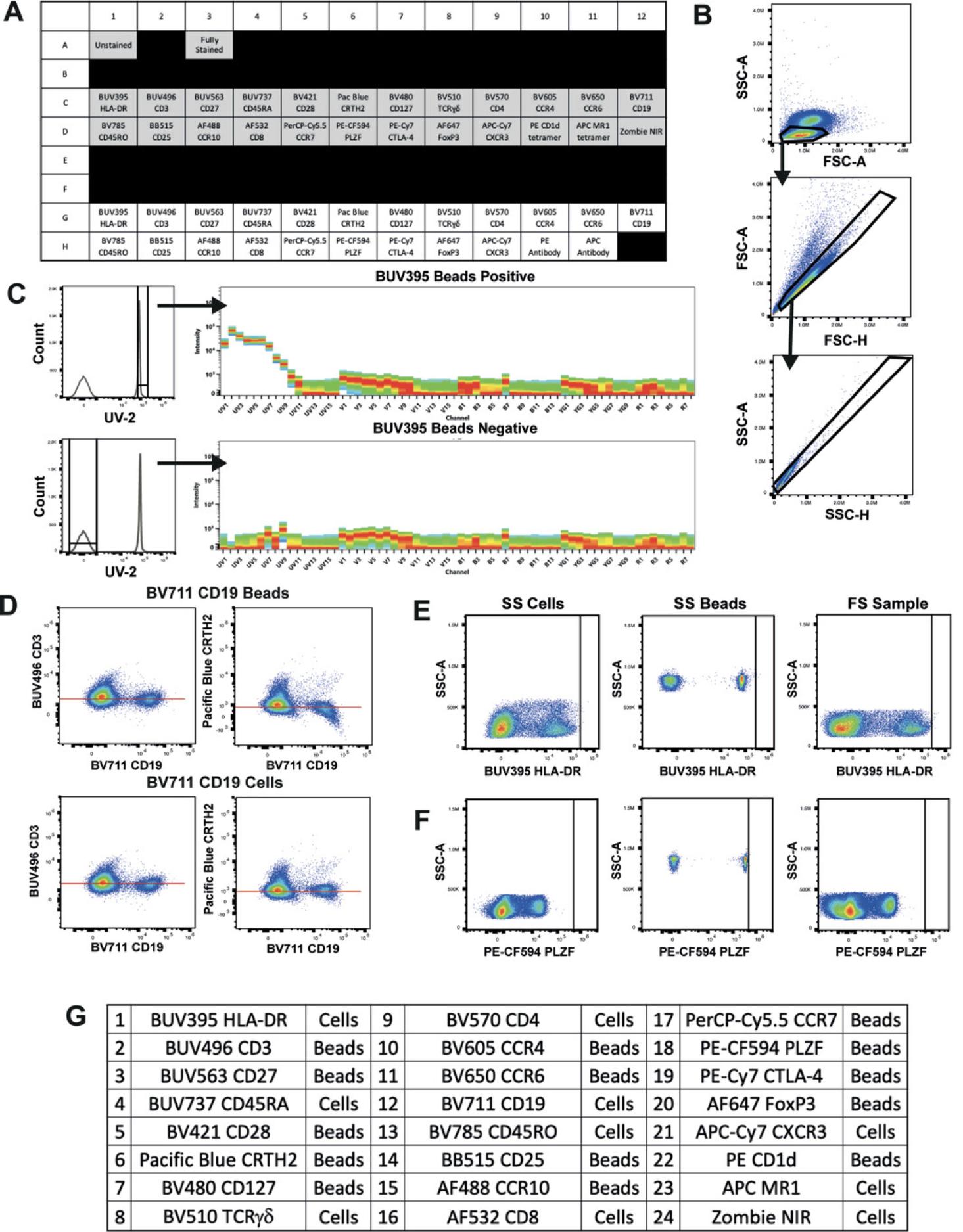
This protocol is divided in two sections. The first is for preparation of SRCs and ensures generation of the high-quality controls required for accurate unmixing. The steps describe the staining of polystyrene compensation beads and cryopreserved PBMCs with surface-labeling antibodies. They can be adapted for other tissues or staining procedures such as intracellular staining. It is important to mention that the preparation procedure will also guide users to prepare a fully stained (FS) sample and fluorescence minus one (FMO) controls as the protocols are very similar; these will be used later for evaluating the unmixing of the FS sample (see Basic Protocol 2). If preferred, FS and FMO samples can be stained separately, but treatment of samples should be kept identical. Importantly, to successfully complete this protocol, SS and FS cells should also be treated the same (antibody concentration, incubation time/temperature, fixed/unfixed, etc.).
The second section of the protocol is for evaluation. This aims first to check the quality of the acquired SRCs and then to evaluate whether there are any spectral mismatches between beads and cells for each fluorophore, which is accomplished by assessing how well the beads unmix the cells using N × N plots for all markers. Unmixing accuracy must be assessed on the actual sample (cells) to be used in the assay. In some cases beads will be acceptable as SRCs and in other cases it may be necessary to use cells. When this protocol is completed, the optimum SRCs (cells or beads) will be determined for future unmixing. If desired, well-characterized and high-quality controls can be stored for future use.
Materials
-
Cryopreserved PBMCs
-
Phosphate-buffered saline (PBS; Gibco, cat. no. 14190-250)
-
Fetal bovine serum (FBS; Gibco, cat. no. 10091-148)
-
Polystyrene compensation beads (e.g., UltraComp eBeads, Life Technologies, cat. no. 01-222-42)
-
FACS staining buffer: PBS with 2% bovine serum albumin (BSA; MP Biochemicals, CAS no. 9048-46-8) and 0.2% sodium azide (Sigma-Aldrich, cat. no. S8032)
-
Zombie NIR Fixable Viability Kit (BioLegend, cat. no. 423106)
-
Human TruStain FcX (Fc Receptor Blocking Solution; BioLegend, cat. no. 422301)
-
Antibodies (see Table 1 for list; see Support Protocol 1 for titration)
-
Brilliant Stain Buffer Plus (BD Biosciences, cat. no. 566385)
-
37°C water bath (e.g., Julabo Ecotemp TW12)
-
Hemocytometer (e.g., Hawksley Counting Chamber) and coverslips
-
96-well U-bottom plate (In Vitro Technologies, cat. no. 353077)
-
Filters with 0.65-μm or smaller pore size (optional)
-
Spectral cytometer (e.g., Cytek Aurora)
-
5-ml polypropylene round-bottom flow tubes (In Vitro Technologies, cat. no. 352008)
-
Cytek SpectroFlo software
-
Data analysis software for analyzing FCS files (e.g., FlowJo or FCS Express)
| Fluorophore | Marker | Supplier | Clone | Catalog number | Concentration (mg/ml) |
|---|---|---|---|---|---|
| BUV395 | HLA-DR | BD Biosciences | G46-6 | 564040 | 1 |
| BUV496 | CD3 | BD Biosciences | UCHT1 | 612940 | 1 |
| BUV563 | CD27 | BD Biosciences | M-T271 | 741336 | 1 |
| BUV737 | CD45RA | BD Biosciences | HI100 | 612846 | 1.25 |
| BV421 | CD28 | BioLegend | CD28.2 | 302930 | 2 |
| Pacific Blue |
CRTH2 (CD294) |
BioLegend | BM16 | 350130 | 8 |
| BV480 | CD127 | BD Biosciences | HIL-7R-M21 | 566101 | 2 |
| BV510 | TCRgd | BioLegend | B1 | 331220 | 15 |
| BV570 | CD4 | BioLegend | RPA-T4 | 300534 | 1 |
| BV605 |
CCR4 (CD194) |
BioLegend | L291H4 | 359418 | 0.6 |
| BV650 |
CCR6 (CD196) |
BD Biosciences | 11A9 | 563922 | 1 |
| BV785 | CD45RO | BioLegend | UCHL1 | 304234 | 1 |
| BB515 | CD25 | BD Biosciences | 2A3 | 564467 | 8.3 |
| AF488 | CCR10 | R&D Systems | 314305 |
RDSFAB3478G 0100 |
0.13 |
| AF532 | CD8 |
Thermo Fisher Scientific |
RPA-T8 | 58-0088-42 | 0.25 |
| PerCP-Cy5.5 | CCR7 | BioLegend | G043H7 | 353220 | 4 |
| PE |
Hu CD1d tetramer |
NIH Tetramer Core Facility |
PBS-57 loaded | 0.81 | |
| PE-CF594 | PLZF | BD Biosciences | R17-809 | 565738 | 0.63 |
| PE-Cy7 | CTLA4 |
eBioscience (TFS) |
14D3 | 25-1529-42 | 0.2 |
| APC |
Hu MR1 tetramer |
NIH Tetramer Core Facility |
5-OP-RU loaded |
0.18 | |
| AF647 | FoxP3 | BioLegend | 259D | 320214 | 0.6 |
| APC-Cy7 |
CXCR3 (CD183) |
Biolegend | 49801.111 | 353722 | 0.13 |
| BV711 | CD19 | BD Biosciences | SJ25C1 | 563036 | 0.25 |
Prepare and acquire samples
1.Thaw PBMCs quickly in a 37°C water bath and add to 5 ml PBS with 2% FBS.
2.Centrifuge 5 min at 500 × g , room temperature, and carefully flick off the supernatant.
3.Resuspend cells in PBS with 2% FBS and count viable cells on a hemocytometer.
4.Centrifuge as before and carefully flick off the supernatant.
5.Resuspend cells in PBS with 2% FBS to a concentration of 5 × 106 viable cells/ml.
6.Distribute 100 μl suspension per well of a 96-well U-bottom plate, allocating one well per fluorophore in the panel and one well for an unstained sample.
7.Add one drop of vortexed compensation beads plus 150 μl FACs staining buffer to a second set of wells, allocating one well per fluorophore in the panel except the viability dye.
8.Centrifuge plate 5 min at 500 × g , 4°C, and flick off the supernatant.
9.Prepare viability stain in PBS according to the titration result (see Support Protocol 1) and add 100 μl to the live/dead cell control and FS sample, if included. Resuspend remaining wells with 100 μl PBS. Incubate for 15-30 min at room temperature, protected from light.
10.Centrifuge as above and flick off the supernatant.
11.Block Fc receptors of the cell samples using 100 μl of 1:40 Fc block in FACS staining buffer. Add an equal volume of FACS staining buffer to the beads. Incubate for 10 min at 4°C.
12.Centrifuge as above and flick off the supernatant.
13.Remove aggregates from antibody stocks by centrifuging 5 min at 16,000-18,000 × g , 4°C (Aass et al., 2011; Ayers et al., 2011; van der Vlist, Nolte-’t Hoen, Stoorvogel, Arkesteijn, & Wauben, 2012) and/or by filtering using a pore size of 0.1-0.65 μm (Inglis et al., 2015).
