Maize Seedling Growth and Hormone Response Assays Using the Rolled Towel Method
Melissa A. Draves, Melissa A. Draves, Rebekah L. Muench, Rebekah L. Muench, Michelle G. Lang, Michelle G. Lang, Dior R. Kelley, Dior R. Kelley
Abstract
Root system architecture is a critical factor in maize health and stress resilience. Determining the genetic and environmental factors that shape maize root system architecture is an active research area. However, the ability to phenotype juvenile root systems is hindered by the use of field-grown and soil-based systems. An alternative to soil- and field-based growing conditions for maize seedlings is a controlled environment with a soil-free medium, which can facilitate root system phenotyping. Here, we describe how to grow maize under soil-free conditions for up to 12 days to facilitate root phenotyping. Maize seeds are sterilized and planted on specialized seed germination paper to minimize fungal contamination and ensure synchronized seedling growth, followed by imaging at the desired time point. The root images are then analyzed to quantify traits of interest, such as primary root length, lateral root density, seminal root length, and seminal root number. In addition, juvenile shoot traits can be quantified using manual annotation methods. We also outline the steps for performing rigorous hormone response assays for four classical phytohormones: auxin, brassinosteroid, cytokinin, and jasmonic acid. This protocol can be rapidly scaled up and is compatible with genetic screens and sample collection for downstream molecular analyses such as transcriptomics and proteomics. © 2022 The Authors. Current Protocols published by Wiley Periodicals LLC.
Basic Protocol 1 : Maize seedling rolled towel assay and phenotyping
Basic Protocol 2 : Maize seedling hormone response assays using the rolled towel assay
INTRODUCTION
Maize is a key crop worldwide that has been described as “needing no introduction” (Hake & Ross-Ibarra, 2015). Genetic and environmental factors shape maize root systems and play critical roles in water use efficiency, nutrient acquisition, and yield (Hochholdinger, Yu, & Marcon, 2018; Liu & von Wirén, 2022). Phenotyping crop root systems from field-grown plants can provide critical insight into regulators of root growth and development (Pauli et al., 2016; Ruiz-Munoz, Nimmagadda, Dowd, Baciak, & Zare, 2020; Seethepalli et al., 2020; Topp, Bray, Ellis, & Liu, 2016). Field-grown root systems, however, are obscured by soil and notoriously difficult to access, especially for crops with extensive crown roots such as maize. Thus, there is significant interest in phenotyping juvenile root traits to overcome the hurdle of extracting field-grown roots from the soil. In addition, examining juvenile maize seedlings grown under controlled environments enables the exploration of hormone and pharmacological treatments that are not possible under field-grown conditions. The ability to grow and collect uniform tissue samples for molecular and biochemical assays in the absence of field contaminants is also an advantage of these assays.
Root growth and development are regulated by several phytohormones (Liu, Moore, Chen, & Lindsey, 2017; Sharma et al., 2021; Yamoune, Cuyacot, Zdarska, & Hejatko, 2021). Specifically, auxin, brassinosteroid, cytokinin, and jasmonic acid have established roles in maize root development and represent the three biosynthetic phytohormone classes (amino acids, isoprenoid pathway, and lipids) (Ahmad et al., 2019; Best, Johal, & Dilkes, 2017; Rivas, Friero, Alarcón, & Salguero, 2022; Zhang et al., 2016).
The rolled towel assay (RTA) has been used for crops such as corn and soybean to assay numerous genotype-phenotype relationships (Bernardi et al., 2018; Ellis, Broders, Paul, & Dorrance, 2011; Kumar, Abdel-Ghani, Reyes-Matamoros, Hochholdinger, & Lübberstedt, 2012; Lanubile, Muppirala, Severin, Marocco, & Munkvold, 2015; Stagnati et al., 2019). The RTA for maize seedling growth is an efficient and space-saving protocol for growing and phenotyping maize seedlings.
Here, we have standardized the maize RTA to maximize germination rates, synchronize seedling growth, and minimize fungal contamination. In addition, we describe how to use this assay to perform reproducible and robust hormone response assays in maize seedlings. The detailed protocols provided herein include a basic rolled towel assay (Basic Protocol 1) and Basic Protocol 2 for performing hormone growth assays with four common growth regulators: indole-3-acetic acid (auxin), brassinolide (brassinosteroid), 5-benzylaminopurine (BAP, a synthetic cytokinin), or jasmonic acid. The initial planting steps in Basic Protocol 1 describe kernel sterilization, planting, scoring for germination, growth conditions for juvenile seedlings, seedling imaging, manual root trait annotation, and statistical analyses of the quantified traits. The hormone treatment protocol (Basic Protocol 2) describes best practices for initiating treatments, examples of treatment regimes, and data interpretation for hormone responses. Using these methods, researchers can obtain extensive phenotypic data for key traits such as primary root length, seminal root number, seminal root length, lateral root number, lateral root density, root hair density, total root area, mesocotyl length, shoot length, and leaf area.
The method described here allows researchers to scale up the number of genotypes and treatments performed as needed for rigorous statistical analysis and phenomic level studies (Bernardi et al., 2018; Ellis et al., 2011; Kumar et al., 2012; Marcon et al., 2015; Stagnati et al., 2019). These assays are optimized for controlled environment growth facilities but can also be modified for greenhouse settings.
Basic Protocol 1: MAIZE SEEDLING ROLLED TOWEL ASSAY AND PHENOTYPING
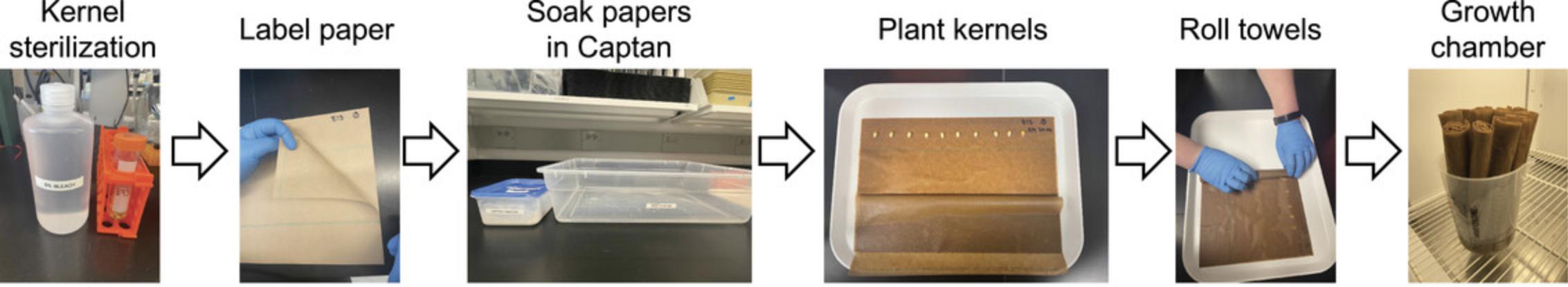
The rolled towel assay for maize seedling growth is an efficient and space-saving protocol for growing and phenotyping juvenile maize seedlings. This protocol can also be used to generate young maize tissue samples for molecular and biochemical assays. Here, we describe how to plant maize kernels, minimize fungal contamination, score for germination and synchronize seedling growth, perform imaging of seedlings, manually annotate traits (using ImageJ), and perform statistical analyses on the quantified traits. At the end of the process, the user will obtain high-quality images of juvenile maize seedlings that can be quantified and analyzed for phenotypes of interest. When done properly, there will be limited mold growth, and users will obtain healthy seedlings for observation and tissue collection for downstream applications. The key steps in this protocol are shown in Figure 1.
