Models of Affective Illness: Chronic Mild Stress in the Rat
Mariusz Papp, Mariusz Papp, Paul Willner, Paul Willner
Abstract
This article describes a chronic mild stress (CMS) model for predicting antidepressant response and investigating mechanisms of antidepressant action in rats. Following exposure to a variety of mild stressors for several weeks, the rats’ behavior is modified in several ways that parallel symptoms of depression. Among these is a substantial reduction in consumption of a 1% sucrose solution, which models the cardinal symptom of major depression, anhedonia. Our standard procedure employs a battery of behavioral tests, comprising weekly assessment of sucrose intake and, at the end of treatment, the elevated plus-maze and novel object recognition tests to assess the anxiogenic and dyscognitive effects of CMS. Chronic administration of antidepressant drugs reverses the decreased sucrose intake and other behavioral changes in these subjects. Also effective are second-generation antipsychotics. The CMS model can be employed in discovery programs to identify anti-anhedonic drugs (e.g., antidepressants and antipsychotics) that act more quickly than existing agents. While most antidepressants require 3 to 5 weeks to normalize behavior, some treatments provide a faster onset of action. For example, the CMS-induced deficits can be reversed by acute or sub-chronic application of treatments that act rapidly in depressed patients, such as deep brain stimulation (DBS), ketamine, and scopolamine, as well as several compounds that have yet to be tested in humans but have fast-onset antidepressant-like effects in animals, such as the 5-HT-1A biased agonists NLX-101 and GLYX-13. Application of the CMS model in Wistar-Kyoto (WKY) rats causes similar behavioral changes to those seen in Wistars, but these are not reversed by antidepressant treatment. However, WKY rats respond to DBS and ketamine, which are effective in patients who are antidepressant non-responders, establishing CMS in WKY rats as a model of treatment-resistant depression. © 2023 The Authors. Current Protocols published by Wiley Periodicals LLC.
Basic Protocol : Induction of chronic mild stress in rats as a model of depression and treatment-resistant depression
INTRODUCTION
Antidepressants elevate mood in patients with affective illness, but it is difficult to identify such agents simply by examining behavioral effects in normal laboratory animals, just as the psychological effects of antidepressant drugs are difficult to detect in non-depressed people. Therefore, efforts have been made to develop animal models of depression. Issues to consider when establishing the relevance of animal models of psychiatric disorders include construct validity (a sound theoretical rationale for the model), face validity (phenomenological similarities between the model and the clinical disorder), and predictive validity (correspondence between compound/drug actions in the model and in the clinic). Desirable features of an animal model of depression include inducing conditions known to be associated with human depression, simulation of core symptoms of the disorder, and appropriate response to antidepressant drugs. Although there are a number of models that display a reasonable pharmacological profile, very few fulfill all three of these validating criteria. Moreover, the majority of animal models of depression involve tests of brief duration, making it difficult to investigate the time course of antidepressant action and to separate the therapeutic and prophylactic effects of drugs and drug candidates. Thus, chronicity is an important feature of animal models of depression. At best, animal models of depression should have demonstrable validity and should display an abnormal state for a prolonged period (weeks or months), during which time antidepressant therapy may be administered.
Of the few models that meet these criteria, the chronic mild stress (CMS) paradigm is the most widely used and the most extensively validated (Willner, 1997, 2017). The Basic Protocol detailed in this article includes the steps necessary to generate this animal model. Data supporting the validity of this test system and a discussion of technical aspects of the procedure are also presented.
NOTE : All protocols involving animals must be reviewed and approved by the appropriate Animal Care and Use Committee and must follow regulations for the care and use of laboratory animals.
STRATEGIC PLANNING
Chronic mild stress as an animal model of depression
In this protocol, animals are exposed to a variety of mild stressors for several weeks, during which time they develop behavioral, biochemical, and physiological changes. The most common way of quantifying the efficacy of the stress is to monitor consumption of a sucrose solution as a measure of anhedonia, a core symptom of depression. Stressed animals consume less sucrose solution compared to control, non-stressed subjects. Upon antidepressant treatments and continued application of the mild stressors, sucrose consumption does not change in control animals, but in stressed rats, it gradually increases to the pre-stress level. Therefore, this model provides a relevant way to assess the clinical effectiveness of a potential antidepressant compound as well as the biological processes underlying the pathophysiology of depression.
While this article presents a single protocol, there are three variables that we should mention at the outset:
Strain
The CMS model of depression is typically implemented in Wistar rats. Wistar-Kyoto rats behave differently. They respond to CMS similarly to Wistars but fail to respond to chronic antidepressant treatment. They do, however, show robust antidepressant-like responses when treated acutely or sub-chronically with DBS or ketamine, which act rapidly in drug-resistant depressed patients. So the implementation of CMS in WKY rats provides a model of treatment-resistant depression.
Duration of treatment
Treatment is usually administered daily over 5 to 6 weeks, as is appropriate for typical slow-acting antidepressant drugs. However, the model responds acutely or sub-chronically to treatments that act rapidly in depressed patients, such as deep brain stimulation (DBS), ketamine, and scopolamine. Hence, the duration of treatment can be curtailed to as little as a few days for investigating rapidly acting antidepressants (RAADs).
Endpoints
In the standard procedure used in our laboratory, the sucrose intake test is supplemented with two additional tests administered at the end of antidepressant treatment: the elevated plus-maze and the novel object recognition test (see, e.g., Strekalova et al., 2022 for review). These tests evaluate, respectively, the anxiogenic and dyscognitive effects of CMS and their reversal by antidepressant treatments.
A number of procedures could be used as alternatives to the sucrose consumption test to demonstrate depressive-like effects. These include sucrose preference (Willner, Towell, Sampson, Sophokleous, and Muscat, 1987), intracranial self-stimulation threshold (Moreau, Jenck, Martin, Mortas, and Haefely, 1992), and place preference conditioning (Papp, Lappas, Muscat, and Willner, 1992). The forced swim and tail suspension tests are also frequently used (in mice, though less so in rats), but these tests are less theoretically grounded and can be difficult to interpret because they respond to acute administration of antidepressants in non-stressed animals. These alternative endpoints are not described in detail as they are not in routine use in our laboratory. We have standardized the procedure using the sucrose consumption test because of its simplicity, relevance to depression, and potential for repeat testing.
