Mouse Islet Perifusion (3-stimuli protocol)
Islet and Pancreas Analysis Core
Abstract
Our perifusion setup consists of 4 independently-driven channels, which allows for up to 4 different groups of islets to be studied simultaneously. Each channel has an intake catheter, a peristaltic pump, a chamber to hold the islets, and a fraction collector.
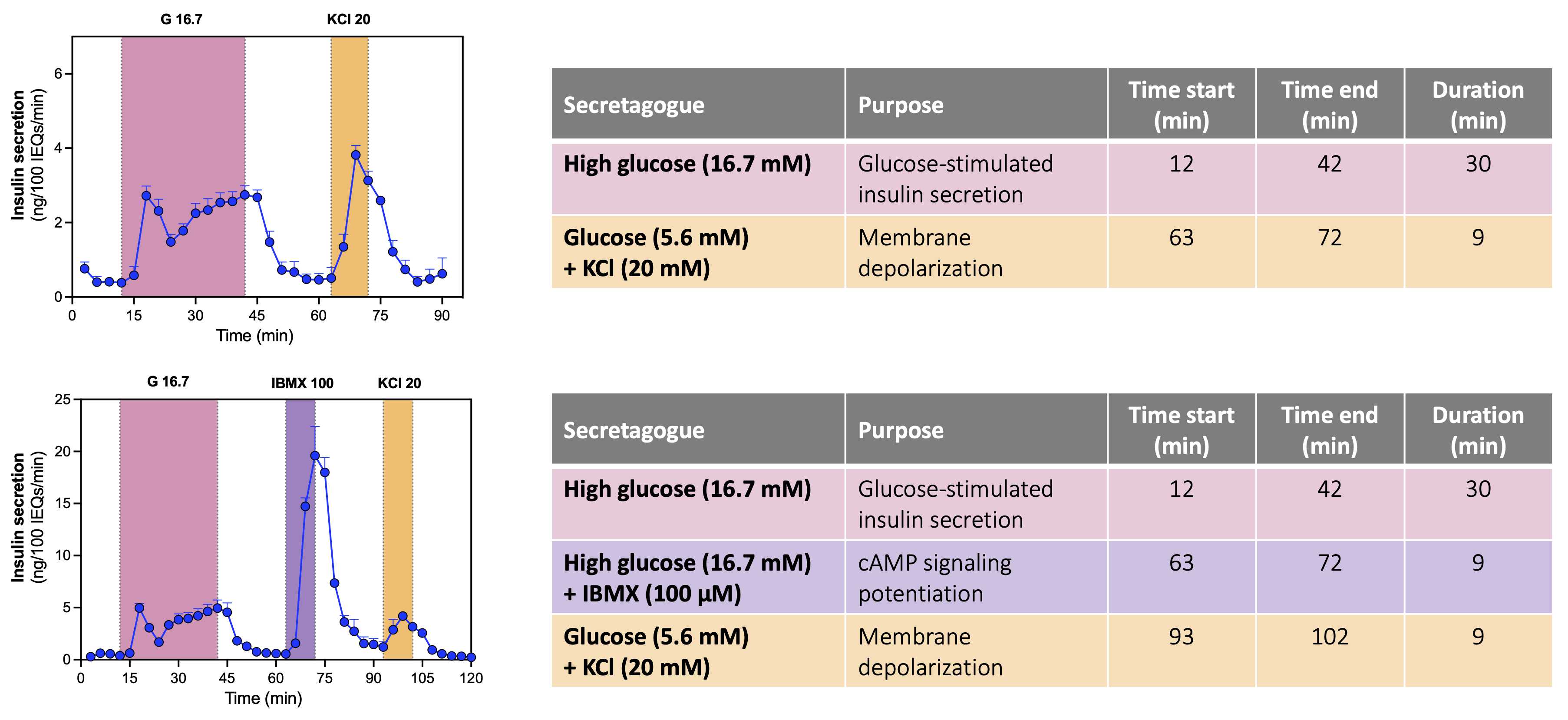
This protocol details stimulation of mouse islets with 3 secretagogues (high glucose, IBMX, and KCl). The flow rate for the perifusion is 1 mL/minute, and fractions are collected every 3 minutes. The animation below illustrates the protocol.

Steps
General Perifusion Startup
Fill perifusion water bath with deionized water to about 1 inch from the top and set the temperature to 37°C.
Label perifusion tubes with date, islet type, fraction number, and any other identifying information. Position the fraction collector trays for perifusion, and load the tubes into the fraction collector.
Rinse the tubing with deionized water at max pump speed for 0h 15m 0s, then place new frits into the islet chamber.
Preparation of Base Perifusion Media and Secretagogues
Prepare base perifusion media by combining reagents below in a 1-L Erlenmeyer flask. Add 1L of deionized water and mix on a magnetic stir plate for at least 0h 15m 0s until dissolved.
3.2g0.58g0.11g1.11g8.28g1g70mgNoteBase media can be made up to 24 hours before perifusion, and stored at 4°C. All subsequent media must be made fresh.