Materials
-
Maize kernels of the desired genotype
-
Bleach solution (see recipe)
-
Captan (Arysta Lifescience, EPA Reg: 66330-234, EPA Est. No: 054675-MEX-001, AD031214V2, 102120-B)
-
MQ water
-
0.5× Linsmaier & Skoog (LS) Medium (see recipe)
-
Sterilized deionized water
-
Sodium hydroxide pellets (Fisher BioReagents, BP359-500)
-
Sterilite 16428012 6QT Storage Box
-
Regular weight seed germination paper, 10 × 15 L (Anchor Paper Company, cat. no. SD3815L)
-
Sharpie
-
Microscope Slide Tough-Tags, 7/8″ × 7/8″, color: white (Diversified Biotech, cat. no. MISL-1000)
-
50-ml conical tubes (Fisherbrand, cat. no. 12-565-271)
-
Cafeteria Tray, MFG tray 3184031537 (18×14×1 in.), Fisher Scientific
-
Laminar flow hood (Labconco Purifier BSC Class II Type A2, or equivalent)
-
Single channel pipettes (20, 200, and 1000 µl) and tips
-
Nalgene polypropylene griffin low-form plastic beaker, 2 L (Fisher Scientific, cat. no. 02-591-10H)
-
Percival E-41L2 (or similar) growth chamber containing Philips F25T8/TL741, 25 W, 36″ bulbs
-
Smartphone camera or DSLR Camera- Canon EOS 80 D (W) with macro lens EF-S 35 mm 1:2.8 IS STM or equivalent
-
Flatbed Scanner-Epson Perfection V600 Photo
-
Ruler or Calibrite ColorChecker Passport Video
-
Photography light box
-
Tripod
-
Black or blue photography backdrops
-
ImageJ/Fiji release 2.5.0 (64-bit for Windows, macOS(x86_64), or 64-bit for Linux)
-
GraphPad Prism (or similar graphing software)
Planting and Seedling Growth
1.For each genotype, create a stack of three germination papers and label the middle paper in the upper right-hand corner. Write your initials, the date, and the genotype. The papers can be labeled with a Sharpie permanent marker or a preprinted ToughTag label.
2.For each genotype, count the number of required maize kernels and place them into a 50-ml conical tube. Set the tubes to the side.
3.Fill each conical tube with 50 ml of freshly prepared 6% (v/v) bleach solution and gently invert the capped tube 2-3 times to evenly distribute the sterilization solution over the kernels. Let the kernels sit in the solution for 10 min at room temperature.
4.While the kernels are being sterilized, make the Captan fungicide solution. The amount of Captan solution depends on the number of rolls being planted. Two (2) liters of Captan solution are sufficient for up to 30 rolls, and each roll will be planted with 10-12 kernels. Twelve rolls can fit into one 4 L beaker. To prepare 2 L of Captan solution, add 5 g of Captan to 2 L of MQ water. Mix the solution until at least some of the fungicide is dissolved. Because the fungicide is wettable, not all of the solid will fully dissolve in the water. Pour this solution into a plastic rectangular storage bin (such as a Sterilite 6QT Storage Box with the lid removed).
5.At this point, the 10-min kernel sterilization (step 3) should be done. Drain the bleach into a beaker.
6.Once all of the tubes are drained, wash each tube three times with sterilized deionized water. Invert the tubes a few times between each rinse to ensure that all of the kernels are fully rinsed.
7.After the kernels are rinsed, planting can begin. To start, pour the Captan solution into the plastic storage box.
8.Dip each stack of three papers into the Captan solution until the paper is soaked through. Pull a corner of the paper to lift the stack of germination papers to drain excess solution off the roll. While the paper should be wet, it should not be dripping.
9.Place the papers on a cafeteria tray or benchtop such that the width is shorter than the length (see Fig. 1). Fold back the first paper towards the long edge so that the label in the upper right-hand corner is visible (Fig. 1).
10.Place the maize kernels on the paper about 2.5 in. from the top. This does not need to be exact but ensures that the seedlings have optimal room for growth.
11.Plant 10 kernels on each roll, taking care to evenly space the kernels, and arrange them with the seed scar pointing down.
12.After the kernels have been placed on the roll, fold the top paper back to cover the kernels and ensure the papers are in line with each other.
13.Fold the far edge of the paper so that it covers the first kernel in line. Creating this initial fold helps to stabilize the rest of the roll.
14.To create the roll, hold the far edges of the paper tightly and begin to roll towards the other short edge. Roll tightly but not so tight that the paper is leaking water.
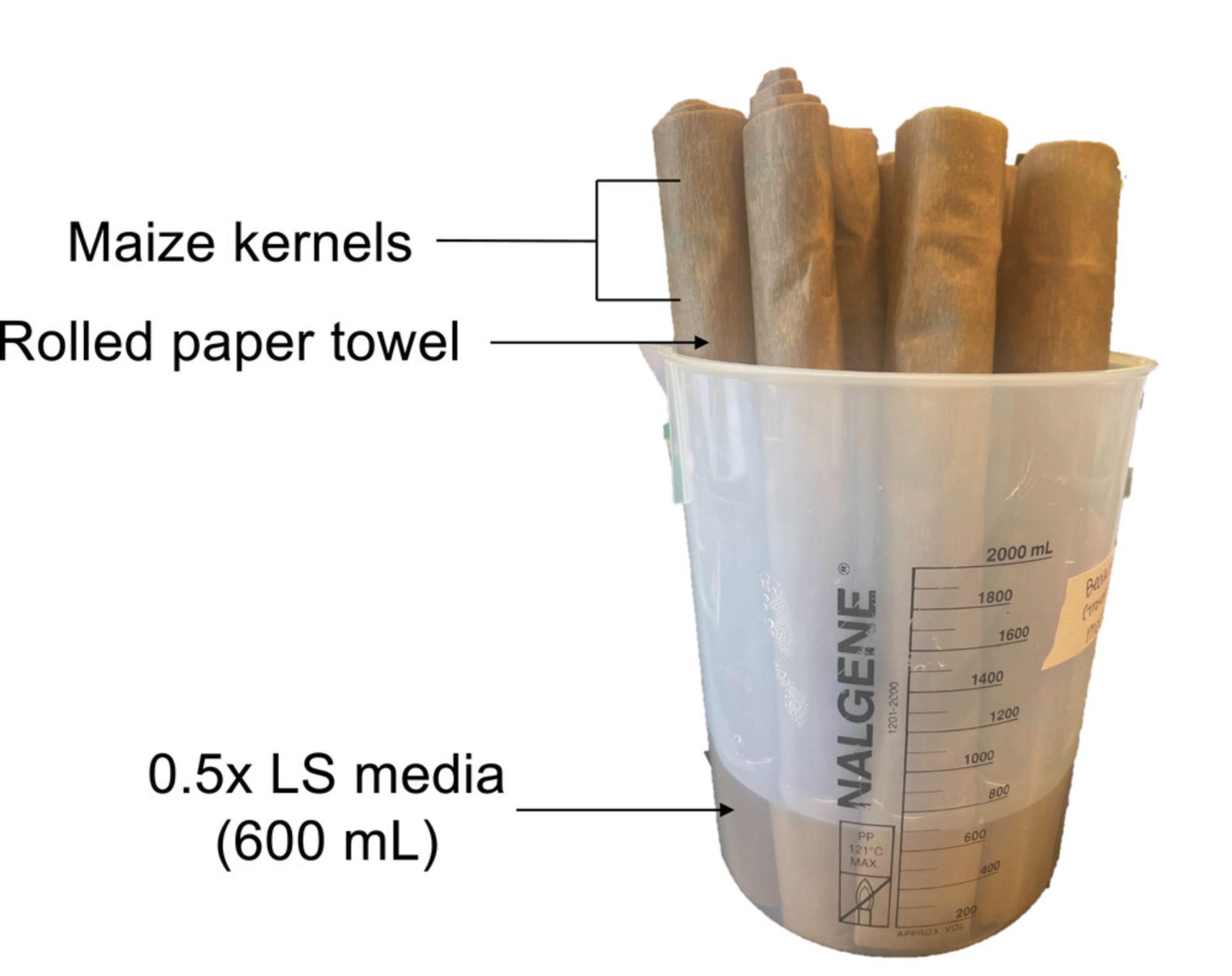
15.Once the roll is done, place the roll upright in a 2-L Nalgene beaker with 600 ml of 0.5× LS, as shown in Figure 2, and place the beaker in a Percival E-41L2 (or similar) growth chamber containing Philips F25T8/TL741, 25 W, 36″ bulbs set at 16/8 hr light/darkness at 23°C and relative humidity. One 2-L Nalgene beaker can fit up to 12 rolls and a Percival E-41L2 can hold 24 beakers.