Basic Protocol: INDUCTION OF CHRONIC MILD STRESS IN RATS AS A MODEL OF DEPRESSION AND TREATMENT-RESISTANT DEPRESSION
Materials
-
∼100 g male or female rats, aged ∼5-6 weeks (e.g., Wistar or Wistar-Kyoto)
-
∼1% (w/v) sucrose solution
-
Drug(s) to be tested and the appropriate vehicle. Experiments typically also include either venlafaxine or escitalopram as a reference compound.
-
Non-transparent plastic home cages (55 × 35 × 20 cm for grouped housing and 40 × 25 × 15 cm for single housing)
-
Sound-proof housing rooms with stable environmental conditions (i.e., light/dark cycle, temperature, and humidity, air exchange rate not higher than 6 per hour)
-
300-ml polyethylene drinking bottles with bungs and stainless-steel spouts containing a ball-bearing to minimize spillage (e.g., North Kent Plastic Cages)
1.Due to differences in reactivity to stressful stimuli, other rat strains (e.g., Long-Evans or Sprague-Dawley) are not recommended. 2.The exact concentration of the sucrose solution can vary between 1% and 2%, depending on the conditions in individual laboratories. The typical sucrose concentration is 1%, and at this concentration, normal non-stressed animals should drink 12 to 18 g of solution per day. The concentration can be increased if they drink less, but the final concentration should not exceed 2%.
Adaptation to laboratory conditions and sucrose consumption
1.After arrival at the laboratory, allow the animals to adapt to environmental conditions for approximately 3 weeks. The number of animals to be ordered will depend on the experimental design but typically should be 8 to 10 animals per treatment group, plus several extras in case of attrition (see below).
During the first 10 days, keep the animals in groups of 10 (in bigger cages of 55 × 35 × 20 cm to avoid overcrowding) and then house them singly (in smaller cages of 40 × 25 × 15 cm). This adaptation period can be prolonged depending on the animals’ weight. At the end of this phase, the animals should weigh ∼200 g.
Sucrose consumption studies
2.Deprive the animals of food and water beginning the evening prior to measuring sucrose consumption at around 18.00 hr.
3.On the morning of the test day, prepare a fresh sucrose solution (final temperature ∼22°C, so not directly from the cold tap), fill the drinking bottles with ∼200 ml of the solution, weigh them, and place them in the cages.
4.Allow the rats to consume the sucrose solution for 1 hr.
5.Remove the bottles, reweigh them, and calculate intake as the difference in weight before and after the test.
6.Carefully wash the bottles and bungs with spouts using warm tap water without detergents.
7.Following the test, allow an interval of at least 1 hr and then return food and water.
8.Repeat steps 2-7 once per week for 7-8 weeks, by which time sucrose intake should have stabilized.
9.On the day following the final baseline test, divide the animals semi-randomly into control and “to-be-stressed” groups (ensuring that mean sucrose intakes are closely matched), move both groups to separate rooms, and start the stress procedure.
Stressor application
10.Subject rats of the stressed group (n = 9-12 initially) to the stress procedure using a selection of stressors listed in Table 1. A typical sequence of stressors used in our Laboratory is presented in Table 2.
| Stressor | Description |
|---|---|
| Food and water deprivation | Use separate periods of food deprivation or water deprivation, and one period of both food and water deprivation. This last stressor precedes each sucrose test. |
| Cage tilting | Tilt the cages backward (∼45°). This stressor should follow periods of food or water deprivation; when the animals are hungry or thirsty, tilting the cages makes access to food or water more difficult. This is usually achieved by tilting individual cages or a whole rack of cages, with measures to ensure they remain secure and do not fall. |
| Grouped housing | Place two animals into one cage, randomly changing the partners (same sex, and using animals from the stressed group, not additional animals from outside the experiment). This powerful stressor is particularly effective if applied at night and after a period of water deprivation. |
| Soiled cage | Pour 250 ml plain water into the sawdust bedding. |
| Stroboscopic illumination | Use low intensity ∼120 flashes/min. Do not forget to switch off the room lights when this stressor is used. |
| Intermittent illumination | Switch the room lights on and off every ∼2 hr. |
| No stress | An unexpected lack of stress is also a stressful stimulus for animals undergoing continuous stress. |
| Time | Stressor |
|---|---|
| Monday morning | Cage cleaning followed by no stress |
| Monday evening | Food and water deprivation |
| Tuesday morning (10.00 AM) | Sucrose test followed by the return of food and water at ∼12.00 PM |
| Tuesday evening | Cage tilting |
| Wednesday morning | Strobe |
| Wednesday evening | No stress |
| Thursday morning | Intermittent illumination |
| Thursday evening | Water or food deprivation |
| Friday morning | Grouped housing |
| Friday evening | Strobe |
| Saturday morning | Food or water deprivation |
| Saturday evening | Cage tilting |
| Sunday morning | Cage tilting continued |
| Sunday evening | Soiled cage |
11.Apply the stressors to single rats in their home cages continuously (i.e., throughout the day and night) and individually (i.e., one stressor at a time).
Provide food and water ad libitum to the control non-stressed animals, except for the overnight period of deprivation preceding each sucrose test.
12.Continue the stress procedure and weekly sucrose tests until the consumption of sucrose solution by the stressed animals falls below their intakes in the final baseline test by approximately 40% to 60% (i.e., from 12-14 g to 6-8 g). (Intakes should be comparable across groups at baseline and week 3 of stress within an experiment but may vary between experiments.)