Use a vacuum-filtration system to filter media, transfer to a side-arm flask, and de-gas at 37°C for at least 0h 30m 0s.
Prepare 5.6 mM glucose media by adding 0.5549g 550mL base perifusion media in a 500-mL bottle and mix until dissolved.
Wait 0h 30m 0s and check glucose levels using a glucose meter.
Reserve 30mL 5.6 mM glucose media in a 50-mL conical tube for islet loading and unloading.
Prepare 16.7 mM high glucose media by adding 0.7522g 250mL base perifusion media in a 250-mL bottle and mix until dissolved.
Wait 0h 30m 0s and check glucose levels using a glucose meter.
Prepare 5.6 mM glucose with 20 mM KCl by adding 0.149g 100mL 5.6 mM glucose media .
Prepare 16.7 mM glucose with 100 µM IBMX by first weighing approximately 10mg
Make 200millimolar (mM) stock by adding the appropriate volume of DMSO (see Table 1 ).
| A | B |
|---|---|
| IBMX weight (mg) | 10 |
| DMSO to add (μl) | =ROUNDDOWN((B1/22.22)*100,1) |
Table 1. Copy and paste into Excel, enter IBMX weight in B1, and volume of DMSO will be automatically returned in B2.
Dilute IBMX stock solution (prepared in step 8.1) to a final concentration of 100micromolar (µM) by adding 100µL IBMX stock solution to 100mL 16.7 mM glucose media .
Setup of Secretagogues in Perifusion Water Bath
Place the bottles of media in the water bath to warm for at least 0h 10m 0s before beginning the perifusion.
Replace Pyrex orange caps with 4-Luer (+1) caps on every bottle of media to be used and tape over the holes so that gas cannot escape.
Turn on gas (95 O2, 5% CO2) and insert one gas line to each bottle cap. Block additional holes in caps with tape. Make sure that gas line is suspended above the media and not submerged in the liquid.
Place intake catheters into baseline media bottle, making sure that they rest on the bottom of the bottle. Run baseline media through the chambers at maximum pump speed for about 0h 10m 0s while islets are being aliquoted for perifusion. Discard flow-through.
Islet Preparation and Loading
Prepare islet picking media (HBSS with 10% FBS) : Remove and discard 50mL from a 500-mL bottle of 50mL
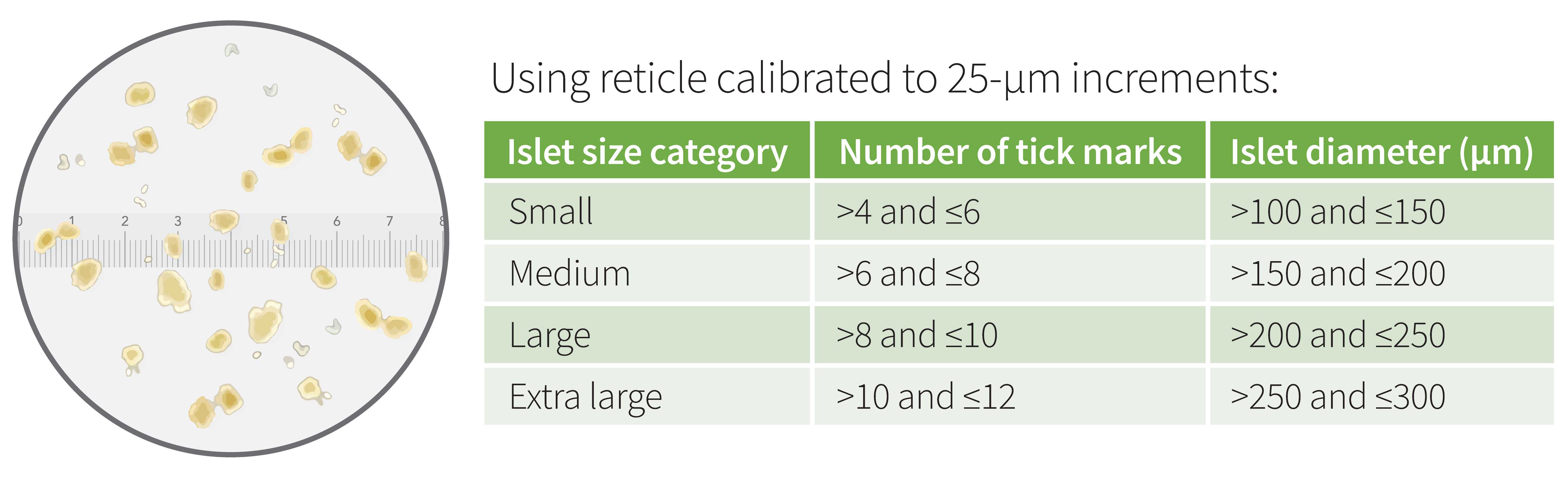
Prepare a 6-cm dish with 5mL islet picking media for each channel to be run. Place freshly-isolated mouse islets on the stage of inverted microscope and view the islets using the 4x objective. Using the reticle in the 10x eyepiece, size the islets according to the chart below.