16.Two days after planting, check the rolls for germination. To do this, unroll each roll and inspect the kernels. Repeat this process daily, at the same time each day, until ≥70% germination per roll has been reached. Remove any ungerminated kernels at this point to avoid non-synchronous growth. Record the first day of germination and keep track of the subsequent growth days as “days after germination,” here abbreviated as DAG.
17.Grow the seedlings for your desired amount of time, depending on the phenotype(s) of interest. Typically, primary root length can be scored beginning at 3 DAG and up to 10 DAG. Lateral root initiation can be scored in 5 to 6 DAG seedlings, while lateral root density should be calculated from 7 to 10 DAG seedlings. For growth assays longer than 2 DAG, replace the 0.5× LS every other day to ensure that the medium remains fresh. To do this, remove the towels from the beaker, pour off any old medium, and pour in 600 ml of fresh 0.5× LS.
Imaging
18.Observe and image seedling phenotypes beginning at one DAG and up to 12 DAG. Most maize genotypes will have extensive root systems that reach the length of the towel at this age.
19.To image, place the roll on a neutral background, such as a countertop or cafeteria tray. Unroll the roll to make the seedlings visible and remove the top sheet of paper. The brown seedling germination paper can serve as a neutral background for photography. Alternatively, the seedlings can be removed from the seedling germination paper and placed on a neutral (black or blue) background suitable for photography.
20.Use a cellphone or DSLR camera to image individuals or groups of seedlings, depending on your needs. Ensure that a ruler is in the frame of the picture for measurements. If a ToughTag was used to label the roll, then a ruler is unnecessary; the ToughTag can serve as a scale marker.
21.If imaging individual seedlings, do this on a neutral photography background (black or blue) using a DSLR camera, and repeat for each genotype and treatment.
22.After imaging the seedlings, collect root and shoot tissues if needed for downstream applications (such as DNA, RNA, or protein extraction). Alternatively, discard the seedlings according to laboratory and institutional procedures.
Image Annotation and Data Analysis
23.Open the image files in ImageJ/Fiji. Set the global scale using the ruler or ToughTag in the image by selecting Analyze > Set Scale. Input the known distance and units, then select “Global”. After the scale has been set for the image, measure the root lengths using the segmented line tool in ImageJ/Fiji. Click on each root (or shoot) and trace the length of the organ using the segmented line tool. Select “Analyze > Measure”. Repeat for every seedling. Export measured values to a spreadsheet.
24.To calculate lateral root number, open the image files and manually count the number of lateral roots observed per seedling and record the values. First, to calculate lateral root density, measure primary root length as described in step 23.then divide the number of lateral roots by the primary root length (cm).
25.Calculate the statistical significance for a given trait of interest using either a t-test statistic (if comparing two genotypes to one another) or an analysis of variance (ANOVA) if comparing genotype and treatment effects.
26.Plot and visualize the measured traits in GraphPad or similar.
Basic Protocol 2: MAIZE SEEDLING HORMONE RESPONSE ASSAYS USING THE ROLLED TOWEL ASSAY
Here, we describe how to use the maize RTA described in Basic Protocol 1 for hormone treatment assays. These experiments can evaluate hormone responses and dependent phenotypes. We outline the steps to perform these assays for brassinosteroids, cytokinin, jasmonic acid, and indole-3-acetic acid (auxin).
Brassinosteroids are classical plant hormones that play key roles in numerous aspects of maize growth and development (Liu et al., 2017; Sharma et al., 2021; Yamoune et al., 2021), including kernel size, drought tolerance, and mesocotyl elongation (Best et al., 2017; Castorina et al., 2018; Castorina & Consonni (2020); Hu et al., 2017; Kir et al., 2015; Kutschera & Wang, 2016; Liu et al., 2021; Sun et al., 2021). Cytokinins (CK) are key regulators of maize growth and development (Cho et al., 2022; Muszynski et al., 2020; Ramireddy et al., 2021; Rivas et al., 2022). Jasmonates are classical phytohormones associated with plant disease and developmental programs, including root elongation and leaf senescence (Huang, Liu, Liu, & Song, 2017; Yan, Huang, Borrego, & Kolomiets, 2014). Auxins are key plant phytohormones essential to the development of juvenile maize seedlings in regards to stress response, vasculature complexity, flower development, stress response, stem cell maintenance, and overall plant growth (Galli et al., 2015; Kutschera & Wang, 2016; LeClere, Schmelz, & Chourey, 2010; Rivas et al., 2022; Wang et al., 2021; Zhang et al., 2015; von Behrens et al., 2011; Gallavotti et al., 2008; Yao et al., 2019; Zhang et al., 2016).
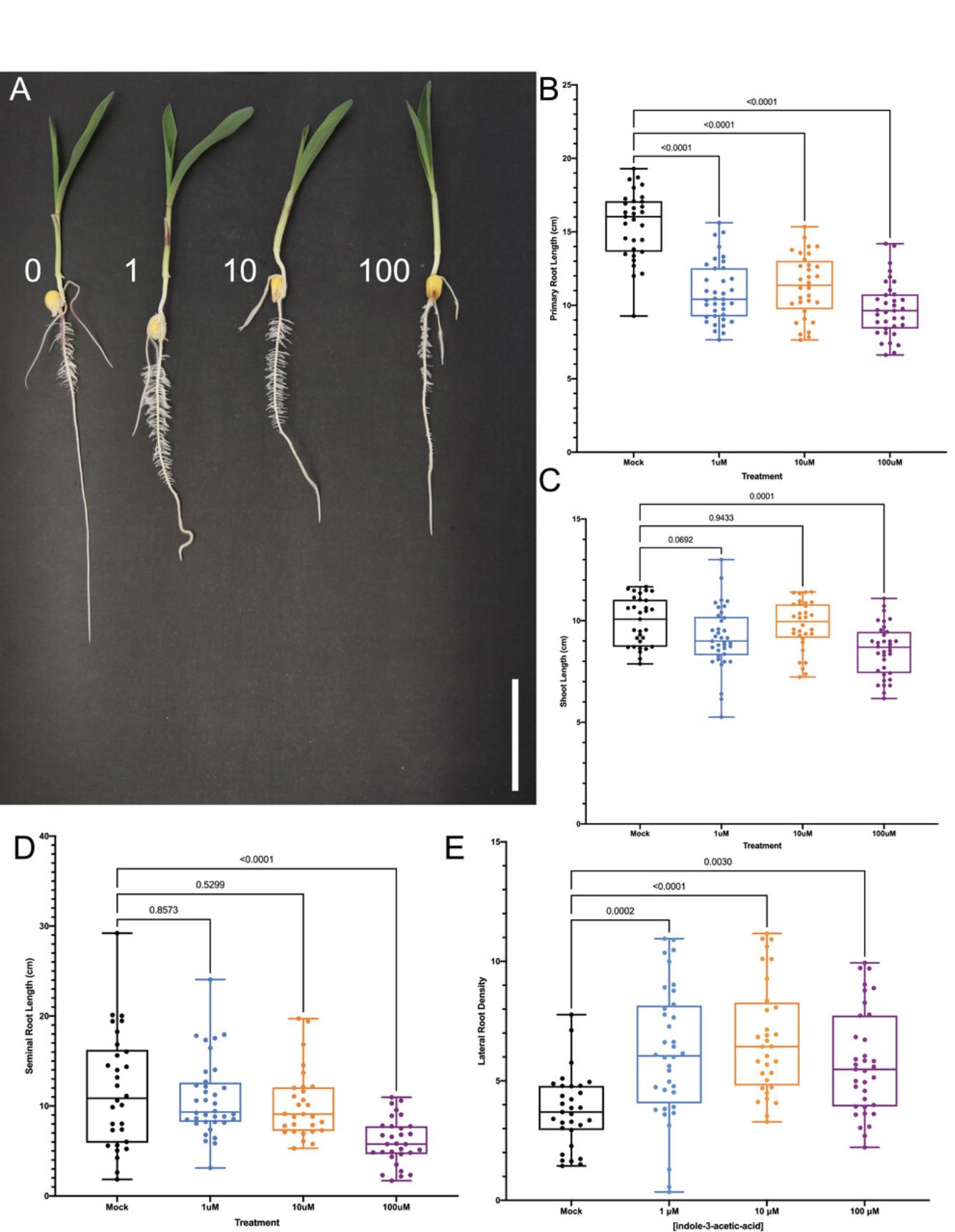
With these assays, users can evaluate numerous traits in response to a hormone of interest, including primary root length inhibition, increased seminal root number, and altered lateral root density (defined as the number of lateral roots per cm of primary root length).