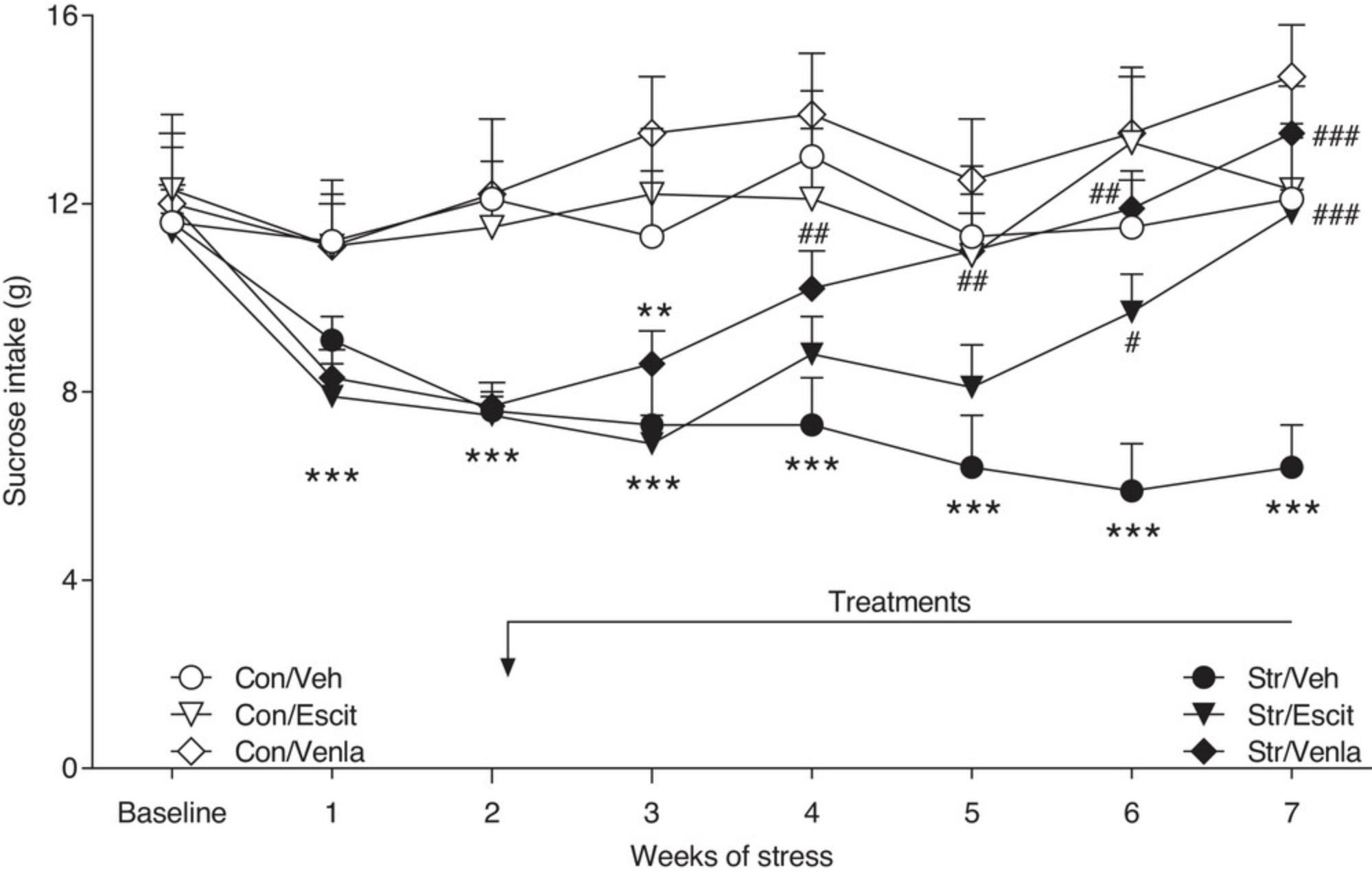
In the majority of studies, such an effect occurs gradually, usually requiring 2 to 3 weeks of stress before the intakes significantly differ from the baseline scores in stressed animals and the intakes of unstressed control subjects.
Test compound administration
13.Based on the sucrose intake following at least 2 weeks of initial stress, divide the animals further into matched treatment groups and begin administration of the test compounds and reference drugs.
14.Administer a reference drug (e.g., venlafaxine or escitalopram, both at 10 mg/kg/day, IP), vehicle (e.g., sterile saline 1 ml/kg/day, IP), and test compounds with appropriate vehicles.
15.Continue weekly sucrose tests on all animals (i.e., vehicle- and drug/compound-treated control and stressed animals).
16.Continue the stress procedure, weekly sucrose tests, and daily treatments until the decrease in the consumption of sucrose in stressed animals is restored to the level of the pre-stress and/or vehicle-treated control group.
- sucrose tests can be continued for 1 to 4 weeks to see how long the effects are sustained after cessation of the treatments.
- a battery of behavioral tests (e.g., Elevated Plus Maze, Novel Object Recognition, Social Interaction) can be conducted to evaluate the effects of a tested compound on stress-induced anxiety and cognitive and social deficits (Papp et al., 2017a, 2017b, 2018, 2021, 2023; Willner et al., 2019).
17.Analyze the sucrose test results using a three-way analysis of variance (ANOVA), followed by a standard test (e.g., Newman-Keuls or Bonferroni test) for post-hoc comparisons of means.
COMMENTARY
Background Information
The CMS model was first employed by Katz (1982), who subjected rats to a variety of highly stressful stimuli over several weeks. In most of these studies, the behavioral effects of chronic stress were evaluated in an open field. However, it was also reported that, unlike control animals, rats subjected to chronic stress did not increase their fluid intake when sucrose or saccharin was added to their drinking water (Katz, 1982). In subsequent studies, Willner and his colleagues observed that a similar effect could be obtained using a milder stress in which each element was, in itself, only slightly stressful. In a series of studies, they found that rats subjected to a variety of low-grade stressors for a prolonged period displayed a substantial decrease in their responsiveness to rewarding stimuli. This deficit was initially monitored by a reduction in the consumption of a highly rewarding weak solution of sucrose (Willner et al., 1987, 1992). This result was subsequently confirmed using other techniques, such as place-preference conditioning (Muscat, Papp, and Willner, 1992; Papp, Willner, and Muscat, 1991; 1992; 1993) or intracranial self-stimulation (Moreau et al., 1992; 1995). Although the CMS-induced impairment of sucrose consumption may be maintained for several weeks, normal behavior is restored with chronic treatment using a wide range of antidepressant drugs and other kinds of antidepressant treatments (see below).