14.Prepare SS, FS, and FMO control antibody mixes by diluting stocks in FACS staining buffer according to titration results (see Support Protocol 1). Be sure to pipette from the top of the liquid to avoid centrifuged aggregates.
15.Stain all cells and beads with 100 μl of the appropriate antibody mix and incubate in the dark using the incubation time and temperature that will be used in the final assay.
16.Centrifuge as above and flick off the supernatant.
17.Wash twice with 200 μl FACS staining buffer, centrifuging again after each wash.
18.Resuspend in 200 μl FACS staining buffer.
19.Acquire on a Cytek Aurora, taking care to meet the following acquisition criteria:
-
Cytek assay settings are used as a starting point for instrument setup.
-
The scatter profiles of cells and beads are on scale and the FSC area scaling factor (ASF) is optimized (see example in Fig.2B).
-
All fluorescence signals are on scale (<4 × 106). This can be assessed in the full-spectrum plot or in individual plots for every detector.
-
All tubes are recorded with the same fluorescence gain settings for each detector.
-
Sufficient events are recorded to find a clear positive signal.
A minimum of 300 events is needed for each positive and negative population. A good starting point is 5000 total events for beads and 30,000-50,000 total events for cell controls, although it may be necessary to record >50,000 cells to get at least 300 positive events of similar fluorescence intensity for rare markers.
Evaluate results (see Video 1)
20.In the SpectroFlo software, check the raw reference control data and verify that the acquisition criteria in the previous steps have been met:
-
The scatter profiles of the cells and beads are on-scale, clean, and easily gated, and the FSC ASF has been optimized (Fig.2B).
-
Cytek assay settings or a close alteration (also see Critical Parameters discussion of Complex Samples and see Support Protocol2) have been used, and all tubes have been recorded with the same settings.
Support Protocol 1: ANTIBODY TITRATION
Antibody titration is the crucial first step in developing high-dimensional flow cytometry panels. Using the incorrect antibody concentration can increase spread, decrease resolution, increase aggregation of reagents, and give rise to nonspecific binding (Stewart & Stewart, 1997), all of which result in poor panel performance and/or inaccurate results. Ideally, titrations should be carried out in the tissue that will be used in the assay. This is not always feasible, however, if the tissue in question is rare or difficult to work with, or the cells of interest are found at low frequencies within the tissue. In such cases, it is suggested to first add a lineage marker to the mix to help identify the cells that express the rarer marker or to use a surrogate tissue in which the marker is more abundant and simpler to process. Results from this type of titration should always be validated using the tissue of interest.
It is important that readout functional markers are titrated under the maximally activated conditions that will be used in the assay. Staining conditions should also be identical between titration and experiment to prevent spectral pattern mismatches and poor unmixing results.
This protocol describes the preparation of cryopreserved peripheral blood mononuclear cells (PBMCs) for titration of extracellular antibodies. It can be easily adapted and used as a general guideline for antibody titration using other tissues or staining procedures.
Materials
-
Cryopreserved PBMCs
-
Phosphate-buffered saline (PBS; Gibco, cat. no. 14190-250)
-
Fetal bovine serum (FBS; Gibco, cat. no. 10091-148)
-
Zombie NIR Fixable Viability Kit (BioLegend, cat. no. 423106)
-
Human TruStain FcX (Fc Receptor Blocking Solution; BioLegend, cat. no. 422301)
-
Polystyrene compensation beads (e.g., UltraComp eBeads, Life Technologies, cat. no. 01-222-42)
-
Antibodies (see Table 1)
-
FACS staining buffer: PBS with 2% bovine serum albumin (BSA; MP Biochemicals, CAS no. 9048-46-8) and 0.2% sodium azide (Sigma-Aldrich, cat. no. S8032)
-
37°C water bath (e.g., Julabo Ecotemp TW12)
-
Hemocytometer (e.g., Hawksley Counting Chamber) and coverslips
-
96-well U-bottom plate (In Vitro Technologies, cat. no. 353077)
-
Filters with 0.65-μm or smaller pore size (optional)
-
Spectral cytometer (e.g., Cytek Aurora)
-
5-ml polypropylene round-bottom flow tubes (In Vitro Technologies, cat. no. 352008)
-
Data analysis software for analyzing FCS files (e.g., FlowJo or FCS Express)
Prepare and acquire samples
1.Thaw PBMCs quickly in a 37°C water bath and add to 5 ml PBS with 2% FBS.
2.Centrifuge 5 min at 500 × g , room temperature, and carefully flick off the supernatant.
3.Resuspend cells in PBS with 2% FBS and count on a hemocytometer.
4.Centrifuge as before and carefully flick off the supernatant.
5.Resuspend cells in PBS with 2% FBS to a concentration of 5 × 106 cells/ml.
6.Distribute 100 μl suspension per well of a 96-well U-bottom plate, allocating six wells to each antibody being titrated. Include additional wells for unstained and live/dead controls (see example plate plan in Fig. 3A).
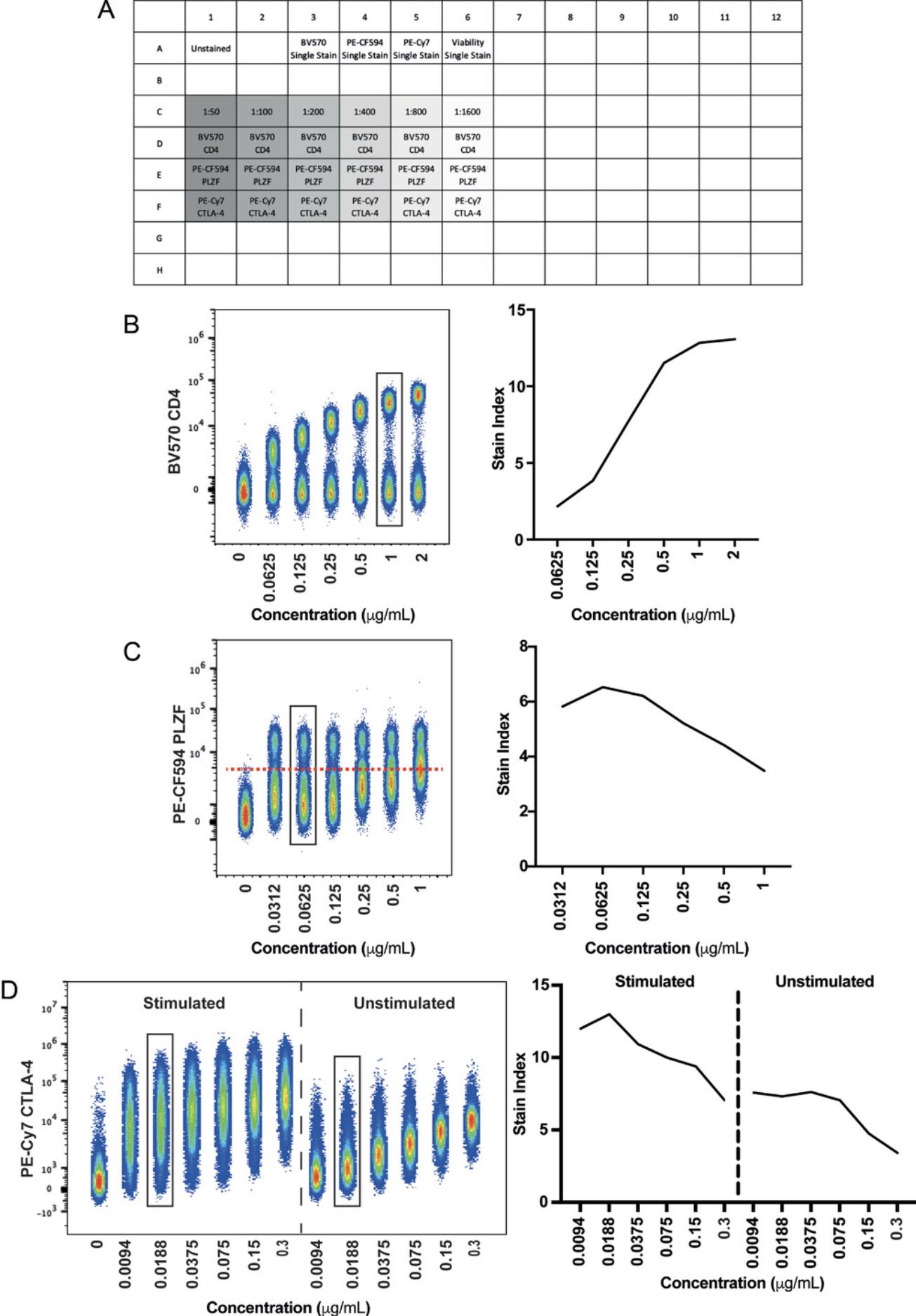
7.Centrifuge plate 5 min at 500 × g , 4°C, and flick off the supernatant.
8.Stain titration samples and live/dead controls with a viability dye that will not cause significant spillover into the fluorophore being titrated.
9.Centrifuge as above and flick off the supernatant.
10.Block Fc receptors by applying 100 μl of a 1:40 dilution of Human TruStain FcX and incubating 10 min at 4°C.
11.Add one drop of vortexed compensation beads per well for the appropriate single stain (SS) controls.
12.Centrifuge as above and flick off the supernatant.
13.Remove aggregates from antibody stocks by centrifuging the vials 5 min at 16,000-18,000 × g , 4°C, or by filtering using a pore size of 0.65 μm or smaller.
14.Create a dilution series for each antibody being titrated by diluting stock solutions in FACS staining buffer. Be sure to pipette from the top of the liquid to avoid the spun-down aggregates.
15.Add 100 μl of each antibody dilution to the corresponding sample in the plate. Add FACS staining buffer to unstained and live/dead controls. For compensation beads, use 100 μl of the 1:100 dilution.
16.Incubate using the time and temperature that will be used in the final assay.
17.Centrifuge as above and flick off the supernatant.
18.Wash twice with 200 μl FACS staining buffer.
19.Resuspend in 200 μl FACS staining buffer and acquire on a Cytek Aurora using Cytek assay settings.
Analyze data
20.Open titration samples in a data analysis software used for analyzing FCS files.
21.Generate median fluorescence intensity (MdFI) values for the positive and negative populations for each concentration.
22.Generate standard deviation (SD) values for the negative population for each concentration.
23.Calculate the stain index (SI) using the equation SI = (MdnFI pos – MdnFI neg) / (2 × SD neg).
24.Select the concentration that gives rise to the best stain index without giving rise to a positive shift of the negative population.