Materials
- Same Materials as in Basic Protocol 1
- 1 M brassinolide (BL, see recipe)
- 10 mM 6-benzylaminopurine (BAP, see recipe)
- 50 mM methyl jasmonate (JA, see recipe)
- 10 mM indole-3-acetic-acid (IAA, see recipe)
Hormone Treatment
1.Begin by following Basic Protocol 1, steps 1-17.
2.At 3 DAG, prepare a fresh set of labeled, Captan-treated paper rolls as described in Basic Protocol step 4.This step is advisable to reduce mold growth and for easy record-keeping between treated and non-treated seedlings. Label fresh germination papers with the experimenter's initials, the initial date of germination, treatment (i.e., mock, BL, BAP, JA, or IAA), and genotype.
3.Divide the seedlings equally between two sets of labeled paper towels as described in step Basic Protocol 1, Step 18.For example, if 20 seedlings germinated, then 10 seedlings should be placed on the “mock” towels and 10 seedlings on the “BL” towels.
4.Prepare the appropriate hormone solution or the mock solvent (vehicle) controls, as follows (see also Table 1):
1.BL * For a 1 µM BL treatment, add 60 µl of a 10 mM BL stock to 600 ml of 0.5× LS medium in a 2-L Nalgene beaker. For the corresponding paired mock treatment, add 60 µl of 95% ethanol into 600 ml of 0.5× LS medium in a 2-L Nalgene beaker. For a 10 µM BL treatment, add 600 µl of a 10 mM BL stock into 600 ml of 0.5× LS medium in a 2-L Nalgene beaker. For the corresponding paired mock treatment, add 600 µl of 95% ethanol into 600 ml of 0.5× LS medium in a 2-L Nalgene beaker. For a 50 µM BL treatment, add 30 µl of a 1 M BL stock into 600 ml of 0.5× LS medium in a 2-L Nalgene beaker. For the corresponding paired mock treatment, add 30 µl of 95% ethanol into 600 ml of 0.5× LS medium in a 2-L Nalgene beaker. 2.BAP * For a 0.5 µM BAP treatment, add 30 µl of a 10 mM BAP stock to 600 ml of 0.5× LS medium in a 2-L Nalgene beaker. For the corresponding paired mock treatment, add 30 µl of 1 M NaOH into 600 ml of 0.5× LS medium in a 2-L Nalgene beaker. For a 2.5 µM BAP treatment, add 150 µl of a 10 mM BAP stock into 600 ml of 0.5× LS medium in a 2-L Nalgene beaker. For the corresponding paired mock treatment, add 150 µl of 1 M NaOH into 600 ml of 0.5× LS medium in a 2-L Nalgene beaker. For a 5 µM BAP treatment, add 300 µl of a 10 mM BAP stock into 600 ml of 0.5× LS medium in a 2-L beaker. For the corresponding mock treatment, add 300 µl of 1 M NaOH into 600 ml of 0.5× LS medium in a 2-L Nalgene beaker. 3.IAA * For a 1 µM IAA treatment, add 60 µl of a 10 mM IAA stock to 600 ml of 0.5× LS medium in a 2-L Nalgene beaker. For the corresponding paired mock treatment, add 60 µl of 95% ethanol into 600 ml of 0.5× LS medium in a 2-L Nalgene beaker. For a 10 µM IAA treatment, add 600 µl of a 10 mM IAA stock into 600 ml of 0.5× LS medium in a 2-L Nalgene beaker. For the corresponding paired mock treatment, add 600 µl of 95% ethanol into 600 ml of 0.5× LS medium in a 2-L Nalgene beaker. For a 100 µM IAA treatment, add 6 ml of a 10 mM IAA stock into 594 ml of 0.5× LS medium in a 2-L Nalgene beaker. For the corresponding paired mock treatment, add 6 ml of 95% ethanol into 594 ml of 0.5× LS medium in a 2-L Nalgene beaker. 4. JA * For a 5 µM JA treatment, add 60 µl of a 50 mM JA stock to 600 ml of 0.5× LS medium in a 2-L Nalgene beaker. For the corresponding paired mock treatment, add 60 µl of 95% ethanol into 600 ml of 0.5× LS medium in a 2-L Nalgene beaker. For a 50 µM JA treatment, add 600 µl of a 50 mM JA stock into 600 ml of 0.5× LS medium in a 2-L Nalgene beaker. For the corresponding paired mock treatment, add 600 µl of 95% ethanol into 600 ml of 0.5× LS medium in a 2-L Nalgene beaker. For a 100 µM JA treatment, add 1.2 ml of a 50 mM JA stock into 600 ml of 0.5× LS medium in a 2-L Nalgene beaker. For the corresponding paired mock treatment, add 1.2 ml of 95% ethanol into 600 ml of 0.5× LS medium in a 2-L Nalgene beaker.
| Hormone | Stock concentration | Solvent control | Root traits to score |
|---|---|---|---|
| Brassinolide | 1 M | 95% ethanol | Primary root length, seminal root number |
| BAP | 10 mM | NaOH | Primary root length, shoot length, lateral root number, lateral root density |
| Indole-3-acetic-acid | 10 mM | 95% ethanol | Primary root length, shoot length, seminal root length, lateral root density |
| Jasmonic acid | 50 mM | 95% ethanol | Primary root length |
5.Label each beaker with the corresponding mock or hormone treatment, along with your initials and the date of germination.
6.Next, replant the seedlings onto the freshly treated germination papers as described in step 18. Place the current roll containing 3 DAG seedlings on a cafeteria tray (or benchtop) and unroll it so the juvenile seedlings are visible. On a second tray, place the labeled fresh Captan-treated germination papers from step 18.
7.Wearing gloves, manually move the seedlings from the old stack of germination papers onto the new stack of papers, roll it up, and place the fresh rolls upright into the corresponding labeled beaker (i.e., mock or hormone). Each 2-L beaker can fit up to 12 rolls.
8.Continue growing the seedlings for an additional 4 to 9 days, replacing both the mock and hormone solutions with freshly prepared medium every other day to account for evaporation and to ensure constant uniform hormone treatment across the duration of the assay.
9.Repeat Basic Protocol 1 steps 23-26 for imaging, data analysis, and plotting.
REAGENTS AND SOLUTIONS
Use milli-Q (MQ) water for all preparations unless otherwise indicated.