This procedure has now been extensively investigated and validated as an animal model of depression. A theoretical rationale for the CMS model is that this procedure simulates anhedonia (a generalized decrease in the sensitivity to rewards or an inability to experience pleasure), a core symptom of depression. This provides construct validity for the model and has been subjected to intensive scrutiny (Willner, et al., 1992, 1997, 2005, 2017; Willner, Muscat, and Papp, 1992). The results of these studies provide strong support for the conclusion that the inhibitory effects of CMS on sucrose drinking reflect a reduction in the rewarding properties of the solution. That is, the stressed animals develop anhedonia.
In addition to inducing anhedonia, CMS causes the appearance of other symptoms of a major depressive disorder, supporting the face validity of this model. The stressed animals show decreased social interactions, decreased sexual and aggressive behavior, deficits in cognitive function, increased anxiety, changes in weight gain, decreased nocturnal locomotor activity during the waking phase of the light-dark cycle, and an advanced phase shift in diurnal rhythms. They also experience and display a variety of sleep disorders, including a decrease in rapid eye movement (REM)–sleep latency and an increased number of REM sleep episodes.
Additional details of the behavioral, biochemical, and physiological changes that occur in stressed animals are presented in reviews by Antoniuk, Bijata, Ponimaskin, and Wlodarczyk, 2019; Willner et al., 1992; and Willner, 1997, 2005, 2017). Together with the generalized decrease in responsiveness to rewards, these parallels with the symptoms of depression are both extensive and comprehensive. Indeed, the only symptoms of depression that have not been demonstrated in animals exposed to the CMS procedure are those revealed in humans only through verbal inquiry.
To establish the predictive validity of the CMS model, studies have been conducted in our laboratory with a wide range of antidepressants and other psychotherapeutic agents. Drugs shown to be effective in reversing stress-induced anhedonia include the tricyclic antidepressants (TCA) imipramine, desipramine, and amitriptyline; the monoamine oxidase inhibitors (MAO) moclobemide and brofaromine; the atypical antidepressants mianserin and tianeptine; the serotonin reuptake inhibitors (SRIs) fluoxetine, fluvoxamine, citalopram, and escitalopram; the noradrenaline reuptake inhibitors (NRIs) maprotiline and reboxetine; the serotonin and noradrenaline reuptake inhibitors (SNRIs) milnacipran and venlafaxine; and the melatonin agonist agomelatine (Brocco, Dekeyne, Papp, and Millan, 2006; Millan et al., 2001; Montgomery, Loft, Sánchez, Reines, and Papp, 2001; Muscat et al., 1992; Papp and Wierońska, 2000; Papp, Moryl, and Willner, 1996; 2000; 2003; 2004; Sánchez, Gruca, and Papp, 2003). A reversal of the CMS-induced deficit in sucrose consumption (i.e., anhedonia) was also observed following repeated administration of second-generation antipsychotics such as amisulpride, risperidone, olanzapine, asenapine, aripiprazole, cariprazine, blonanserin, and lurasidone (Adham et al., 2010; Begni et al., 2022; Brivio et al., 2021; Calabrese, Savino, Papp, Molteni, and Riva, 2016; Haduch, Rysz, Papp, and Daniel, 2018; Marston et al., 2010; Paladini et al., 2021; Papp and Wieronska, 2000; Papp et al., 2014; 2017a). These findings suggest that the CMS procedure is sensitive not only to antidepressant drugs, but also seems to respond to drugs with anti-anhedonic properties. Anhedonia, defined as a reduction in the ability to experience pleasure, is one of the core clinical features of depression but is also one of the negative symptoms of schizophrenia (Der-Avakian and Markou, 2012). Therefore, the activity in this test of drugs used to treat anhedonia in humans further supports the notion that the CMS procedure produces an animal model of anhedonia that can be effectively reversed by appropriate, clinically relevant treatments, e.g., antidepressants and atypical antipsychotics.
Agents ineffective in this model include chlordiazepoxide, amphetamine, morphine, and haloperidol (Papp et al., 1996; Willner et al., 1992). None of these drugs are clinically effective as antidepressants.
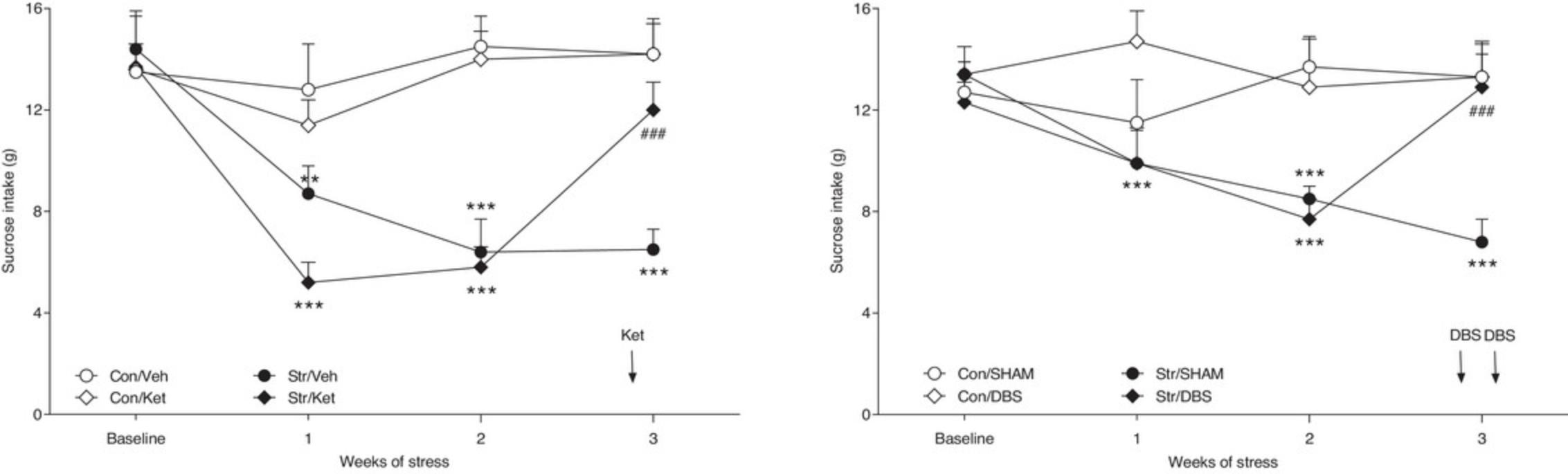
The normalization of CMS-induced behavioral deficits following traditional antidepressant therapy required long-term administration of drugs. However, a series of recent studies conducted in our laboratory found that the effects of stress can also be reversed by a single dose of ketamine or acute application of deep brain stimulation (DBS) of the medial prefrontal cortex (Papp, Gruca, Lason-Tyburkiewicz, and Willner, 2017b; 2018; Willner et al., 2019; unpublished). These effects were observed in both the Wistar and Wistar-Kyoto rat strains. The significance of WKY is that these rats have traditionally been considered antidepressant non-responsive, an observation that we have confirmed in the CMS model (Willner et al., 2019). However, the fact that WKY rats do respond to treatments that are effective in antidepressant-resistant patients validates the use of CMS in WKY as a model of treatment-resistant depression. We have recently used optogenetic stimulation techniques to show that their non-responsiveness to antidepressant treatment is mediated by an inability of antidepressant treatment to activate transmission from the ventral hippocampus to the medial prefrontal cortex (Papp et al., 2021; 2022).