Support Protocol 2: CHANGING INSTRUMENT SETTINGS
In some cases an adjustment of fluorescent gain settings away from the optimized settings cannot be avoided, for instance, if a bright reporter protein (e.g., eGFP) is off-scale. Such adjustments must be carried out carefully to minimize impact on all spectral signatures within the panel. Adjustments can be made detector by detector or to a whole detector array at one time, where all detectors of a given laser line are changed simultaneously. Reduction of the whole array is the recommended approach, as manual alteration of single detectors is prone to distortion of spectral signatures, where the ratio of brightness from detector to detector is not maintained. Regardless, reducing whole detector arrays can lead to peak emissions of spectral signatures being shifted to an incorrect detector (potentially on a different laser line). Therefore, all changes must be checked to ensure that signatures remain as expected based on Cytek's Full Spectrum Viewer, published fluorophore guidelines, or historical data.
1.Observe which fluorescent channel(s) has an off-scale signal.
2.Use trial and error to determine the gain setting that is appropriate to bring the emission peak on-scale (i.e., <4 × 106 on the Aurora).
3.Reduce secondary detector arrays with off-scale signals by the same percentage as the reduction made in step 2.
4.Use Cytek's Full Spectrum Viewer, published fluorophore guidelines, or historical data to check that the altered spectral signature retains the correct overall pattern and that the primary emission peak has not been reduced below any secondary emission peaks.
5.If adjustments to other laser lines are required, continue reducing the respective detector arrays by the same percentage as in step 2.
6.Repeat steps 4 and 5 until the spectral signature appears as expected or all arrays have been reduced by the same percentage.
7.Record all samples at the adjusted settings.
Basic Protocol 2: UNMIXING EVALUATION OF FULLY STAINED SAMPLE
Before any analysis can be undertaken, the FS sample must be checked to ensure that clean data can be obtained through removal of artefacts such as doublets, dead cells, and aggregates. It is also necessary to verify that there is positive staining for all markers in the panel, taking into consideration the biology of each marker. Once this has been asserted, it must be determined whether the SRCs selected (either beads or cells) successfully unmix the SS cells in Basic Protocol 1 can also be used to successfully unmix the FS sample (Video 2).
Basic Protocol 3: EVALUATION OF MARKER RESOLUTION
Once the best possible unmixing has been achieved using Basic Protocols 1 and 2, the resolution of each marker in the FS sample must be compared to the SS cell controls. Assessing the spread of the negative population and/or shifts in the positive signal will provide an indication of whether there is any loss of resolution of markers in the panel when fully stained. This assessment is best achieved by overlaying each marker in the SS cell sample onto the FS sample. If any reduction in resolution is seen, the impact of this can be further investigated to identify if it will impact the ability to identify populations of interest using an established gating strategy. Ideally, the same number of cells from the same tissue type should be stained and acquired for all samples. In practice, however, this is not often feasible, and downsampling can be used to achieve identical cell numbers across samples during analysis. In theory, the only difference between the SS and FS cells should be the number of antibodies in the tube (Video 3). Additionally, AF extraction should be tested to determine if marker resolution can be improved (Video 4).
Support Protocol 3: MANAGING HETEREOGENEOUS AUTOFLUORESCENCE (Video 5)
The following protocol is divided into three sections: Discover, Distinguish and Designate.
Discover
1.Observe N × N plot permutations of raw channel data.
-
On the Aurora, draw as many pseudocolor or dot plots as channels.
-
Leave thexaxis unchanged.
-
Set eachyaxis to the different channels, so each plot shows a different raw combination.
An example of a SpectroFlo N × N raw template is shown in Figure8.
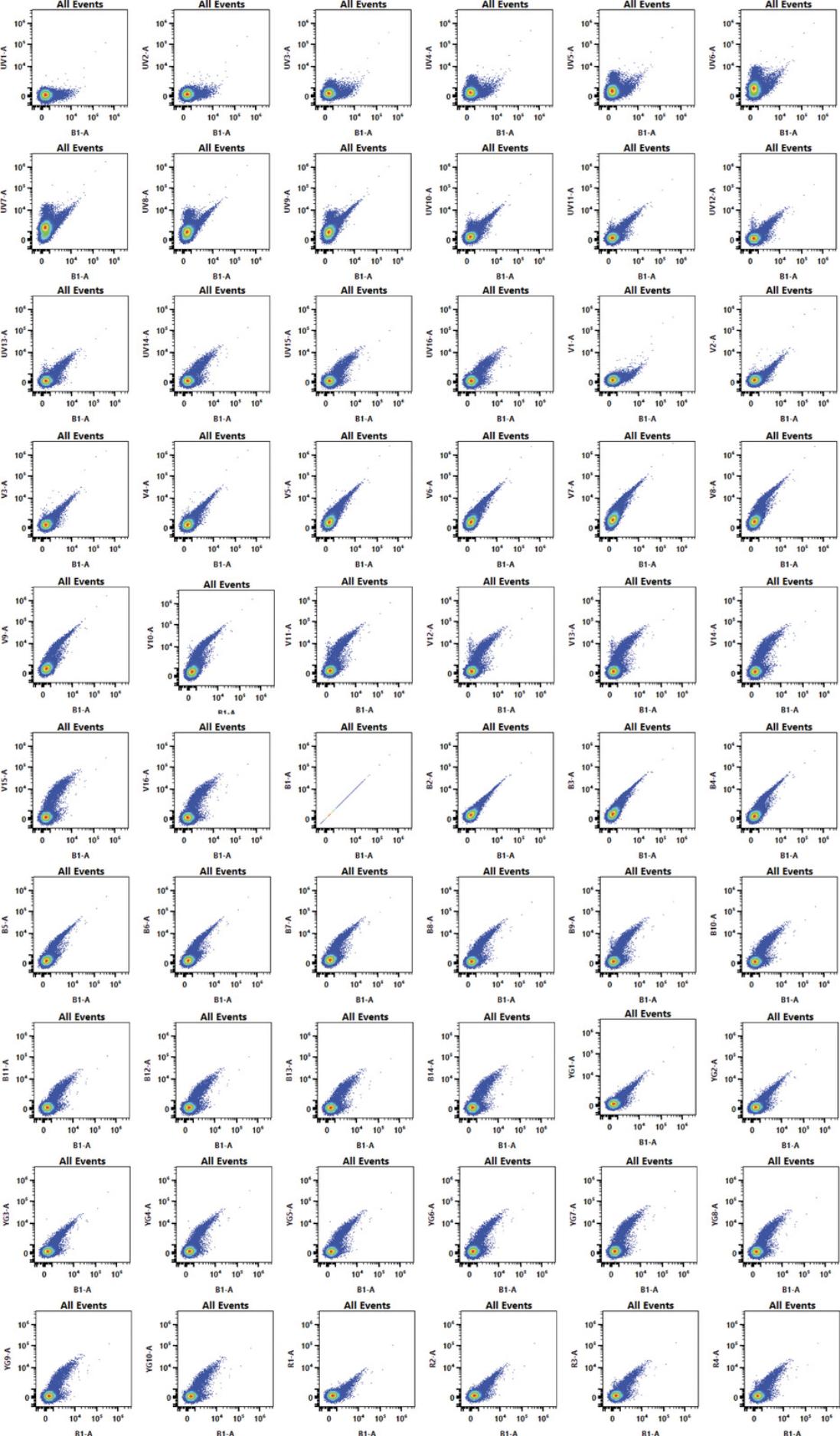
2.Find a combination that separates the greatest number of populations from one another.
Distinguish
3.Choose the plot with highest degree of separation.
4.Place gates around all unique populations and observe the scatter properties and spectral signatures on adjacent spectral plots and select only the populations that have unique spectral characteristics.
5.Find the peak fluorescent channel for each unique population and then display said population on a histogram using this channel.
6.Gate the brightest 300-500 cells only, in order to derive the brightest AF SS control.
7.Right-click on the gate and export events as a new FCS file.
8.Repeat for all unique populations, producing an FCS file for each.
9.Export an AF dim population (dimmest AF spectral signature found in the sample) to be used as the negative control for the previously exported populations.
Designate
10.Designate these new AF signatures as fluorescence tags in the SpectroFlo Library.
- Create a new AF group.
This will facilitate filtering and exporting these fluorophores for offline analysis, as well as identification when designing experiments.
-
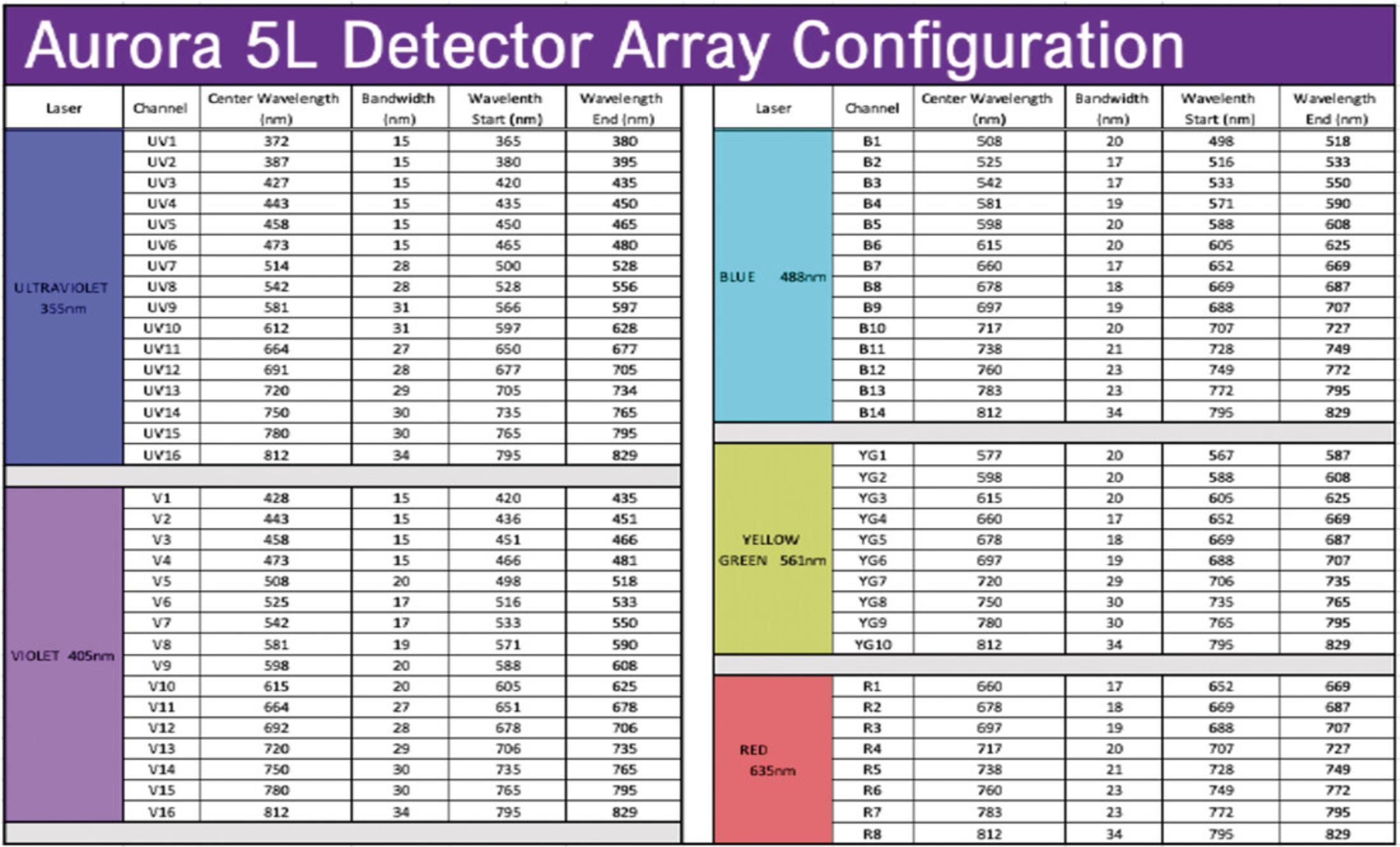
Assign the AF tag a name, choose the excitation laser, and assign an emission wavelength (use the optical configuration in Figure9to determine wavelength based on peak emission channel).