6-Benzylaminopurine (BAP), 10 mM
- 0.11 g BAP (Sigma Aldrich, B3408-100 MG)
- 1 ml 1 M NaOH
- Store at −20°C for up to 1 month in 1-ml aliquots
Bleach solution, 6%
- 25 ml of standard household bleach
- 475 ml of MQ water
- Mix and store in 500-ml glass or plastic bottles such as Pyrex or Nalgene
- Store at room temperature for up to 6 months
Brassinolide (BL), 1 M
- 10 mg brassinolide (Millipore Sigma, cat. no. B1439)
- 1 ml 95% ethanol
- Store at −20°C for up to 1 month in 1-ml aliquots
Indole-3-acetic acid (IAA), 10 mM
- 35 mg indole-3-acetic acid (MP Biomedicals, cat. no. 102037)
- 20 ml 95% ethanol
- Store at −20°C for up to 1 month in 1-ml aliquots
Linsmaier & Skoog (LS), 0.5×
- 2.35 g Linsmaier and Skoog powder (Caisson, Fisher Scientific cat. no. NC1522332))
- 1 L MQ water
- Mix in a 1-L glass bottle
- Autoclave for 20 min
- Store at room temperature for up to 3 months
Methyl jasmonate (JA), 50 mM
- 0.29 µl methyl jasmonate, 95% solution (Sigma Aldrich, 392707-5 ML)
- 0.71 µl 95% ethanol
- Store at −20°C for up to 1 month in 1-ml aliquots
COMMENTARY
Background Information
Maize seedlings have been assayed and grown via a paper roll method for many years (Hochholdinger, 2009; Kumar et al., 2012). In the improved version described here, we include key details to ensure synchronous seedling growth, such as scoring for germination and removing ungerminated kernels, and treating both kernels and seed germination paper to minimize mold contamination. In addition, we provide new detailed hormone treatment assays using this rolled towel method across several tested concentrations of four key phytohormones. Specifically, these protocols allow for the monitoring of root traits in response to four key phytohormones: brassinosteroid, cytokinin, jasmonic acid, and indole-3-acetic acid (auxin). These hormones were chosen because they are central to plant growth and development.
With these established growth assays, researchers can perform forward and reverse genetic screens, examine the effects of exogenous hormone treatment on maize growth and development, and quantify juvenile phenotypes. These assays enable large-scale genome-wide association studies (GWAS), quantitative trait loci (QTL) approaches, phenomics, and gene expression analyses.
Critical Parameters
A key factor when growing maize seedlings on paper towel rolls is to synchronize the germination time in order to have standardized growth. To properly synchronize the germination day, the seedlings should be scored for germination every day after planting. Checking for germination at the same time each day can assist in synchronizing the kernels. Care should be taken to remove ungerminated kernels. In addition, seed quality can vary between batches, and germination rates will need to be determined for each seed lot.
A critical component of the hormone treatments is to replant the seedlings onto new, fungicide-treated seed germination papers at the start of the treatment regime. This step is laborious but can help immensely in combating fungal contamination.
Finally, the mock and hormone solutions must be replaced every other day during the treatment period to maintain optimal plant growth. It is also essential that hormone stocks are carefully prepared at the correct concentrations and stored under the recommended conditions; old (>1 month old) hormone stocks can lose efficacy.