In summary, the CMS model has traditionally been considered an animal model of depression with a relatively high degree of construct, face, and predictive validities, based in part on its ability to mimic both the delayed and rapid onset of action observed with clinically effective antidepressants. The observation that atypical antipsychotic compounds are also active in this test suggests that it may also model aspects of anhedonia that are associated with schizophrenia. It is interesting to note, however, that two atypical antipsychotics, olanzapine and aripiprazole, have recently been approved as adjunct therapies for treatment-resistant depression. Together with the efficacy of DBS and ketamine, these data suggest that the CMS model may serve as a suitable research tool for evaluating novel compounds with potential antidepressant properties and in investigating the psychobiological mechanisms underlying depression and treatment-resistant depression.
Critical Parameters and Troubleshooting
The CMS model has in the past attracted frequent criticism for a lack of reliability. A recent survey of users of the CMS model found that these issues are now far less prevalent (Willner, 2017), but some laboratories still experience difficulty implementing the procedure. Attention to the issues outlined below should help.
Sucrose test
The sucrose test is a relatively simple and valid measure of the animals’ response to chronic stress. Moreover, it allows for continuous observation of the same animals undergoing chronic antidepressant therapy, enabling evaluation of the onset of action of tested drugs and compounds. Therefore, it is employed in most studies with the CMS model, at least in our laboratory. However, despite its technical simplicity, the sucrose consumption test is extremely sensitive to environmental influences, and failure to account for this sensitivity may lead to unclear and unstable results. Therefore, all efforts should be made to minimize the inflow of any external olfactory and sound stimuli into the rooms where animals are housed and tested. No other animals should be housed with the CMS animals, and no other experiments should be conducted in these rooms. In addition, no detergents should be used to clean drinking bottles and home cages, and no perfumes or deodorants should be used by laboratory staff.
According to our long experience with the CMS procedure, the rats show much more stable sucrose consumption when tested shortly after the end of the dark phase of the non-reversed 12-hr light/dark cycle with the lights on at 8.00. Therefore, it is recommended that the animals are deprived of food and water from 18.00 to 20.00, and the sucrose tests are performed the following day at 10.00. Extending the deprivation and/or test duration can lead to a marked reduction in the difference between control and stressed animals. It is also important to keep an interval of approximately 1 hr between the end of the test and the return of food and water; this interval will prevent the animals from making an association between the sucrose test and the return of food and water.
Approximately 15% of the animals either do not show a stable consumption of the sucrose solution in the baseline tests or have very high or low scores. Such animals should be eliminated before initiating the chronic stress procedure.
Stress procedure
Stressors and application schedules other than those listed above can be used. However, remember that the idea of the CMS model is that the level of stress represents a relatively realistic analog of the stresses that people encounter in their daily lives. Therefore, it is not the severity of the stressors but their variety and prolonged presentation that are essential for this procedure. A significant increase in stress intensity often leads to a paradoxical increase rather than a decrease in sucrose consumption in stressed animals. Also, remember that some stressors (e.g., wetting or grouping) are more severe, while others are similar in nature (e.g., intermittent illumination and strobe), so these should not be applied in successive blocks but rather evenly scheduled throughout the week.
Test compound administration
In the CMS model, traditional antidepressants and novel compounds are administered continuously for several weeks, and their efficacy is monitored weekly by changes in the consumption of sucrose solution relative to the responses of control and stressed animals receiving vehicle. Both the route of administration and the type of vehicle used can influence the results. It is recommended that intraperitoneal or oral routes of administration are employed rather than subcutaneous injections, which may cause skin damage after repeat administration.
It is occasionally observed that compounds tested in the CMS model change the consumption of sucrose solution in control non-stressed animals, which may suggest a nonspecific effect of the compound on thirst motivation or fluid intake. If this is the case, an additional experiment should be performed in which plain water is presented instead of the sucrose solution. However, it is important that such a study is conducted on both control and stressed animals under conditions similar to those used for the sucrose test.
If the CMS model is used to identify potential novel antidepressants, a conventional antidepressant drug, e.g., venlafaxine or escitalopram (both 10 mg/kg/day, IP), should be used as a reference.
In the majority of studies, 5 weeks of drug administration are sufficient to achieve full recovery from the stress-induced deficit in sucrose consumption. Thus, if no effect is observed within this period, it may be assumed that the treatment is ineffective in this model or at this dose.
Alternative CMS procedures
There are many variants of CMS in the literature, which appear under names such as chronic unpredictable stress (CUS), chronic unpredictable mild stress (CUMS or UCMS), or chronic variable stress (CVS). While the names vary, the procedures, by and large, do not (Willner, 2017). For example, the designation of a procedure as CUMS does not imply that the procedure is any less predictable than procedures described simply as CMS. The version of the procedure outlined in this article is little changed from the original CMS procedure described by Willner et al. in 1987 and 1992 and has been found to be reliable and productive over the intervening 35 years.
Anticipated Results
The reversal of the CMS-induced anhedonia occurs gradually, requiring ∼3 to 5 weeks of continuous daily administration, which resembles the clinical time course of antidepressant action. In addition, the antidepressants should act only in animals exposed to the stress procedure and should not alter the behavior of non-stressed control subjects. Treatment with antidepressants should not commence until a hedonic deficit has been established, demonstrating, again, as in the clinical situation, the therapeutic, rather than prophylactic, activity of antidepressants. Exemplary gradual effects of escitalopram and venlafaxine, obtained in our laboratory, are presented in Figure 1. The fast effects of ketamine and DBS in the CMS model are shown in Figure 2.