11.Open the experiment of interest and add the new AF tag(s) as if it were a fluorophore in the experiment.
12.In the reference controls tab, add an additional negative for the AF tags that will be the AF dim.
13.Import the populations exported during the Distinguish steps as SRCs for the corresponding tags, and import the AF dim population as an additional negative for the AF SRCs.
14.Use the Unmixing Wizard QC tools to assess AF signature similarity.
15.Unmix the experiment and evaluate the unmixed N × N matrix.
Basic Protocol 4: ASSESS DATA QUALITY USING EXPERT GATING AND DIMENSIONALITY REDUCTION ALGORITHMS
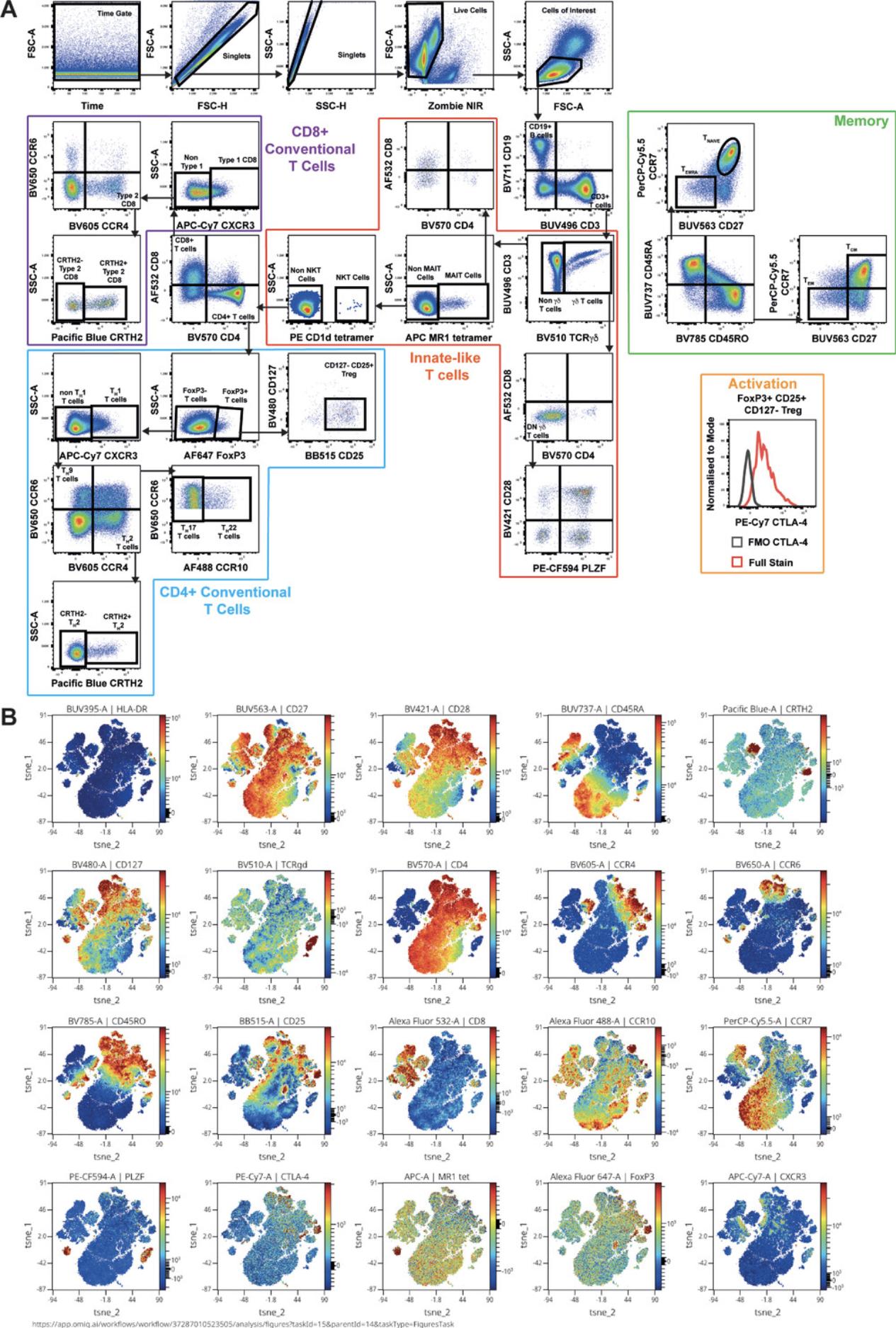
Informed gating is a useful way to check panel quality. It is necessary to check that all populations of interest can be identified and to investigate how well they can be resolved (Fig. 10A, Video 6). The biology of the sample should be considered to ensure that populations seen in the sample are as expected and that markers are expressed on all the expected cell types. This protocol indicates whether the panel is ready for use on experimental samples. Additionally, dimensionality reduction algorithms can be useful tools for investigating panel quality, as they simplify the data for visualization while also exposing artefacts that may be missed through expert gating. Using t -distributed stochastic neighbor embedding (t -SNE; van der Maaten & Hinton, 2008; Fig. 10B), markers that are usually co-expressed should be checked to see they are found in similar regions of the t -SNE plot (Brummelman et al., 2019).
Materials
- Data analysis software (e.g., FlowJo, FCS Express, or any other software that allows gating of data)
- FCS files generated in Basic Protocol 3
Perform expert gating
1.Open the unmixed FS sample in data analysis software used for analyzing FCS files.
2.Follow steps 2-6 of Basic Protocol 2 to gate on live single cells of interest.
3.Gate the rest of the markers based on panel design, prior knowledge, and published literature.
4.Check the gating strategy for unexpected marker combinations or cell populations (Fig. 10A).
5.Evaluate whether all populations of interest have clear positive signals that can be easily resolved from the negative.
6.Ensure readout markers (used for experimental readout) can be quantified in each of the cell types of interest.
Apply dimensionality reduction algorithm (t-SNE)
7.Using the cleaned data from step 2, run a t -SNE at the default settings (iterations: 1000; perplexity: 30) utilizing all fluorescence parameters. Exclude non-informative parameters such as those used in the data cleaning steps (e.g., viability and phenotypic markers for identifying cells of interest).
8.Assess marker co-localization one by one in viSNE plots by coloring the t -SNE based on the expression of each marker (Fig. 10B).
9.As in step 4, check for the appearance of unexpected marker combinations on specific cell types.
COMMENTARY
Background Information
The first step of building a successful large multicolor panel is good theoretical panel design. Full-spectrum flow cytometry panel design has been previously described (Ferrer-Font, Pellefigues, et al., 2020; Park et al., 2020). In summary, in the context of successful theoretical panel design, it is very important to have a clear experimental question and to know the biology of the markers that are included in the assay, including their expression and co-expression patterns. It is important to understand the instrument configuration (i.e., number of lasers on board) to know what the spectral signatures for each fluorophore will look like with the given configuration, as fluorophore brightness will vary depending on the excitation wavelength available. This information, coupled with the amount of spread (both given and received) of each fluorophore in the panel (detailed in Nguyen, Perfetto, Mahnke, Chattopadhyay, & Roederer, 2013), allows fluorophores to be optimally assigned to the different markers of the immunophenotyping panel.
After the panel has been theoretically designed, performance of QC steps is strongly recommended. This process is necessary to theoretically validate the panel before proceeding to a full experiment in order to reduce preventable issues as much as possible. It is advised to review the panel on a marker-by-marker and population-by-population basis, making sure that fluorophores inducing considerable amounts of spread (which could impair marker resolution) are allocated to non-co-expressed markers, and that dim markers receive a minimal amount of spread, while fluorophore brightness and antigen expression levels are well matched (high-expressing antigens with dim fluorophores and low-expressing antigens with bright fluorophores). To address potential issues, markers that are available with multiple fluorophores can be substituted to see if spillover spread can be minimized. Additionally, fluorophores that create (but do not receive) the most spillover can be designated to dump or viability channels. It is strongly recommended to complete panel optimization before working with an actual biological experiment, as it is a wise investment in terms of time, effort, and cost, without jeopardizing precious experimental samples.
Critical Parameters
Multiple factors influence the success of this protocol. The importance of good sample preparation—addressing sample complexity issues and the use of high-quality controls—must be emphasized.
Working with complex samples
When working with more complex samples than PBMCs (e.g., skin, tumor, fat tissue), the steps for panel optimization are the same, but there are some extra considerations.
Quality of single-cell suspension. An important consideration that is sometimes underestimated is the quality of the single-cell suspension that will be used to run the immunophenotyping panel. As different immune cell subsets have varying susceptibility to cell death, the single-cell suspension should have a viability of 80% or more to ensure proportional representation of the original sample (Costantini et al., 2003). Cell death can occur for different reasons, including how the sample has been treated before staining. Cryopreservation and harsh digestion protocols can affect sample quality and these procedures also need to be optimized. Indeed, when working with digested tissue, epitopes for the markers of interest should be verified to ensure the digestion protocol has not negatively impacted them. For example, if the same epitopes of interest exist in the spleen, one should compare marker staining and epitope preservation between digested and undigested spleen (Ferrer-Font, Mehta, et al., 2020).