Overall, the consistent timing of germination checks and medium replacement will aid in obtaining reproducible results for these protocols.
Troubleshooting
See Table 2 for a list of common problems with the protocols, their causes, and potential solutions.
| Problem | Possible cause | Solution |
|---|---|---|
| Poor kernel germination | Growth chamber temperature is suboptimal | Check growth conditions to ensure temperature is correct. |
| Poor seed (kernel) quality | Source alternative seeds for the desired genotype; re-bulk seed sources. | |
| Fungal contamination | Seeds not sterilized well or poor seed storage | Prepare fresh bleach solutions and ensure uniform paper wetting with Captan fungicide; transfer seedlings to fresh paper towels after germination. |
| Control (wild-type) seedlings do not show a hormone response | Old hormone stock solution | Prepare fresh stock solutions and note day to use by on the tube. |
| Hormone working solutions were incorrectly prepared | Double check your math and dilution calculations. | |
| Lack of statistical significance in control genotype(s) by treatments | Low replication number or wide variance in measured trait | Perform a power analysis to determine the smallest sample size that is suitable to detect the examine the phenotype of interest. |
Understanding Results
The growth assays described here will produce images from which traits (such as primary root length) can be quantified using software such as ImageJ/Fiji. Biological replicates across genotypes and treatments must be performed to enable a statistical analysis to inform conclusions (Brady et al., 2015); the number of biological replicates to be performed can vary between genotype and treatment, but a recommended starting point is to use 10 replicates per genotype or treatment. A power analysis can be performed prior to beginning the assay to ensure a sufficient number of replicates are assayed per genotype/treatment. Assays should be repeated independently at least three times to ensure reproducibility. It is also advisable to include control reference genotypes, such as B73, which exhibit known growth patterns and hormone responses, as presented in Figures 3-6. Data visualization can be performed using GraphPad Prism as described in Basic Protocol 1.
Sample data is presented for each hormone in this protocol. All data are interpreted as being significant based on the ANOVA results for each assay. Here, we ascribe statistical significance to any comparison with a p -value < 0.05.
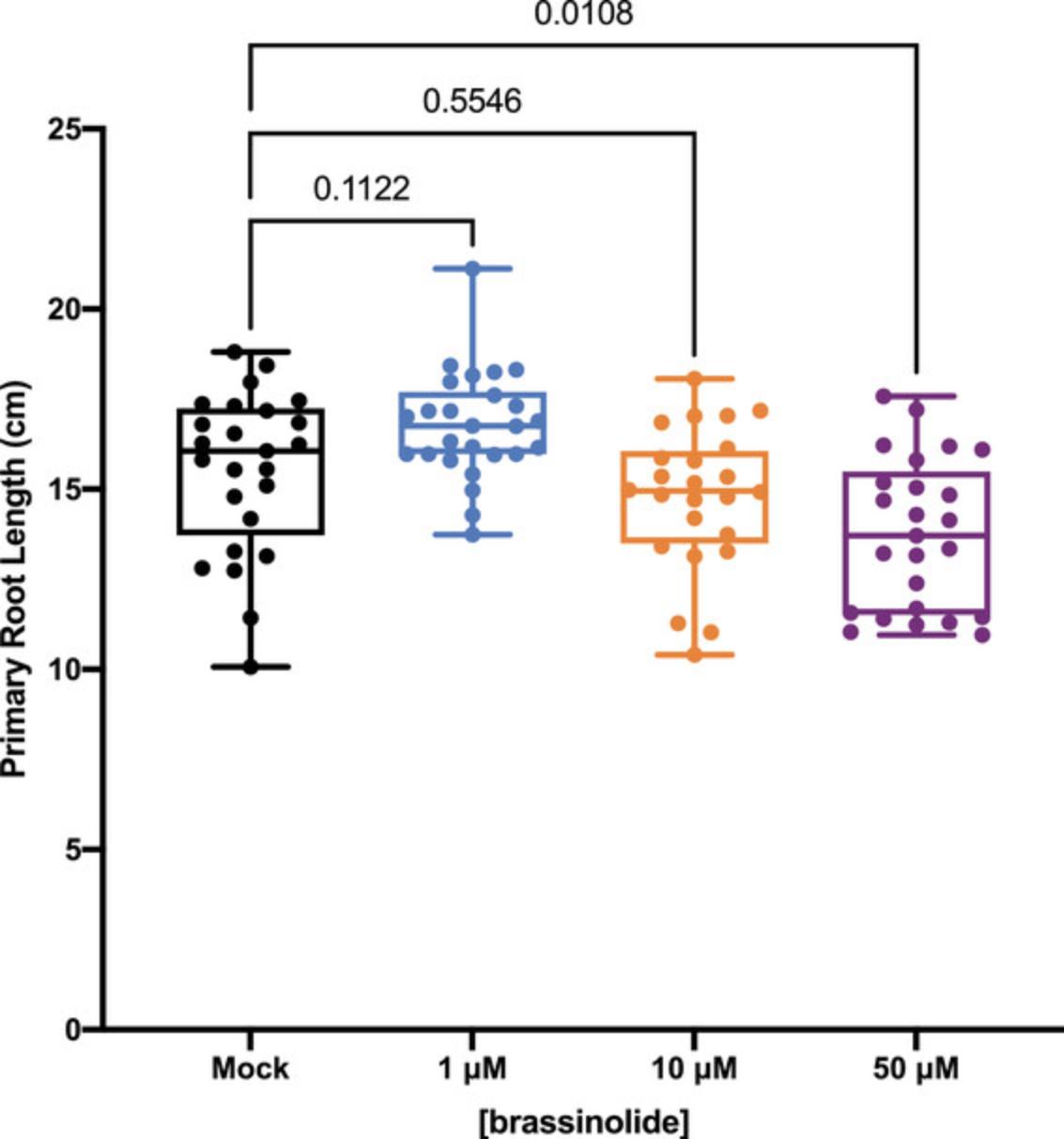
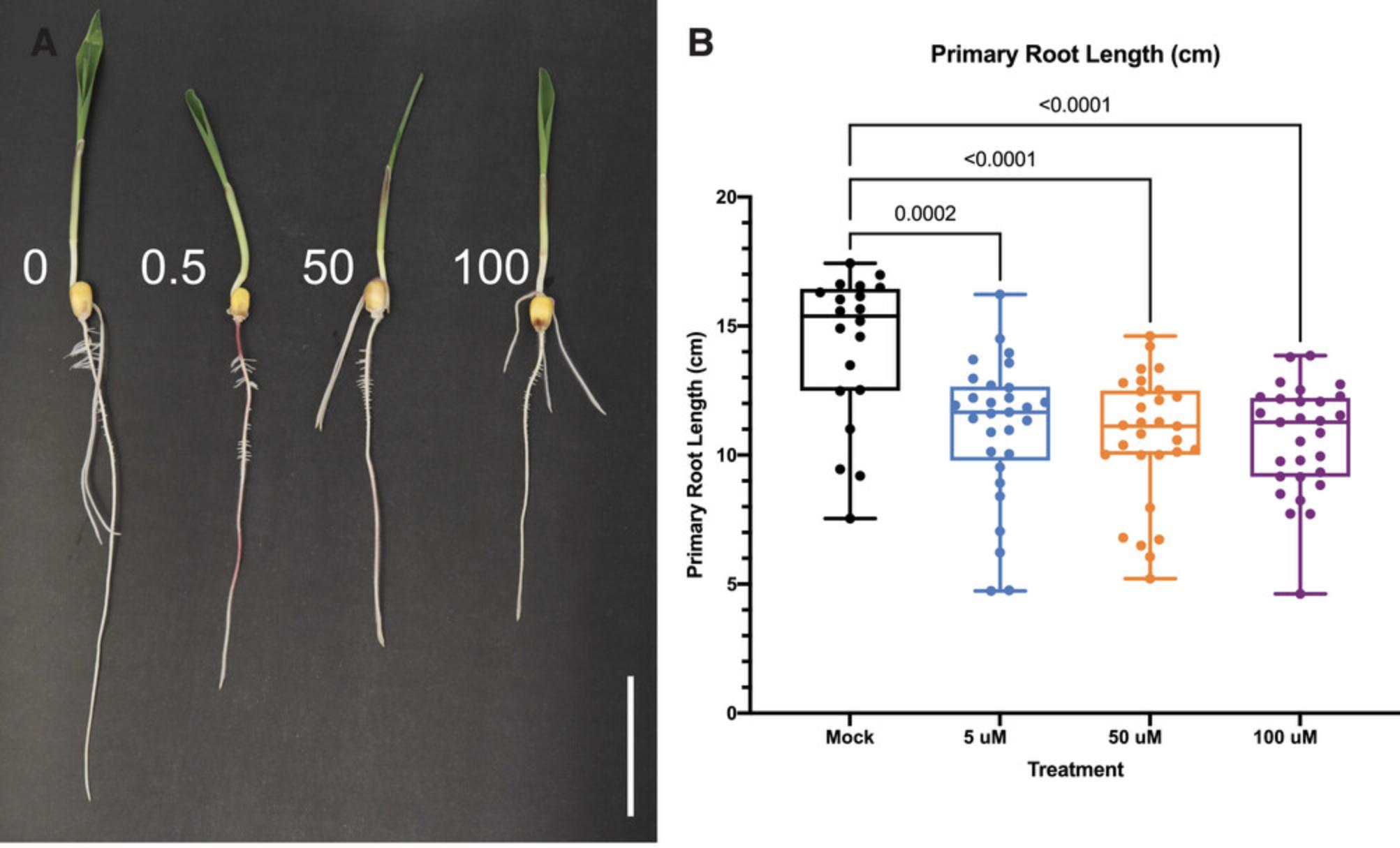
Figure 3 shows the effect of brassinosteroid (BL) treatment on 5-day-old maize seedlings (i.e., seedlings treated at 3 DAG for 2 days), showing how primary root length is inhibited after 50 μM BL treatment.
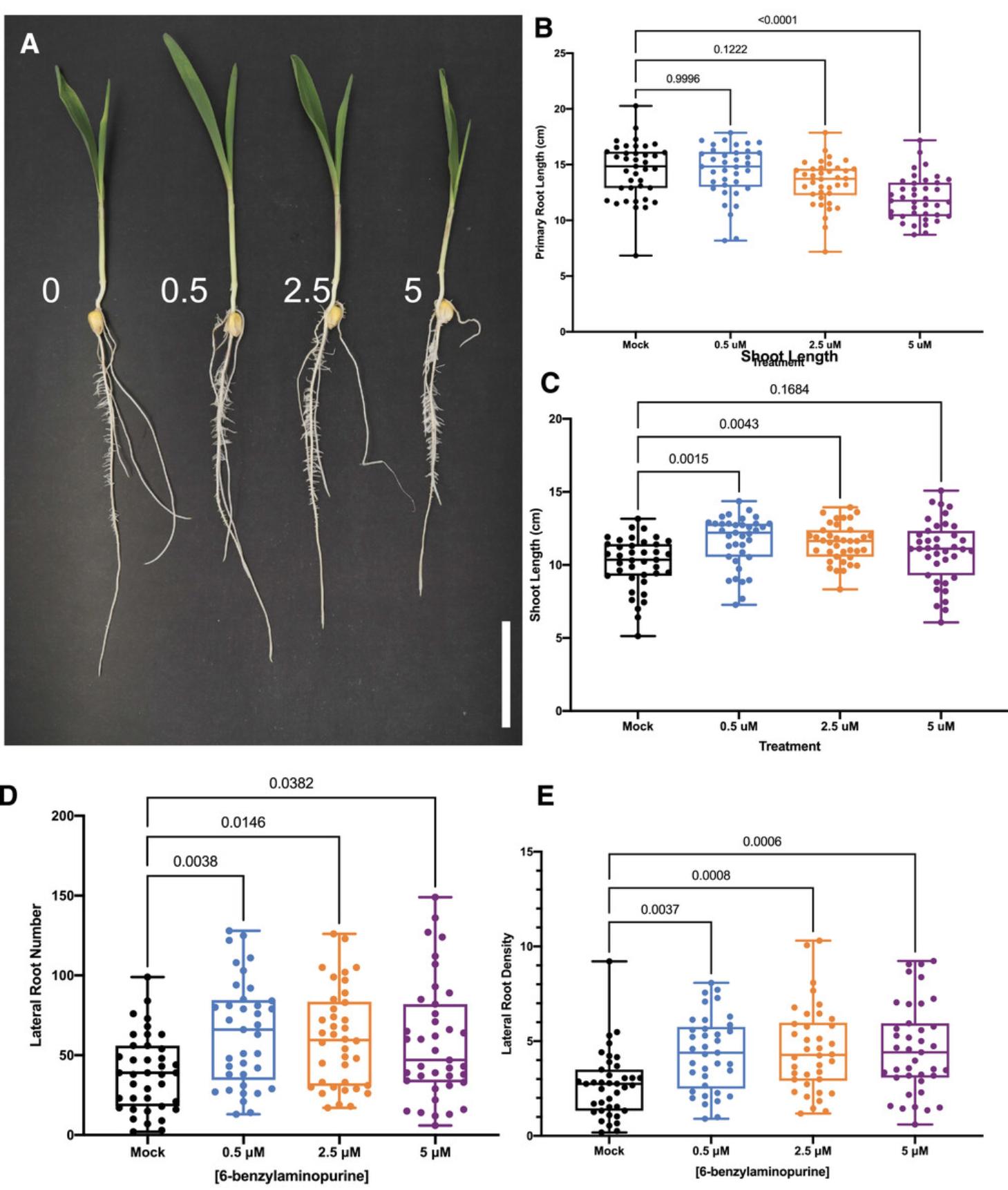
Figure 4 presents several key traits that are regulated by exogenous cytokinin. These data were obtained following Basic Protocol 2 and include inhibition of the primary root after 5 µM BAP treatment for 2 days (Fig. 4B) and increased lateral root density after 2 days of BAP treatment at all concentrations tested (Fig. 4E). Other observable phenotypes include an increase in lateral root number after BAP treatment for 2 days (Fig. 4D), and an increase in shoot length after 0.5 and 2.5 µM BAP treatment for 2 days (Fig. 4C).