Handpick islets using a 200-µL micropipette and transfer to the 6-cm tissue culture dish containing islet picking media . Record islet number and size to calculate IEQ.
Using a 200-µL micropipette, transfer all islets from the center of the dish to a clear 1.5-mL microcentrifuge tube for loading into perifusion chamber.
Perform islet imaging:
Place the dish containing clean islets on the stage of a stereomicroscope equipped with a high-resolution camera and swirl until all islets are in the camera field of view at 10x magnification. Using cellSens software, capture brightfield images at approximately 12 ms exposure and darkfield images at approximately 400 ms exposure, each at 10x magnification. Ensure all islets are present in image. Save all image files.
Ensure perifusion intake and air uptake pumps are off. Turn the stopcock on the air uptake lines so that the waste pathway is open, and close the stopcock on the outlet line at the fraction collector.
Remove the chamber from its mounting, turn it upside down, and remove the red end piece (inlet). Remove and discard two thirds of the media from the chamber.
Using a 1-mL micropipette, transfer the slurry of islets from the microcentrifuge tube to the perifusion chamber. Rinse the tube at least 3 times with baseline media and transfer to the chamber.
Place the chamber back onto the mounting, and fill it up with baseline media until there is a convex meniscus. Tap the sides of the column lightly to dislodge air bubbles from the walls, and collect and discard any bubbles from the top of the meniscus.
When all the bubbles have been removed, carefully replace the inlet plunger.
Turn the chamber right side up, and put it back on the mounting rack. Open the outlet line at the fraction collector and close the waste line on the air uptake line.
Turn on the intake and air uptake pumps, set the fraction collector to 0h 3m 0s and flip the collector arm so that it is over the first collection tube. Press “Start” on the collector to start the timer.
Tighten column end pieces, and end fittings, and make sure there are no leaks. Lower the column mounting rack into the water bath, and tighten the clamp to prevent wobbling.
Collection of Perifusate Fractions
Collect 10 preliminary fractions using 5.6 mM glucose media to rinse the islets and to synchronize intake pump speed to deliver 1 mL per minute fractions, collecting 3mL (3 minute) per fraction.
Record each intake pump speed on the perifusion worksheet in the perifusion logbook.
Begin to collect fractions, changing media/secretagogues at predetermined timepoint based on fraction collector timer (see sample protocol below).When the fraction collector moves at the desired timepoint, pause the intake pump.
Move the media intake catheters from one secretagogue to the next, making sure to not tangle the tubing and that the catheter is resting on the bottom of the bottle.
Restart intake pump until next secretagogue change.
When perifusion is complete, cap the perifusion fractions and store at -20°C.
Islet Recovery and System Cleanup
When perifusion is complete, stop intake and air uptakes pumps and close stopcocks to air uptake and media outlet lines.
Raise the mounting rack from the water bath and wait approximately 0h 2m 0s so that islets can settle to the bottom of the chamber.
Carefully remove the blue outlet end piece from the islet chamber. Pipet the islets and media from the chamber into a 6-cm untreated tissue culture dish and rinse the chamber and the blue column end piece with 5 times with 1mL each of baseline media into the dish.
Rinse the chamber with deionized water and remove frits from chamber.
Reassemble column and rinse all lines, including waste lines, with 10% bleach at maximum pump speed for approximately 0h 15m 0s.
Rinse all lines, including waste lines, with deionized water at maximum pump speed for approximately 1h 30m 0s.
Turn off intake and air uptake pumps, drain the water bath, turn off gas, and record the perifusion date, details, and any errors or problems in the perifusion logbook.
Islet Hormone Extraction
Using 6-cm dish with recovered islets, quantify IEQ () and perform imaging () to calculate IEQ recovered.
Using a pipette, transfer all islets from the center of the dish to a clear 1.5-mL microcentrifuge tube for islet hormone extraction.
Centrifuge the tube at 200rcf and aspirate the supernatant using a pipette, being careful not to disturb the islet pellet. Place tube On ice.
Prepare fresh acid alcohol for hormone extraction by adding 50µL 12M HCl to 5.5mL 95% ethanol.
Add 200µL acid ethanol (prepared in step 41) to islet pellet and incubate at 4°C 24h 0m 0s or for up to 24h 0m 0s.
Centrifuge tube at 3000rpm and transfer three 50µL aliquots of supernatant to 2-mL screwcap tubes. Store at -80°C.
Data Collection and Analysis
Open the darkfield image in the cellSens software. Using the manual HSV threshold function, segment the islet tissue channel.
Use the custom Count and Measure algorithm to determine islet count and mean islet diameter. Split adjacent but discrete islets using the Manually Split Objects tool to get an accurate islet count and mean diameter measurements.
Use the mean diameter measurements to assign islets to a diameter group using the chart provided ( ).
Perform insulin and/or glucagon assays on perifusion fractions and extracts.