Assessing instrument setup. Wherever possible, the instrument settings for fluorescence detector gains should remain unaltered from the Cytek Assay Settings (CAS). When using fluorescently labeled antibodies, this is achieved by carefully pairing expression levels to fluorophore brightness during panel design, and then by antibody titration to ensure fluorescence signals remain on-scale at the optimized settings. In certain applications where fluorescence intensity is not tunable, such as fluorescent reporter protein expression within the cells of interest, the instrument settings may need to be adjusted to accommodate off-scale fluorescence signal. Any such change will impact not only the spectral signature being accommodated, but also other signatures with emission in the same area. Reducing fluorescence gains may lead to increased similarity between spectral signatures, which can give rise to more spillover spreading error and thus negatively impact marker resolution (Ferrer-Font, Pellefigues, et al., 2020). See Support Protocol 2 for steps required to alter fluorescence gain settings to accommodate off-scale signals. If using something other than the Cytek Aurora, it will be necessary to optimize the settings based on manufacturer's recommendations.
Autofluorescence. Samples with heterogeneous (Fig. 7C) or very bright homogeneous AF signatures (Fig. 7B) can make unmixing challenging. To improve the accuracy of the unmixing and to improve marker resolution in the case of heterogeneous AF samples, multiple AF reference controls can be created for each of the different AF signatures present in the sample (as if they were individual fluorophores included in the panel). The steps required for this process are divided in three sections called Discover, Distinguish and Designate. The goal is to Discover unique spectral signatures within the unstained control through use of a raw N × N plots; Distinguish these unique signatures into separate SRCs by exporting each population as a new FCS file which can then be reimported; and Designate each signature as unique fluorophores within the software to be unmixed as if they were part of the original panel. Care must be taken to ensure only clearly unique signatures (with similarity index <0.98) with at least 300 events of similar fluorescence intensity are distinguished to ensure high quality SRCs are generated (for a detailed protocol, see Support Protocol 3 and Video 4). For very bright homogeneous AF, the steps outlined in Basic Protocol 3 and Video 4 can be followed to improve unmixing and markers resolution outcomes.
Controls
The quality of controls will directly translate to the quality of unmixing and the data obtained. It is therefore worth investing the time to optimize them. It is of utmost importance to include all necessary controls from the beginning of a project. A complete overview about controls can be found in Maecker & Trotter (2006). Below is a summary of the categories of controls that should be considered when optimizing a flow cytometry panel.
Unstained controls. The unstained control is meant for AF assessment and should have a clean signature with no contamination from other fluorophores and match the tissue or sample type being analyzed. If, for example, different tissue types are used in an experiment, multiple unstained sample controls must be used for each tissue type. Similarly, if samples are being treated differently (e.g., fixed/fresh or stimulated/unstimulated), an unstained control should be included for each condition. It is not advised to mix samples from different conditions and collect only one unstained control, as it may not be possible to have enough events to generate clear AF signatures.
Spectral reference controls. Appropriate single-stained SRC samples are required for optimal unmixing of the fully stained sample. The purpose is to provide a signature of each fluorophore to be used by the unmixing algorithm. Briefly, SRCs should have positive and negative populations that are clearly separated or a universal negative; positive populations should be brighter than the fully stained sample; the negative and positive populations should have identical AF characteristics; sufficient events for both populations should be collected; and the fluorescence spectrum of the positive control needs to be identical to the one in the fully stained sample. To fulfill these best practices, cells and beads should be compared and the best option should be used. Special considerations for viability staining are important, as live and dead cells have different AF signatures, which means the positive and negative controls will not have the same AF signature. To overcome this issue, it is possible to kill all cells in the viability SRC (e.g., heat-killing at 55°-70°C for 5-10 min), stain only half of them, and mix these with the unstained cells. In this case, all the cells will be dead and the AF will be the same for the positive and negative controls.
SS cell controls. SS cell controls are used not only as reference controls but also to assess the performance of each marker compared to the FS tube. SS cells are gold-standard for each antibody performance and are used to quantify the spread of the negative population and/or shifts in the positive signal and thus any subsequent loss of resolution of markers in the panel.
Gating controls. A fluorescence minus one (FMO) control is a sample stained with all fluorophores used in the experiment except one. Analyzing the FMO control for each fluorophore in the panel is not required for panel optimization, but FMOs can be used as a guide to set the boundary between positive and negative events if it is ambiguous. FMOs also aid in the assessment of spread between positive and negative events and are an important tool for assessing panel performance. A good alternative for large 20+ color spectral panels is the use of fluorescence minus multiple (FMM) controls, as recently described by Jensen & Wnek (2020).
Core versus FS panel
When optimizing a high-dimensional immunophenotyping panel, it is recommended to first complete Basic Protocols 1-4 with a core panel. A core panel is limited to only the essential lineage markers required to identify the cell types of interest. This will reduce confounding factors when attempting to understand the source of errors. Once a core panel has been optimized, additional markers can be added (including readout functional markers, intracellular markers, etc.) and optimized with the knowledge that issues are not originating from the core panel.
Fixation/permeabilization buffers
Intracellular staining procedures also require special consideration. It is important to source the fixation/permeabilization reagent that is most appropriate for the markers being detected (e.g., transcription factors, cytokines, or intracellular proteins) while also considering their relative locations (cytosolic or nuclear). The type of fixation buffer can influence the staining of both intracellular and surface markers. The type of fixative can have an impact in many aspects of staining: damaging the epitopes, altering the fluor stability and resulting in a different optimal titer, altering the background fluorescence without altering the positive signal (leading to reduced resolution between positive and negative populations), and altering the fluorescence spectral signatures (causing spectral signature mismatches between FS samples and SRCs, resulting in unmixing issues). It is recommended that antibodies be titrated using the same fixation/permeabilization buffer conditions used for the final staining of FS samples and SRCs.
Troubleshooting
The term troubleshooting is used when one or more issues are found in the panel and the source of these issues needs to be identified and steps included to rectify them. In this regard, the exact steps provided for validating the panel can also be applied for troubleshooting. By following these clear steps, the user will gain a better understanding of the quality of the panel and identify issues prior to acquiring experimental samples.
In general, 80%-90% of unmixing issues that arise can be traced to suboptimal controls. Therefore, it is important to use controls that are well characterized, high quality, and appropriate for the experiment (i.e., matched to the conditions of the experimental sample). This is particularly vital if they are going to be reused.
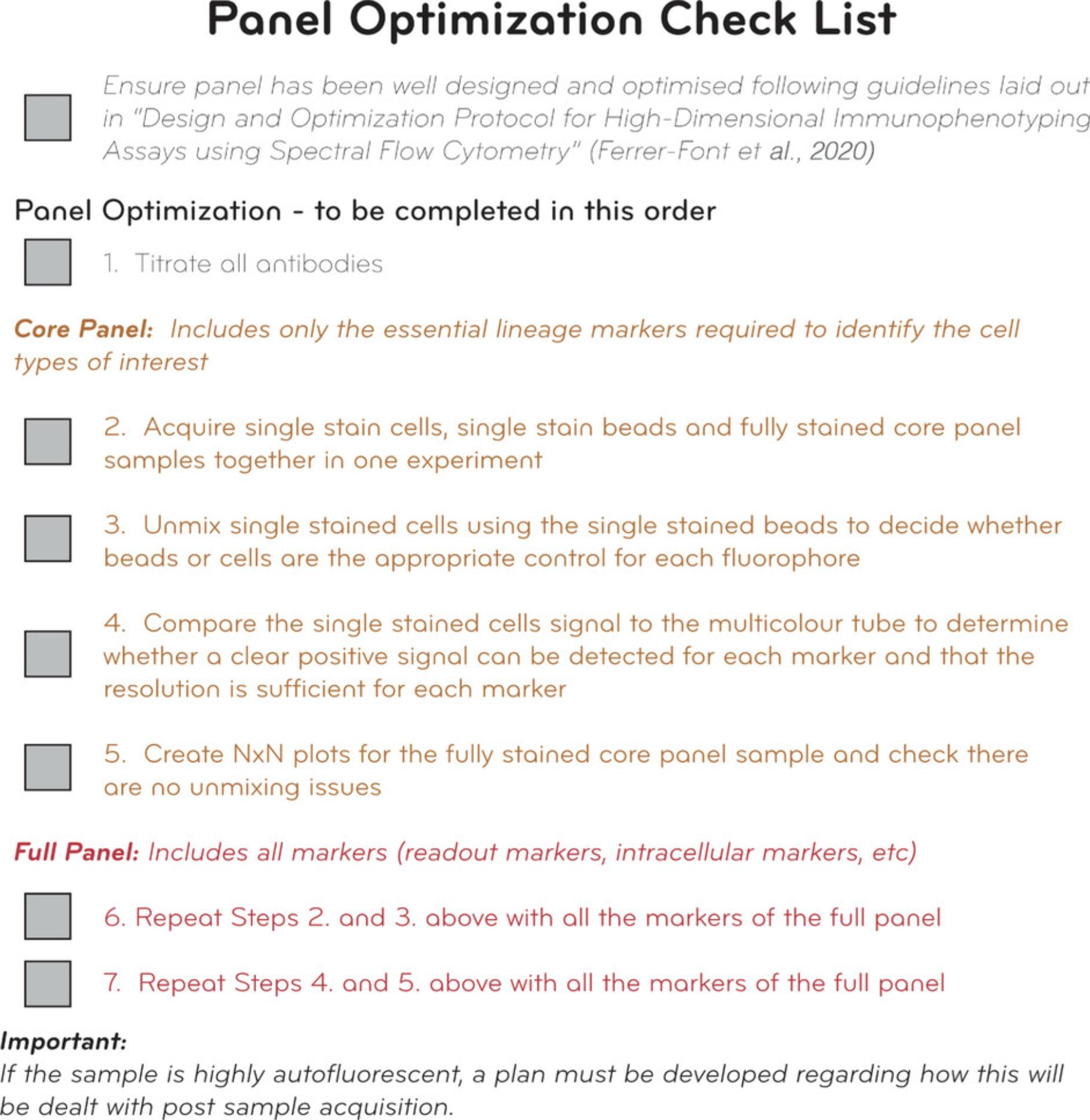
The checklist provided in Figure 11 aims to help users follow clear steps to optimize and troubleshoot their panels. By following these steps, users should be able to discover issues if they exist and have a pathway and alternatives to address and resolve them. Additional troubleshooting for general issues that may arise during panel optimization can be found in Tables 2-5.