Various neurochemical systems have been implicated as primarily responsible for the behavioral sequelae of the CMS procedure and the therapeutic action of antidepressants. The most interesting changes that are consistently observed in stressed animals are found within the monoaminergic and glutamatergic neurotransmitter systems. These alternations include down- or up-regulation of receptors, modifications in intracellular signaling, structural and molecular alterations, changes in gene expression, neuroinflammation, and activation of inflammatory, oxidative, apoptotic, and antineurogenic mechanisms. Most can be reversed by antidepressant therapies. It is beyond the scope of this article to review all those findings, but they are available in the PubMed database (see, e.g., Hill, Hellemans, Verma, Gorzalka, and Weinberg, 2012; Kubera, Obuchowicz, Goehler, Brzeszcz, and Maes, 2011, Khan, Geiger, Wiborg, and Czéh, 2020). All these findings seem to confirm that the CMS model of depression may provide valuable information to evaluate the mechanisms involved in the pathophysiology of depression and/or anhedonia, their therapy, and their resistance to antidepressant treatment.
Time Considerations
The CMS model is a long-term procedure. A typical experiment with a conventional antidepressant drug requires more than 4 months. This includes adaptation to laboratory conditions (up to 3 weeks) and the consumption of sucrose solution (8 to 10 weeks), followed by a period of 2 to 3 weeks of initial stress to obtain a stable decrease of sucrose intake in stressed animals, and another 5 weeks of drug administration to determine whether the stress-induced effect on sucrose consumption can be reversed.
Acknowledgments
The study was financially supported by the NCN grant no. 2019/35/B/NZ7/00787.
Author Contributions
Mariusz Papp : Conceptualization, project administration, validation, original draft writing; Paul Willner : Validation, draft review and editing.
Conflict of Interest
The authors declare no biomedical financial interests and no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Literature Cited
- Adham, N., Caron, M. G., Gyertyán, I., Gruca, P., Kiss, B., Lason-Tyburkiewicz, M., … Papp, M. (2010). Cariprazine, a D3-preferring dopamine D3/D2 receptor partial agonist, has antidepressant-like activity with fast onset of action in the chronic mild stress-induced anhedonia model. Poster Session I. Neuropsychopharmacology , 35, S171. doi: 10.1038/npp.2010.216
- Antoniuk, S., Bijata, M., Ponimaskin, E., & Wlodarczyk, J. (2019). Chronic unpredictable mild stress for modeling depression in rodents: Meta-analysis of model reliability. Neuroscience and Biobehavioral Reviews , 99, 101–116. doi: 10.1016/j.neubiorev.2018.12.002
- Begni, V., Pisano, I., Marizzoni, M., Marchisella, F., Creutzberg, K. C., De Rosa, F., … Riva, M. A. (2022). Exposure to chronic stress impairs the ability to cope with an acute challenge: Modulation by lurasidone treatment. European Neuropsychopharmacology , 61, 78–90. doi: 10.1016/j.euroneuro.2022.06.005
- Brivio, P., Sbrini, G., Tarantini, L., Parravicini, C., Gruca, P., Lason, M., … Calabrese, F. (2021). Stress modifies the expression of glucocorticoid-responsive genes by acting at epigenetic levels in the rat prefrontal cortex: Modulatory activity of lurasidone. International Journal of Molecular Sciences , 22(12), 6197. doi: 10.3390/ijms22126197
- Brocco, M., Dekeyne, A., Papp, M., & Millan, M. J. (2006). Antidepressant-like properties of the anti-Parkinson agent, piribedil, in rodents: Mediation by dopamine D2 receptors. Behavioural Pharmacology , 17, 559–572. doi: 10.1097/01.fbp.0000236267.41806.5b
- Calabrese, F., Savino, E., Papp, M., Molteni, R., & Riva, M. A. (2016). Chronic mild stress-induced alterations of clock gene expression in rat prefrontal cortex: Modulatory effects of prolonged lurasidone treatment. Pharmacological Research , 104, 140–150. doi: 10.1016/j.phrs.2015.12.023
- Der-Avakian, A., & Markou, A. (2012). The neurobiology of anhedonia and other reward-related deficits. Trends in Neuroscience (Tins) , 35, 68–77. doi: 10.1016/j.tins.2011.11.005
- Haduch, A., Rysz, M., Papp, M., & Daniel, W. A. (2018). The activity of brain and liver cytochrome P450 2D (CYP2D) is differently affected by antidepressants in the chronic mild stress (CMS) model of depression in the rat. Biochemical Pharmacology , 156, 398–405. doi: 10.1016/j.bcp.2018.09.005
- Hill, M. N., Hellemans, K. G., Verma, P., Gorzalka, B. B., & Weinberg, J. (2012). Neurobiology of chronic mild stress: Parallels to major depression. Neuroscience & Biobehavioral Reviews, 36, 2085–2117. doi: 10.1016/j.neubiorev.2012.07.001
- Katz, R. J. (1982). Animal model of depression: Pharmacological sensitivity of a hedonic deficit. Pharmacology Biochemistry and Behavior , 16, 965–968. doi: 10.1016/0091-3057(82)90053-3
- Khan, A. R., Geiger, L., Wiborg, O., & Czéh, B. (2020). Stress-induced morphological, cellular and molecular changes in the brain—lessons learned from the chronic mild stress model of depression. Cells , 9, 1026. doi: 10.3390/cells9041026
- Kubera, M., Obuchowicz, E., Goehler, L., Brzeszcz, J., & Maes, M. (2011). In animal models, psychosocial stress-induced (neuro)inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 35, 744–759. doi: 10.1016/j.pnpbp.2010.08.026
- Marston, H. M., Martin, F. D., Papp, M., Gold, L., Wong, E. H., & Shahid, M. (2010). Attenuation of chronic mild stress-induced “anhedonia” by asenapine is not associated with a “hedonic” profile in intracranial self-stimulation. Journal of Psychopharmacology , 25, 1388–1398. doi: 10.1177/0269881110376684
- Millan, M. J., Dekeyne, A., Papp, M., Rochelle, C. D., MacSweeny, C., & Brocco, M. (2001). S33005, a novel ligand at both serotonin and norepinephrine transporters: Ii. behavioral profile in comparison with venlafaxine, reboxetine, citalopram, and clomipramine. Journal of Pharmacology and Experimental Therapeutics , 298, 581–591.