Exogenous treatment with JA for 2 days at 5, 50, or 100 µM can lead to inhibition of primary root growth (Fig. 5B).
Key traits shown to be regulated by auxin include primary root length inhibition under 1, 10, and 100 µM indole-3-acetic acid (IAA) treatments after 2 days (Fig. 6B), inhibition of shoot length under 100 µM IAA treatment after 2 days (Fig. 6C), inhibition of seminal root number under 100 µM IAA treatment after 2 days (Fig. 6D), and increased lateral root density under 1, 10, and 100 µM IAA treatments after 2 days (Fig. 6E).
Time Considerations
Planting should usually take place earlier in the week to allow time for germination checks throughout the typical work week (i.e., Monday-Friday). Rolled towel assays typically take 2 to 2.5 weeks to complete from the initial planting date and require that the medium and hormone solution is replaced every other day. Thus, a planting, checking, and replacement schedule should be planned out prior to sterilization and planting.
Acknowledgments
This work is a product of the Iowa Agriculture and Home Economics Experiment Station, Ames, Iowa, Project No. IOW03649 and was sponsored by the Hatch Act, State of Iowa, and start-up funds from Iowa State University to Dior R. Kelley. DRK is also supported by the US Department of Agriculture National Institute of Food and Agriculture (NIFA) Agriculture and Food Research Initiative (AFRI) Grant no. 12907916.
Open access funding provided by the Iowa State University Library.
Author Contributions
Melissa A. Draves : Conceptualization, Formal analysis, Investigation, Methodology, Validation, Visualization, Original draft, review, and editing; Rebekah L. Muench : Conceptualization, Formal analysis, Investigation, Methodology, Validation, Visualization, Review and editing; Michelle G. Lang : Conceptualization, Methodology; Dior R. Kelley : Conceptualization, Project administration, Supervision, Original draft, review, and editing.
Conflict of Interest
The authors have no financial or personal relationships or supporting sponsors to disclose.
Open Research
Data Availability Statement
Raw data and metadata for the growth assays quantified in Figures 3-6 are available upon request.
Literature Cited
- Ahmad, R. M., Cheng, C., Sheng, J., Wang, W., Ren, H., Aslam, M., & Yan, Y. (2019). Interruption of jasmonic acid biosynthesis causes differential responses in the roots and shoots of maize seedlings against salt stress. International Journal of Molecular Sciences , 20, 6202. doi: 10.3390/ijms20246202
- von Behrens, I., Komatsu, M., Zhang, Y., Berendzen, K. W., Niu, X., Sakai, H., … Hochholdinger, F. (2011). Rootless with undetectable meristem 1 encodes a monocot-specific AUX/IAA protein that controls embryonic seminal and post-embryonic lateral root initiation in maize: AUX/IAA regulation of maize root development. The Plant Journal , 66, 341–353. doi: 10.1111/j.1365-313X.2011.04495.x
- Bernardi, J., Stagnati, L., Lucini, L., Rocchetti, G., Lanubile, A., Cortellini, C., … Marocco, A. (2018). Phenolic profile and susceptibility to Fusarium infection of pigmented maize cultivars. Frontiers in Plant Science , 9, 1189. doi: 10.3389/fpls.2018.01189
- Best, N. B., Johal, G., & Dilkes, B. P. (2017). Phytohormone inhibitor treatments phenocopy brassinosteroid–gibberellin dwarf mutant interactions in maize. Plant Direct , 1, pld3.9. doi: 10.1002/pld3.9
- Brady, S. M., Burow, M., Busch, W., Carlborg, Ö., Denby, K. J., Glazebrook, J., … Kliebenstein, D. J. (2015). Reassess the t test: Interact with all your data via ANOVA. The Plant Cell , 27, 2088–2094. doi: 10.1105/tpc.15.00238
- Castorina, G., & Consonni, G. (2020). The role of Brassinosteroids in controlling plant height in Poaceae: A genetic perspective. International Journal of Molecular Sciences , 21(4), 1191. doi: 10.3390/ijms21041191
- Castorina, G., Persico, M., Zilio, M., Sangiorgio, S., Carabelli, L., & Consonni, G. (2018). The maize lilliputian1 (lil1) gene, encoding a brassinosteroid cytochrome P450 C-6 oxidase, is involved in plant growth and drought response. Annals of Botany , 122, 227–238. doi: 10.1093/aob/mcy047
- Cho, L., Yoon, J., Tun, W., Baek, G., Peng, X., Hong, W., … An, G. (2022). Cytokinin increases vegetative growth period by suppressing florigen expression in rice and maize. The Plant Journal , 110(6), 1619–1635. doi: 10.1111/tpj.15760
- Ellis, M. L., Broders, K. D., Paul, P. A., & Dorrance, A. E. (2011). Infection of soybean seed by Fusarium graminearum and effect of seed treatments on disease under controlled conditions. Plant Disease , 95, 401–407. doi: 10.1094/PDIS-05-10-0317
- Gallavotti, A., Barazesh, S., Malcomber, S., Hall, D., Jackson, D., Schmidt, R. J., & McSteen, P. (2008). Sparse inflorescence1 encodes a monocot-specific YUCCA -like gene required for vegetative and reproductive development in maize. Proceedings of the National Academy of Sciences , 105, 15196–15201. doi: 10.1073/pnas.0805596105
- Galli, M., Liu, Q., Moss, B. L., Malcomber, S., Li, W., Gaines, C., … Gallavotti, A. (2015). Auxin signaling modules regulate maize inflorescence architecture. Proceedings of the National Academy of Sciences of the United States of America , 112, 13372–13377. doi: 10.1073/pnas.1516473112
- Hake, S., & Ross-Ibarra, J. (2015). Genetic, evolutionary and plant breeding insights from the domestication of maize. eLife , 4, e05861. doi: 10.7554/eLife.05861
- Hochholdinger, F. (2009). The maize root system: Morphology, anatomy, and genetics. In J.L. Bennetzen & S.C. Hake (Eds.), Handbook of maize: Its biology (pp. 145–160). New York, NY: Springer New York. doi: 10.1007/978-0-387-79418-1_8
- Hochholdinger, F., Yu, P., & Marcon, C. (2018). Genetic control of root system development in maize. Trends in Plant Science , 23, 79–88. doi: 10.1016/j.tplants.2017.10.004
- Hu, S., Sanchez, D. L., Wang, C., Lipka, A. E., Yin, Y., Gardner, C. A. C., & Lübberstedt, T. (2017). Brassinosteroid and gibberellin control of seedling traits in maize (Zea mays L.). Plant Science , 263, 132–141. doi: 10.1016/j.plantsci.2017.07.011
- Huang, H., Liu, B., Liu, L., & Song, S. (2017). Jasmonate action in plant growth and development. Journal of Experimental Botany , 68, 1349–1359. doi: 10.1093/jxb/erw495
- Kir, G., Ye, H., Nelissen, H., Neelakandan, A. K., Kusnandar, A. S., Luo, A., … Becraft, P. W. (2015). RNA interference knockdown of BRASSINOSTEROID INSENSITIVE1 in maize reveals novel functions for brassinosteroid signaling in controlling plant architecture. Plant Physiology , 169, 826–839. doi: 10.1104/pp.15.00367