| Problem | Cause | Potential solution |
|---|---|---|
| Spectral signature of SRC does not match expected signature for fluorophore | Contamination of control with another fluorescent antibody | Preferably prepare new SRC; alternatively, in SpectroFlo software using the raw SRC, exclude the contaminating signature, export the clean file, and import this FCS file as the correct SRC |
| Carryover from previous SRCs on the cytometer | Preferably prepare a new SRC; alternatively, in the SpectroFlo software using the raw SRC, exclude the contaminating signature, export the clean file and import this FCS file as the correct SRC | |
| Tandem dye degradation | Find the cause of degradation (issue with fixative, how long samples are stored in fixative, temperature of incubation, light exposure during protocol, etc.) and make necessary corrections (modifying staining procedure or buy a new vial) | |
| Brilliant Stain Buffer added to bead controls | Remake bead SRCs without addition of Brilliant Stain Buffer | |
| Wrong tube was recorded (signature matches different fluorophore) | Read the correct control or import the correct SRC FCS file | |
| More than one spectral signature is visible | Autofluorescence signature | If positive and negative populations have the same secondary signature(s), this may be autofluorescence. See Basic Protocol 3 or Support Protocol 3 to decide on an approach for mitigating AF. |
| Contamination of control with another fluorescent antibody | If there are multiple positive populations and gating on each one produces a distinct signature, then it is likely that there are two fluorophores in the SRC. Prepare a new SRC or exclude the signature of the contamination and import this FCS file as the correct SRC, if possible | |
| Fluorescent signal in the negative population of the SRC | Nonspecific binding of antibody to negative beads | Revisit antibody titer to make sure the optimal titration is used; alternatively, use the universal negative feature in the software |
| Inadequate sample preparation/wash procedure | Wash SRC controls well in the presence of excess wash solution such as FACS Staining Buffer (see Basic Protocol 1) | |
| Carryover of samples from previous SRC | Look at time vs. fluorescent signal, export a cleaned FCS file removing the contaminating signal, and import this FCS file as the correct SRC | |
| Unmixing errors in SRCs | Gates were not set correctly in the Unmixing Wizard | Ensure that the P1 gate is set on the population with the highest expression of the marker. Place the positive histogram gate on the brightest signal (this may be different than gating on all positive signals). Better results can be obtained with tighter gates that do not include a side variety of cell sizes and/or fluorescence intensities. |
| Contamination of control with another fluorescent antibody | Preferably prepare a new SRC; alternatively, in SpectroFlo software using the raw SRC, exclude the contaminating signature, export the clean file, and import this FCS file as the correct SRC | |
| No positive signal can be detected | Gates were not set correctly in the Unmixing Wizard | Move P1 gate to population that expresses marker and/or move histogram to peak detector |
| Not enough events were recorded | The unmixing algorithm requires a minimum of 300 positive events; record more total events | |
| Antibody was not added to SRC | Prepare new SRC |
| Problem | Potential cause | Potential solution |
|---|---|---|
| Unmixing Wizard unmixes SRCs cells correctly, but unmixing errors are present in FS cells | SRCs are dimmer than fully stained sample | Use brighter SRCs; ensure staining protocol is exactly the same for SRCs and FS cells (antibody concentration, incubation time/temperature, use of fixative, stimulation of cells, etc.). Note that the optimal antibody concentration for compensation beads is often different than for cells. Pipetting error can easily occur when pipetting small volumes (<1-2 μl) for SRCs compared to using a master mix for FS samples. In this case, it is recommended to dilute antibodies to avoid pipetting small volumes. Other common mistakes include not using fix/perm buffer on compensation beads or using a tissue for SRCs that has lower marker expression than FS sample. |
| Beads were used for all the SRCs and there may be a mismatch in the emission spectra between beads and cells | Use SRC stained cells instead. | |
| Polymer dyes (more than 2) are included in the panel without using Brilliant Stain Buffer or Super Bright Stain Buffer | Use Brilliant Stain Buffer when more than one polymer dye is added in the same tube following manufacturer recommendations. | |
| AF signature in FS sample is complex and different to the controls | Use AF extraction and see information regarding complex AF samples. Ensure the unstained control used for AF extraction is treated the same as the FS stained sample. AF signature may change with treatment (fixation, stimulation, timepoint, etc.). With human samples there may be patient-to-patient variability in AF signatures, so if using AF extraction for complex AF samples, an unstained control for each patient may be required. Also ensure that enough events are recorded in the unstained control to assess AF. | |
| Wrong tube was recorded (signature matches different fluorophore) | Read the correct control or import the correct SRC FCS file | |
| Some FS samples unmixed correctly while others have unmixing errors | Biological variation; marker level of expression greatly changes across donors or across experimental conditions. | Try using beads as SRCs. If they are not optimal, use donor with the highest level of expression as control. |
| Problem | Potential cause | Potential solution |
|---|---|---|
| Low signal in certain markers (in FS and SS samples) | Marker expression too low or non-existent | Check that the marker is expected to be expressed on the particular cells or animal model of interest. Compare to Technical Data Sheet (TDS) or published literature. |
| Experimental design (i.e., timepoint or stimulation) does not elicit certain markers | Stimulate cells with a positive control (e.g., PMC-ionomycin) to ensure cells are capable of expressing the markers of interest. | |
| Epitope is damaged by digestion procedure | Test different clone or modify digestion conditions | |
| Epitope is damaged by staining procedure | Test different clone or modify staining conditions | |
| Fluorophores chosen were too dim | Choose a brighter fluorophore for the specific marker and optimize concentration used | |
| Tandem dyes have degraded or decoupled | Find cause of degradation (issue with fixative, how long samples are stored in fixative, temperature of incubation, light exposure during protocol, etc.) and replace tandem dye with a new vial | |
| FS sample stained less (lower MFI) than SS sample for a given marker | Binding site blocked by other reagents in the panel or different Ab/receptor binding kinetics | Test sequential staining, perform experiments to identify antibodies that are interfering with each other or try different clones |
| Saturation is not achieved at optimal titer for SRCs | Increase titer to reach saturation | |
| Pipetting error when pipetting antibody cocktails | Repeat experiment to double-check | |
| Antibody/antibodies were trapped in the column when filtering the antibody cocktail | Optimize centrifuge spinning time and speed | |
| Unstained, SS, and FS cells have high AF background | Cells of interest are highly autofluorescent | Extract autofluorescence |
| Suboptimal separation/resolution between negative and positive populations | Antibody concentration is too low or too high | Optimize antibody concentration based on antibody titration results |
| Spread | Check SSM and use FMOs to confirm source of spread | |
| Unbound antibodies were not adequately washed from samples | Add additional centrifugation and FACS Staining Buffer wash step | |
| Viability dye concentration is too high and live cells are stained with dye | If live cells are stained with viability dye, the spreading error from the viability dye must be accounted for and this may cause problems with marker resolution. Ideally, viability dye should be titrated so that live cells do not stain with viability dye. |
| Problem | Potential cause | Potential solution |
|---|---|---|
| Appearance of unexpected biological patterns | Inadequate cleaning gates | Make sure you are using time gates, excluding doublets, dead cells, and antibody aggregates, and gating on cells of interest |
| Viability dye concentration is too high and live cells are stained with dye | Titrate viability dye to make sure the optimal concentration is used | |
| Lack of viability dye in panel and dead cells nonspecifically bind to antibodies | Titrate and add viability dye to FS samples | |
| Lack of addition of Brilliant Stain Buffer | Use Brilliant Stain Buffer when more than one polymer dye is added in the same tube following manufacturer recommendations |
Understanding Results
The example panel optimized using this protocol and presented here aims to identify conventional T cell subsets in peripheral blood of healthy participants. The participants were infected with low-dose human hookworm as part of a longitudinal study. It is well established that during early parasitic infection there is an increase in T-helper Type 2 immune responses (Th2) in the T cell compartments due to the primary role these cells play in host responses to parasites (McSorley & Loukas, 2010). However, the wider effects on the human immune system of long-term chronic parasitic infection with the gut-residing hookworm Necator americanus (Na) have not been investigated. Therefore, a panel was designed and optimized to look at the following conventional and unconventional T cell subsets of interest: CD4+ T cells (Th1, Th2, Th17, Th9, Th22, and Tregs), CD8+ T cells (Tc1 and Tc2), and innate-like T cells (γδ T, mucosal associate invariant T [MAIT], and natural killer T [NKT] cells). This panel can assess the frequency of these cell subsets as well as their memory and activation phenotypes, using both expert gating and high-dimensional data analysis.
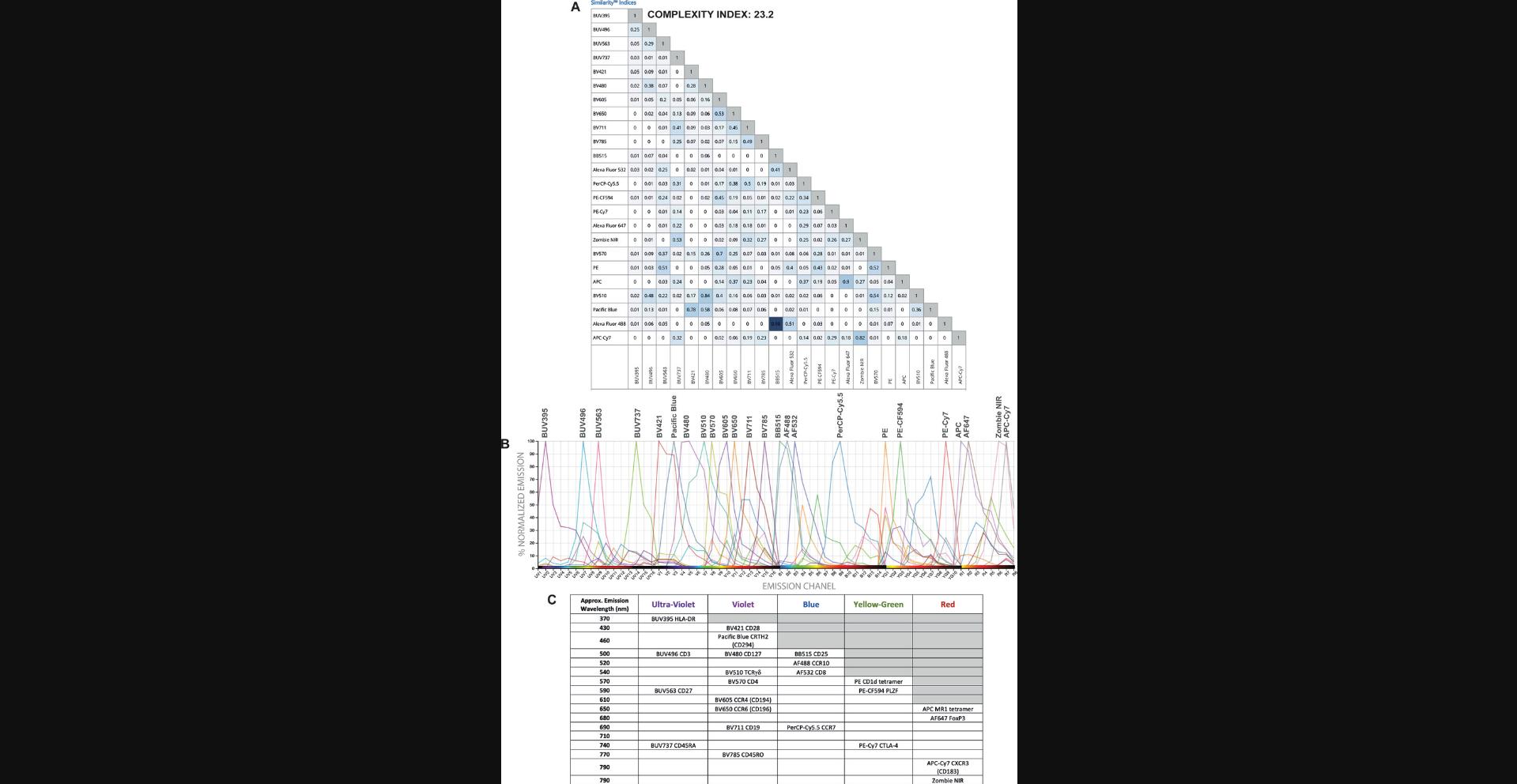
Some characteristics of the fluorophore selection for a certain panel including the similarity and complexity indices of the panel (Fig. 12A) and the spectral signatures of fluorophores used (Fig. 12B) have been included. Although some fluorophores have a high similarity index, they have been allocated to different cell types and therefore the impact of the expected spreading error should be minimal (Fig. 12). A panel distribution table has also been included (Fig. 12C) to show the peak emission wavelengths for the fluorophores and markers assigned to the panel.