- Montgomery, S. A., Loft, H., Sánchez, C., Reines, E. H., & Papp, M. (2001). Escitalopram (S-enantiomer of citalopram): Clinical efficacy and onset of action predicted from a rat model. Pharmacology & Toxicology, 88, 282–286. doi: 10.1111/j.1600-0773.2001.880511.x
- Moreau, J.-L., Jenck, F., Martin, J. R., Mortas, P., & Haefely, W. E. (1992). Antidepressant treatment prevents chronic mild stress-induced anhedonia as assessed by ventral tegmentum self-stimulation behaviour in rats. European Neuropsychopharmacology , 2, 43–49. doi: 10.1016/0924-977X(92)90035-7
- Moreau, J.-L., Scherschlicht, R., Jenck, F., & Martin, J. R. (1995). Chronic mild stress-induced anhedonia model of depression: Sleep abnormalities and curative effects of electroshock treatment. Behavioural Pharmacology , 6, 682–687. doi: 10.1097/00008877-199511000-00003
- Muscat, R., Papp, M., & Willner, P. (1992). Reversal of stress-induced anhedonia by the atypical antidepressants, fluoxetine and maprotiline. Psychopharmacology , 109, 433–438. doi: 10.1007/BF02247719
- Paladini, M. S., Spero, V., Begni, V., Marchisella, F., Guidi, A., Gruca, P., … Molteni, R. (2021). Behavioral and molecular effects of the antipsychotic drug blonanserin in the chronic mild stress model, Pharmacological Research , 163, 105330. doi: 10.1016/j.phrs.2020.105330
- Papp, M., Willner, P., & Muscat, R. (1991). An animal model of anhedonia: Attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology , 104, 255–259. doi: 10.1007/BF02244188
- Papp, M., Lappas, S., Muscat, R., & Willner, P. (1992). Attenuation of place preference conditioning but not place aversion conditioning by chronic mild stress. Journal of Psychopharmacology , 6, 352–356. doi: 10.1177/026988119200600302
- Papp, M., Muscat, R., & Willner, P. (1993). Sub-sensitivity to rewarding and locomotor stimulant effects of a dopamine agonist following chronic mild stress. Psychopharmacology , 110, 152–158. doi: 10.1007/BF02246965
- Papp, M., Moryl, E., & Willner, P. (1996). Pharmacological validation of the chronic mild stress model of depression. European Journal of Pharmacology , 296, 129–136. doi: 10.1016/0014-2999(95)00697-4
- Papp, M., & Wieronska, J. (2000). Antidepressant-like effects of amisulpride in two animal models of depression. Journal of Psychopharmacology , 14, 46–52. doi: 10.1177/026988110001400106
- Papp, M., Gruca, P., Boyer, P - A., & Mocaër, E. (2003). Effect of agomelatine in the chronic mild stress model of depression in the rat. Neuropsychopharmacology , 28, 694–703. doi: 10.1038/sj.npp.1300091
- Papp, M., Panconi, E., & Gruca, P. (2004). Effects of the novel antidepressant milnacipran in a chronic mild stress model of depression. Drug Development Research , 61, 101–106. doi: 10.1002/ddr.10340
- Papp, M., Gruca, P., Lasoń-Tyburkiewicz, M., Adham, N., Kiss, B., & Gyertyán, I. (2014). Attenuation of anhedonia by cariprazine in the chronic mild stress model of depression. Behavioural Pharmacology , 25, 567–574. doi: 10.1097/FBP.0000000000000070
- Papp, M., Gruca, P., Lason-Tyburkiewicz, M., Litwa, E., Niemczyk, M., Tota-Glowczyk, K., & Willner, P. (2017a). Dopaminergic mechanisms in memory consolidation and antidepressant reversal of a chronic mild stress-induced cognitive impairment. Psychopharmacology , 234, 2571–2585. doi: 10.1007/s00213-017-4651-4
- Papp, M., Gruca, P., Lason-Tyburkiewicz, M., & Willner, P. (2017b). Antidepressant, anxiolytic and procognitive effects of subacute and chronic ketamine in the chronic mild stress model of depression. Behavioural Pharmacology , 28, 1–8. doi: 10.1097/FBP.0000000000000259
- Papp, M., Gruca, P., Lason-Tyburkiewicz, M., Niemczyk, M., Tota-Glowczyk, K., & Willner, P. (2018). Rapid antidepressant effects of deep brain stimulation of the pre-frontal cortex in an animal model of treatment-resistant depression. Journal of Psychopharmacology , 32, 1133–1140. doi: 10.1177/0269881118791737
- Papp, M., Gruca, P., Lason, M., Litwa, E., Solecki, W., & Willner, P. (2021). Insufficiency of ventral hippocampus to medial prefrontal cortex transmission explains antidepressant non-response, Journal of Psychopharmacology , 35, 1253–1264. doi: 10.1177/02698811211048281
- Papp, M., Gruca, P., Lason, M., Litwa, E., Solecki, W., & Willner, P. (2022). Optogenetic stimulation of medial prefrontal cortex excites GABAergic cells in the nucleus accumbens and hippocampus of Wistar-Kyoto rats exposed to chronic mild stress. Psychopharmacol , 239(7), 2299–2307. doi: 10.1007/s00213-022-06116-6
- Papp, M., Gruca, P., Litwa, E., Lason, M., & Willner, P. (2023). Optogenetic stimulation of transmission from prelimbic cortex to nucleus accumbens core overcomes resistance to venlafaxine in an animal model of treatment-resistant depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 123, 110715. doi: 10.1016/j.pnpbp.2023.110715
- Sánchez, C., Gruca, P., & Papp, M. (2003). R-citalopram counteracts the antidepressant-like effect of escitalopram in a rat chronic mild stress model. Behavioural Pharmacology , 14, 465–470. doi: 10.1097/01.fbp.0000087733.21047.60