- Kumar, B., Abdel-Ghani, A. H., Reyes-Matamoros, J., Hochholdinger, F., & Lübberstedt, T. (2012). Genotypic variation for root architecture traits in seedlings of maize (Zea mays L.) inbred lines: Genotypic variation for root traits in maize lines. Plant Breeding , 131, 465–478. doi: 10.1111/j.1439-0523.2012.01980.x
- Kutschera, U., & Wang, Z.-Y. (2016). Growth-limiting proteins in maize coleoptiles and the auxin-brassinosteroid hypothesis of mesocotyl elongation. Protoplasma , 253, 3–14. doi: 10.1007/s00709-015-0787-4
- Lanubile, A., Muppirala, U. K., Severin, A. J., Marocco, A., & Munkvold, G. P. (2015). Transcriptome profiling of soybean (Glycine max) roots challenged with pathogenic and non-pathogenic isolates of Fusarium oxysporum. BMC Genomics , 16, 1089. doi: 10.1186/s12864-015-2318-2
- LeClere, S., Schmelz, E. A., & Chourey, P. S. (2010). Sugar levels regulate tryptophan-dependent auxin biosynthesis in developing maize kernels. Plant Physiology , 153, 306–318. doi: 10.1104/pp.110.155226
- Liu, J., Moore, S., Chen, C., & Lindsey, K. (2017). Crosstalk complexities between auxin, cytokinin, and ethylene in Arabidopsis root development: From experiments to systems modeling, and back again. Molecular Plant , 10, 1480–1496. doi: 10.1016/j.molp.2017.11.002
- Liu, L., Xiang, Y., Yan, J., Di, P., Li, J., Sun, X., … Zhang, A. (2021). BRASSINOSTEROID-SIGNALING KINASE 1 phosphorylating CALCIUM/CALMODULIN-DEPENDENT PROTEIN KINASE functions in drought tolerance in maize. New Phytologist , 231, 695–712. doi: 10.1111/nph.17403
- Liu, Y., & von Wirén, N. (2022). Integration of nutrient and water availabilities via auxin into the root developmental program. Current Opinion in Plant Biology , 65, 102117. doi: 10.1016/j.pbi.2021.102117
- Marcon, C., Malik, W. A., Walley, J. W., Shen, Z., Paschold, A., Smith, L. G., … Hochholdinger, F. (2015). A high-resolution tissue-specific proteome and phosphoproteome atlas of maize primary roots reveals functional gradients along the root axes. Plant Physiology , 168, 233–246. doi: 10.1104/pp.15.00138
- Muszynski, M. G., Moss-Taylor, L., Chudalayandi, S., Cahill, J., Del Valle-Echevarria, A. R., Alvarez-Castro, I., … Brugière, N. (2020). The maize Hairy Sheath Frayed1 (Hsf1) mutation alters leaf patterning through increased cytokinin signaling. The Plant Cell , 32, 1501–1518. doi: 10.1105/tpc.19.00677
- Pauli, D., Chapman, S. C., Bart, R., Topp, C. N., Lawrence-Dill, C. J., Poland, J., & Gore, M. A. (2016). The quest for understanding phenotypic variation via integrated approaches in the field environment. Plant Physiology , 172(2), 622–634.
- Ramireddy, E., Nelissen, H., Leuendorf, J. E., Van Lijsebettens, M., Inzé, D., & Schmülling, T. (2021). Root engineering in maize by increasing cytokinin degradation causes enhanced root growth and leaf mineral enrichment. Plant Molecular Biology , 106, 555–567. doi: 10.1007/s11103-021-01173-5
- Rivas, M. Á., Friero, I., Alarcón, M. V., & Salguero, J. (2022). Auxin-cytokinin balance shapes maize root architecture by controlling primary root elongation and lateral root development. Frontiers in Plant Science , 13, 836592. doi: 10.3389/fpls.2022.836592
- Ruiz-Munoz, J. F., Nimmagadda, J. K., Dowd, T. G., Baciak, J. E., & Zare, A. (2020). Super resolution for root imaging. Applications in Plant Sciences , 8(7), e11374. doi: 10.1002/aps3.11374
- Seethepalli, A., Guo, H., Liu, X., Griffiths, M., Almtarfi, H., Li, Z., … York, L. M. (2020). RhizoVision crown: An integrated hardware and software platform for root crown phenotyping. Plant Phenomics , 2020, 3074916. doi: 10.34133/2020/3074916
- Sharma, M., Singh, D., Saksena, H. B., Sharma, M., Tiwari, A., Awasthi, P., … Laxmi, A. (2021). Understanding the intricate web of phytohormone signalling in modulating root system architecture. International Journal of Molecular Sciences , 22, 5508. doi: 10.3390/ijms22115508
- Stagnati, L., Lanubile, A., Samayoa, L. F., Bragalanti, M., Giorni, P., Busconi, M., … Marocco, A. (2019). A genome wide association study reveals markers and genes associated with resistance to Fusarium verticillioides infection of seedlings in a maize diversity panel. G3 Genes|Genomes|Genetics , 9, 571–579. doi: 10.1534/g3.118.200916
- Sun, F., Ding, L., Feng, W., Cao, Y., Lu, F., Yang, Q., … Yu, H. (2021). Maize transcription factor ZmBES1/BZR1-5 positively regulates kernel size. Journal of Experimental Botany , 72, 1714–1726. doi: 10.1093/jxb/eraa544
- Topp, C. N., Bray, A. L., Ellis, N. A., & Liu, Z. (2016). How can we harness quantitative genetic variation in crop root systems for agricultural improvement? Journal of Integrative Plant Biology , 58, 213–225. doi: 10.1111/jipb.12470
- Wang, Y., Sun, H., Wang, H., Yang, X., Xu, Y., Yang, Z., … Li, P. (2021). Integrating transcriptome, co-expression and QTL-seq analysis reveals that primary root growth in maize is regulated via flavonoid biosynthesis and auxin signal transduction. Journal of Experimental Botany , 72, 4773–4795. doi: 10.1093/jxb/erab177
- Yamoune, A., Cuyacot, A. R., Zdarska, M., & Hejatko, J. (2021). Hormonal orchestration of root apical meristem formation and maintenance in Arabidopsis. Journal of Experimental Botany , 72, 6768–6788. doi: 10.1093/jxb/erab360
- Yan, Y., Huang, P.-C., Borrego, E., & Kolomiets, M. (2014). New perspectives into jasmonate roles in maize. Plant Signaling & Behavior, 9, e970442.
- Yao, H., Skirpan, A., Wardell, B., Matthes, M. S., Best, N. B., McCubbin, T., … McSteen, P. (2019). The barren stalk2 gene is required for axillary meristem development in maize. Molecular Plant , 12, 374–389. doi: 10.1016/j.molp.2018.12.024
- Zhang, Y., von Behrens, I., Zimmermann, R., Ludwig, Y., Hey, S., & Hochholdinger, F. (2015). LATERAL ROOT PRIMORDIA 1 of maize acts as a transcriptional activator in auxin signalling downstream of the Aux/IAA gene rootless with undetectable meristem 1. Journal of Experimental Botany , 66, 3855–3863. doi: 10.1093/jxb/erv187
- Zhang, Y., Marcon, C., Tai, H., von Behrens, I., Ludwig, Y., Hey, S., … Hochholdinger, F. (2016). Conserved and unique features of the homeologous maize Aux/IAA proteins ROOTLESS WITH UNDETECTABLE MERISTEM 1 and RUM1-like 1. Journal of Experimental Botany , 67, 1137–1147. doi: 10.1093/jxb/erv519
Internet Resources
- ImageJ (also known as Fiji) Image Processing and Analysis in Java tool for measuring organ lengths.