After following all the steps of Basic Protocols 1-4 for our panel shown here, and resolving any issues that arose (such as one tandem degradation or implementing the sequential staining for some of the markers), high-quality full-spectrum flow cytometry data as defined by clear resolution of all expected populations was achieved. The optimized staining protocol for this panel is detailed in the Supplementary Material. Sequential staining was applied, following the order given in the protocol, for markers that showed a reduction in positive signal in the FS compared to the SS control, starting at the marker with the greatest reduction and working toward the one with the least reduction. This approach provided satisfactory results and did not require the testing of other combinations. To reduce staining procedure time, further optimization would be required to determine whether some markers could be added at the same time instead of using all of them sequentially.
A clean and clear positive population can be detected for all markers in the panel, with no signal resolution loss when all antibodies are combined. In some cases, such as for the activation markers, the use of FMOs is necessary to assist in evaluating gates to determine positive staining with confidence. All populations of interest could be found using expert gating, and the populations resemble the expected expression patterns and frequencies. Additionally, high-dimensional data analysis algorithms were successfully used without the appearance of artefacts, confirming the high quality of the data.
Time Considerations
The time needed for designing, optimizing, and analyzing a high-dimensional flow cytometry panel can be highly variable and can depend on the assay complexity, number of markers, wait-time required for reagents (geographically dependent), sample access and frequency of sample delivery (particularly for patient samples), duration of the disease model being investigated, and more. As an example, it took five months to optimize the digestion, design, and optimization of a 23-color spectral flow cytometry panel in gut tissue (Ferret-Font, Mehta, et al., 2020), whereas one month may be sufficient for a PBMC panel where digestion does not need optimization. It is therefore advisable to develop certain core panels that can be applied to several experimental questions and models.
Acknowledgments
This work was enabled by the Hugh Green Cytometry Centre at the Malaghan Institute of Medical Research. We wish to thank the Hugh Green Foundation, the Marjorie Barclay Trust, the Dinies Foundation and the HRC Independent Research Organisation (18/1003) for funding this study. We are also very grateful to the NIH Core Tetramer Facility for supplying the tetramers used in this panel and to Olivia Burn and Kathryn Hally for feedback on the manuscript.
Author Contributions
Laura Ferrer-Font : Conceptualization; investigation; methodology; project administration; writing–original draft; Sam J. Small : Investigation; methodology; writing–original draft; Brittany Lewer : Investigation; writing–review & editing; Katherine R Pilkington : Resources; writing–review & editing; Laura K. Johnston : Writing–review & editing; Lily M. Park : Writing–review & editing; Joanne Lannigan : Methodology; writing–review & editing; Maria C. Jaimes : Conceptualization; methodology; resources; supervision; writing–review & editing; Kylie M. Price : Funding acquisition; project administration; supervision; writing–review & editing.
Conflicts of Interest
Kate Pilkington, Lily Park, Maria C. Jaimes are employees of Cytek Biosciences, Inc., the manufacturer of the Aurora full-spectrum flow cytometer used in these studies. Joanne Lannigan is a paid consultant for Cytek Biosciences, Inc.
Open Research
Data Availability Statement
Data available on request due to privacy/ethical restrictions.
Supporting Information
| Filename | Description |
|---|---|
| cpz1222-sup-0007-SuppMat.docx22.4 KB | Supplementary Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
Literature Cited
- Aass, H. C. D., Øvstebø, R., Trøseid, A. M. S., Kierulf, P., Berg, J. P., & Henriksson, C. E. (2011). Fluorescent particles in the antibody solution result in false TF- and CD14-positive microparticles in flow cytometric analysis. Cytometry Part A , 79 A, 990–999. doi: 10.1002/cyto.a.21147.
- Ashhurst, T. M., Smith, A. L., & King, N. J. C. (2017). High-dimensional fluorescence cytometry. Current Protocols in Immunology , 2017, 5.8.1–5.8.38. doi: 10.1002/cpim.37.
- Ayers, L., Kohler, M., Harrison, P., Sargent, I., Dragovic, R., Schaap, M., … Ferry, B. (2011). Measurement of circulating cell-derived microparticles by flow cytometry: Sources of variability within the assay. Thrombosis Research , 127, 370–377. doi: 10.1016/j.thromres.2010.12.014.
- Brummelman, J., Haftmann, C., Núñez, N. G., Alvisi, G., Mazza, E. M. C., Becher, B., & Lugli, E. (2019). Development, application and computational analysis of high-dimensional fluorescent antibody panels for single-cell flow cytometry. Nature Protocols , 14, 1946–1969. doi: 10.1038/s41596-019-0166-2.
- Costantini, A., Mancini, S., Giuliodoro, S., Butini, L., Regnery, C. M., Silvestri, G., & Montroni, M. (2003). Effects of cryopreservation on lymphocyte immunophenotype and function. Journal of Immunological Methods , 278, 145–155. doi: 10.1016/S0022-1759(03)00202-3.
- Disis, M., dela Rosa, C., Goodell, V., & Ling-Yu, K. (2006). Maximizing the retention of antigen specific lymphocyte function after cryopreservation. Journal of Immunological Methods , 308, 13–18. doi: 10.1016/J.JIM.2005.09.011.
- Feher, K., von Volkmann, K., Kirsch, J., Radbruch, A., Popien, J., & Kaiser, T. (2016). Multispectral flow cytometry: The consequences of increased light collection. Cytometry Part A , 89, 681–689. doi: 10.1002/cyto.a.22888.
- Ferrer-Font, L., Mehta, P., Harmos, P., Schmidt, A. J., Chappell, S., Price, K. M., … Mayer, J. U. (2020). High-dimensional analysis of intestinal immune cells during helminth infection. eLife , 9, e51678. doi: 10.7554/eLife.51678.
- Ferrer-Font, L., Pellefigues, C., Mayer, J. U., Small, S. J., Jaimes, M. C., & Price, K. M. (2020). Panel design and optimization for high-dimensional immunophenotyping assays using spectral flow cytometry. Current Protocols in Cytometry , 92, 1–25. doi: 10.1002/cpcy.70.
- Inglis, H. C., Danesh, A., Shah, A., Lacroix, J., Spinella, P. C., & Norris, P. J. (2015). Techniques to improve detection and analysis of extracellular vesicles using flow cytometry. Cytometry Part A , 87, 1052–1063. doi: 10.1002/cyto.a.22649.
- Jalbert, E., Shikuma, C. M., Ndhlovu, L. C., & Barbour, J. D. (2013). Sequential staining improves detection of CCR2 and CX3CR1 on monocytes when simultaneously evaluating CCR5 by multicolor flow cytometry. Cytometry Part A , 83 A, 280–286. doi: 10.1002/cyto.a.22257.
- Jensen, H. A., & Wnek, R. (2020). Analytical performance of a 25-marker spectral cytometry immune monitoring assay in peripheral blood. Cytometry Part A , 99 ( 2 ) , 180-193. doi: 10.1002/cyto.a.24290.
- Maecker, H. T., & Trotter, J. (2006). Flow cytometry controls, instrument setup, and the determination of positivity. Cytometry Part A , 69, 1037–1042. doi: 10.1002/cyto.a.20333.
- Mayeno, A. N., Hamann, K. J., & Gleich, G. J. (1992). Granule-associated flavin adenine dinucleotide (FAD) is responsible for eosinophil autofluorescence. Journal of Leukocyte Biology , 51, 172–175. doi: 10.1002/jlb.51.2.172.
- McSorley, H. J., & Loukas, A. (2010). The immunology of human hookworm infections. Parasite Immunology , 32, 549–559. doi: 10.1111/j.1365-3024.2010.01224.x.
- Nguyen, R., Perfetto, S., Mahnke, Y. D., Chattopadhyay, P., & Roederer, M. (2013). Quantifying spillover spreading for comparing instrument performance and aiding in multicolor panel design. Cytometry Part A , 83 A, 306–315. doi: 10.1002/cyto.a.22251.
- Park, L. M., Lannigan, J., & Jaimes, M. C. (2020). OMIP-069: Forty-color full spectrum flow cytometry panel for deep immunophenotyping of major cell subsets in human peripheral blood. Cytometry Part A , 97, 1044–1051. doi: 10.1002/cyto.a.22251.
- Petriz, J., Bradford, J. A., & Ward, M. D. (2018). No lyse no wash flow cytometry for maximizing minimal sample preparation. Methods , 134–135, 149–163. doi: 10.1016/j.ymeth.2017.12.012.
- Roederer, M. (2016). Distributions of autofluorescence after compensation: Be panglossian, fret not. Cytometry Part A , 89, 398–402. doi: 10.1002/cyto.a.22820.