- Strekalova, T., Liu, Y., Kiselev, D., Khairuddin, S., Chiu, J. L. Y., Lam, J., … Lim, L. W. (2022). Chronic mild stress paradigm as a rat model of depression: Facts, artifacts, and future perspectives. Psychopharmacology , 239, 663–693. doi: 10.1007/s00213-021-05982-w
- Willner, P. (1997). Validity, reliability and utility of the chronic mild stress model of depression: A 10-year review and evaluation. Psychopharmacology , 134, 319–329. doi: 10.1007/s002130050456
- Willner, P. (2005). Chronic mild stress (CMS) revisited: Consistency and behavioural neurobiological concordance in the effects of CMS. Neuropsychobiology , 52, 90–110. doi: 10.1159/000087097
- Willner, P. (2017). The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiology of Stress , 24, 78–93. doi: 10.1016/j.ynstr.2016.08.002
- Willner, P., Towell, A., Sampson, D., Sophokleous, S., & Muscat, R. (1987). Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology , 93, 358–364. doi: 10.1007/BF00187257
- Willner, P., Muscat, R., & Papp, M. (1992). Chronic mild stress-induced anhedonia: A realistic animal model of depression. Neuroscience and Biobehavioral Reviews , 16, 525–534. doi: 10.1016/S0149-7634(05)80194-0
- Willner, P., Gruca, P., Lason, M., Tota-Glowczyk, K., Litwa, E., Niemczyk, M., & Papp, M. (2019). Validation of chronic mild stress in the Wistar-Kyoto rat as an animal model of treatment-resistant depression. Behavioural Pharmacology , 30, 235–250. doi: 10.1097/FBP.0000000000000431
Citing Literature
Number of times cited according to CrossRef: 11
- Michał Walaszek, Zofia Kachlik, Wiesław Jerzy Cubała, Low-carbohydrate diet as a nutritional intervention in a major depression disorder: focus on relapse prevention, Nutritional Neuroscience, 10.1080/1028415X.2024.2303218, 27 , 10, (1185-1198), (2024).
- Adam K. Klein, Eric W. Austin, Michael J. Cunningham, Dino Dvorak, Silvia Gatti, Sarah K. Hulls, Laszlo Kiss, Andrew C. Kruegel, Gerard J. Marek, Mariusz Papp, Jonathan Sporn, Zoë A. Hughes, GM-1020: a novel, orally bioavailable NMDA receptor antagonist with rapid and robust antidepressant-like effects at well-tolerated doses in rodents, Neuropsychopharmacology, 10.1038/s41386-023-01783-1, 49 , 6, (905-914), (2024).
- Sara Derosa, Paulina Misztak, Jessica Mingardi, Giulia Mazzini, Heidi Kaastrup Müller, Laura Musazzi, Changes in neurotrophic signaling pathways in brain areas of the chronic mild stress rat model of depression as a signature of ketamine fast antidepressant response/non-response, Progress in Neuro-Psychopharmacology and Biological Psychiatry, 10.1016/j.pnpbp.2023.110871, 128 , (110871), (2024).
- Zofia Kachlik, Michał Walaszek, Wiesław Jerzy Cubała, Low-carbohydrate diet as a disease modifier for relapse prevention of treatment-resistant depression. Spotlight on neuroplasticity and brain-derived neurotrophic factor, Medical Hypotheses, 10.1016/j.mehy.2024.111356, 187 , (111356), (2024).
- Guowei Gong, Kumar Ganesan, Yongjie Wang, Zhenxia Zhang, Yaqun Liu, Junli Wang, Fenglian Yang, Yuzhong Zheng, Ononin ameliorates depression-like behaviors by regulating BDNF-TrkB-CREB signaling in vitro and in vivo, Journal of Ethnopharmacology, 10.1016/j.jep.2023.117375, 320 , (117375), (2024).
- Shweta Sharma, Shivani Chawla, Praveen Kumar, Rizwan Ahmad, Prabhakar Kumar Verma, The chronic unpredictable mild stress (CUMS) Paradigm: Bridging the gap in depression research from bench to bedside, Brain Research, 10.1016/j.brainres.2024.149123, 1843 , (149123), (2024).
- Daniil Grinchii, Kristína Janáková Csatlósová, Mireia Viñas-Noguera, Roman Dekhtiarenko, Ruslan Paliokha, Ľubica Lacinová, Eliyahu Dremencov, Michal Dubovický, Effects of pre-gestational exposure to the stressors and perinatal bupropion administration on the firing activity of serotonergic neurons and anxiety-like behavior in rats, Behavioural Brain Research, 10.1016/j.bbr.2023.114796, 459 , (114796), (2024).
- Chloe J. Jordan, Susan L. Andersen, The relative invulnerability of juvenile rats to addiction: Longitudinal assessment of risk behaviors and their relationship to cocaine self-administration, Addiction Neuroscience, 10.1016/j.addicn.2024.100161, 12 , (100161), (2024).
- Adam Bielawski, Agnieszka Zelek-Molik, Katarzyna Rafa-Zabłocka, Marta Kowalska, Piotr Gruca, Mariusz Papp, Irena Nalepa, Elevated Expression of HSP72 in the Prefrontal Cortex and Hippocampus of Rats Subjected to Chronic Mild Stress and Treated with Imipramine, International Journal of Molecular Sciences, 10.3390/ijms25010243, 25 , 1, (243), (2023).
- Zheng-Wei Zhang, Pei Han, Jie Fu, Hang Yu, Hui Xu, Jia-Chun Hu, Jin-Yue Lu, Xin-Yu Yang, Hao-Jian Zhang, Meng-Meng Bu, Jian-Dong Jiang, Yan Wang, Gut microbiota-based metabolites of Xiaoyao Pills (a typical Traditional Chinese medicine) ameliorate depression by inhibiting fatty acid amide hydrolase levels in brain, Journal of Ethnopharmacology, 10.1016/j.jep.2023.116555, 313 , (116555), (2023).
- Magdalena Kolasa, Agata Faron-Górecka, Preclinical models of treatment-resistant depression: challenges and perspectives, Pharmacological Reports, 10.1007/s43440-023-00542-9, 75 , 6, (1326-1340), (2023).