- Sahir, F., Mateo, J. M., Steinhoff, M., & Siveen, K. S. (2020). Development of a 43 color panel for the characterization of conventional and unconventional T-cell subsets, B cells, NK cells, monocytes, dendritic cells, and innate lymphoid cells using spectral flow cytometry. Cytometry Part A , 2020, Dec 18. doi: 10.1002/cyto.a.24288.
- Schmutz, S., Valente, M., Cumano, A., & Novault, S. (2016). Spectral cytometry has unique properties allowing multicolor analysis of cell suspensions isolated from solid tissues. PLoS One , 11, e0159961. doi: 10.1371/journal.pone.0159961.
- Shi, L., Lu, L., Harvey, G., Harvey, T., Rodríguez-Contreras, A., & Alfano, R. R. (2017). Label-free fluorescence spectroscopy for detecting key biomolecules in brain tissue from a mouse model of Alzheimer's disease. Scientific Reports , 7, 1–7. doi: 10.1038/s41598-017-02673-5.
- Stewart, C. C., & Stewart, S. J. (1997). Titering antibodies. Current Protocols in Cytometry , 00 ( 1 ) , 4.1.1–4.1.13. doi: 10.1002/0471142956.cy0401s14.
- van der Maaten, L., & Hinton, G. (2008). Visualizing data using t-SNE. Journal of Machine Learning Research , 9, 2579–2605.
- van der Vlist, E. J., Nolte-’t Hoen, E. N. M., Stoorvogel, W., Arkesteijn, G. J. A., & Wauben, M. H. M. (2012). Fluorescent labeling of nano-sized vesicles released by cells and subsequent quantitative and qualitative analysis by high-resolution flow cytometry. Nature Protocols , 7, 1311–1326. doi: 10.1038/nprot.2012.065.
Citing Literature
Number of times cited according to CrossRef: 26
- Gabriel DeNiro, Kathryn Que, Trevor Fujimoto, Soo Min Koo, Bridget Schneider, Anandaroop Mukhopadhyay, Jeong Kim, Anandi Sawant, Tuan Andrew Nguyen, OMIP‐105: A 30‐color full‐spectrum flow cytometry panel to characterize the immune cell landscape in spleen and tumor within a syngeneic MC‐38 murine colon carcinoma model, Cytometry Part A, 10.1002/cyto.a.24886, 105 , 9, (659-665), (2024).
- Janna E. G. Roet, Aleksandra M. Mikula, Michael Kok, Cora H. Chadick, Juan J. Garcia Vallejo, Henk P. Roest, Luc J. W. Laan, Charlotte M. Winde, Reina E. Mebius, Unbiased method for spectral analysis of cells with great diversity of autofluorescence spectra, Cytometry Part A, 10.1002/cyto.a.24856, 105 , 8, (595-606), (2024).
- Andrew J. Konecny, Peter L. Mage, Aaron J. Tyznik, Martin Prlic, Florian Mair, OMIP‐102: 50‐color phenotyping of the human immune system with in‐depth assessment of T cells and dendritic cells, Cytometry Part A, 10.1002/cyto.a.24841, 105 , 6, (430-436), (2024).
- Yaroslava Shevchenko, Isabella Lurje, Frank Tacke, Linda Hammerich, Fluorochrome‐dependent specific changes in spectral profiles using different compensation beads or primary cells in full spectrum cytometry, Cytometry Part A, 10.1002/cyto.a.24836, 105 , 6, (458-463), (2024).
- Alanna G. Spiteri, Katherine R. Pilkington, Claire L. Wishart, Laurence Macia, Nicholas J.C. King, High‐Dimensional Methods of Single‐Cell Microglial Profiling to Enhance Understanding of Neuropathological Disease, Current Protocols, 10.1002/cpz1.985, 4 , 3, (2024).
- Praveen Sharma, Parveen Bose, Nabhajit Mallik, Dikshat Gopal Gupta, Suneel Rachagiri, Arun Kumar, Jasbir Kaur, Pankaj Malhotra, Neelam Varma, Man Updesh Singh Sachdeva, FLAER as a standalone reagent for paroxysmal nocturnal hemoglobinuria: Do we need to reconsider the guidelines for testing?, International Journal of Laboratory Hematology, 10.1111/ijlh.14213, 46 , 2, (383-389), (2023).
- Hemei Qi, Li Qin, Yuefeng Li, Fujun Jin, Zhongkui Kang, Jianghou Hou, Yifei Wang, A 16-color full spectrum flow cytometric analysis for comprehensive evaluation of T-cell reconstitution in SIV-infected rhesus macaques, Journal of Immunological Methods, 10.1016/j.jim.2022.113404, 514 , (113404), (2023).
- V. Paget, O. Guipaud, A. François, F. Milliat, Detection of radiation-induced senescence by the Debacq-Chainiaux protocol: Improvements and upgrade in the detection of positive events, 10.1016/bs.mcb.2022.10.015, (2023).
- François Santinon, Yoon Kow Young, Sonia V. del Rincón, Koren K. Mann, Analyzing the Tumor-Immune Microenvironment by Flow Cytometry, The Tumor Microenvironment, 10.1007/978-1-0716-2914-7_2, (17-36), (2023).
- Hendrik Fokken, Julian Waclawski, Nadine Kattre, Arnold Kloos, Sebastian Müller, Max Ettinger, Tim Kacprowski, Michael Heuser, Tobias Maetzig, Adrian Schwarzer, A 19‐color single‐tube full spectrum flow cytometry assay for the detection of measurable residual disease in acute myeloid leukemia, Cytometry Part A, 10.1002/cyto.a.24811, 105 , 3, (181-195), (2023).
- Xiaoshan Shi, Wei Fan, Majid Mehrpouyan, Yu Chen, Louise M. D'Cruz, Stephanie J. Widmann, Aaron J. Tyznik, Flow cytometry analysis of protein expression using antibody‐derived tags followed by CITE‐Seq, Cytometry Part A, 10.1002/cyto.a.24792, 105 , 1, (62-73), (2023).
- Alexander Heubeck, Adam Savage, Katherine Henderson, Charles Roll, Veronica Hernandez, Troy Torgerson, Thomas Bumol, Julian Reading, Cross‐platform immunophenotyping of human peripheral blood mononuclear cells with four high‐dimensional flow cytometry panels, Cytometry Part A, 10.1002/cyto.a.24715, 103 , 6, (500-517), (2023).
- Benjamin E. J. Spurgeon, Andrew L. Frelinger, Platelet Phenotyping by Full Spectrum Flow Cytometry, Current Protocols, 10.1002/cpz1.687, 3 , 2, (2023).
- Laura Ferrer‐Font, Geoffrey Kraker, Kathryn E. Hally, Kylie M. Price, Ensuring Full Spectrum Flow Cytometry Data Quality for High‐Dimensional Data Analysis, Current Protocols, 10.1002/cpz1.657, 3 , 2, (2023).
- Andreia Peixoto, Andreia Miranda, Lúcio Lara Santos, José Alexandre Ferreira, A roadmap for translational cancer glycoimmunology at single cell resolution, Journal of Experimental & Clinical Cancer Research, 10.1186/s13046-022-02335-z, 41 , 1, (2022).
- Kef K Prasit, Laura Ferrer-Font, Olivia K Burn, Regan J Anderson, Benjamin J Compton, Alfonso J Schmidt, Johannes U Mayer, Chun-Jen J Chen, Nathaniel Dasyam, David S Ritchie, Dale I Godfrey, Stephen R Mattarollo, P Rod Dunbar, Gavin F Painter, Ian F Hermans, Intratumoural administration of an NKT cell agonist with CpG promotes NKT cell infiltration associated with an enhanced antitumour response and abscopal effect, OncoImmunology, 10.1080/2162402X.2022.2081009, 11 , 1, (2022).
- Matthew C. Woodruff, Doan C. Nguyen, Caterina E. Faliti, Ankur Singh Saini, F. Eun-Hyung Lee, Ignacio Sanz, Response under pressure: deploying emerging technologies to understand B-cell-mediated immunity in COVID-19, Nature Methods, 10.1038/s41592-022-01450-1, 19 , 4, (387-391), (2022).
- Holly A. Jensen, Jeong Kim, iCoreDrop: A robust immune monitoring spectral cytometry assay with six open channels for biomarker flexibility, Cytometry Part A, 10.1002/cyto.a.24708, 103 , 5, (405-418), (2022).
- Joanne Lannigan, Flow cytometry has seen the light: All of it, Cytometry Part A, 10.1002/cyto.a.24694, 101 , 10, (809-811), (2022).
- David Novo, A comparison of spectral unmixing to conventional compensation for the calculation of fluorochrome abundances from flow cytometric data, Cytometry Part A, 10.1002/cyto.a.24669, 101 , 11, (885-891), (2022).
- Maria C. Jaimes, Michael Leipold, Geoffrey Kraker, El‐ad Amir, Holden Maecker, Joanne Lannigan, Full spectrum flow cytometry and mass cytometry: A 32‐marker panel comparison, Cytometry Part A, 10.1002/cyto.a.24565, 101 , 11, (942-959), (2022).
- Vanta J. Jameson, Tina Luke, Yuting Yan, Angela Hind, Maximilien Evrard, Kevin Man, Laura K. Mackay, Axel Kallies, Jose A. Villadangos, Hamish E. G. McWilliam, Alexis Perez‐Gonzalez, Unlocking autofluorescence in the era of full spectrum analysis: Implications for immunophenotype discovery projects, Cytometry Part A, 10.1002/cyto.a.24555, 101 , 11, (922-941), (2022).
- Lindsey B. Crawford, Human Embryonic Stem Cells as a Model for Hematopoietic Stem Cell Differentiation and Viral Infection, Current Protocols, 10.1002/cpz1.622, 2 , 12, (2022).
- Carly E. Whyte, Damon J. Tumes, Adrian Liston, Oliver T. Burton, Do more with Less: Improving High Parameter Cytometry Through Overnight Staining, Current Protocols, 10.1002/cpz1.589, 2 , 11, (2022).
- Kathryn Farrand, Lauren E. Holz, Laura Ferrer‐Font, Michael D. Wilson, Mitch Ganley, Jordan J. Minnell, Ching‐Wen Tang, Gavin F. Painter, William R. Heath, Ian F. Hermans, Olivia K. Burn, Using Full‐Spectrum Flow Cytometry to Phenotype Memory T and NKT Cell Subsets with Optimized Tissue‐Specific Preparation Protocols, Current Protocols, 10.1002/cpz1.482, 2 , 7, (2022).
- Arianne C. Richard, Gordon L. Frazer, Claire Y. Ma, Gillian M. Griffiths, Staggered starts in the race to T cell activation, Trends in Immunology, 10.1016/j.it.2021.09.004, 42 , 11, (994-1008), (2021).

