In vitro digestion method for Atlantic salmon
Gopika Radhakrishnan, marta Silva, Ikram Belghit
Abstract
This in vitro digestion method was used to evaluate amino acid solubility of different black soldier fly larvae (BSFL) meals and experimental diets for Atlantic salmon. Three types of insect meal that had been through different processing techniques included: a microwave full fat BSFL (BSFM), defatted BSFL meal with an enzymatic pre-treatment (BSFE) and a defatted BSFL meal without enzymatic pre-treatment (BSFH).
Steps
Extraction of crude salmon enzymes
Due to biological variability, use the viscera from at least 6 fish. The exact number of fish required will depend on the quantity of enzyme needed for the experiment.
Feed the fish 40 g of feed 4 hours before collecting tissues (e.g. commercial feed (Supreme Plus15, Skretting, crude protein 51%)).
After 4 h, the fish were anaethetised (e.g. imersing the fish in water containing 100 mg/L of MS222). This step was followed by a quick cephalic concussion.
Dissect the fish by separating stomach and pyloric ceca + intestine.

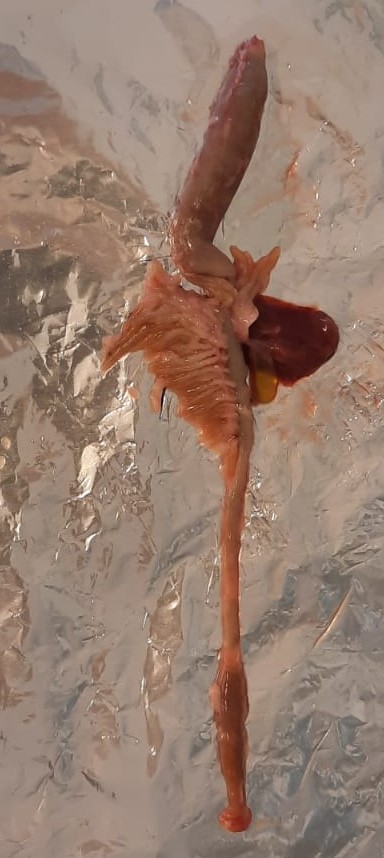
Note the pH of the stomach and intestines before the excision.
Note: All the steps from 4 to 6 should be performed at low temperature, by possible keeping on ice.
Wash the stomach and the intestine along with pyloric caeca with cold milliQ water.
Note: This step is meant to remove blood stains and fat.
Chop the tissues into smaller pieces and homogenise with cold (4°C) milliQ water in 1:10 ratio using a tissue homogeniser (Polytron PT 2100).

Note: To prevent overheating, the homogenization was conducted in several 30-second pulses. Throughout this process, the homogenate was kept in a glass beaker on ice to preserve the integrity of the tissue's proteins and enzymes.
Collected supernatant which constituted the crude enzyme extract were stored at -80°C until further use. Discard pellet (these are mostly tissue debris).
Dialysis
Prior to measuring enzyme activity or conducting in vitro experiments, the crude enzyme extracts were further concentrated through dialysis using 10 MWCO dialysis tubes.
Determination of pepsin and protease activity
The assay was initiated by adding 5 mL of the substrate into the glass tubes named blank and test. All the tubes were placed at 37°C for approximately 10 min to equilibrate.
This was followed by addition of 1 mL of enzyme solution into the test tubes and were placed at 37°C for 10 min to incubate.
One unit of pepsin activity was defined as the change in absorbance of 0.001 per min. at pH 2 at 37°C measured as TCA soluble products. All measurements were conducted in duplicate.
Determination of protease activity
In this assay, the protease activity of the stock solution was measured using casein as the standard substrate.
To begin with, 20 µL of enzyme solution was mixed with 0.5 mL of 0.1M Tris-HCl buffer (pH 8) at room temperature.
The reaction was initiated by the addition of 0.5 mL of 1% casein and kept for 30 min.
Afterwards, the reaction was terminated by adding 0.5 mL of 20% TCA. The mixtures were then left to stand for 10 minutes at room temperature, followed by centrifugation at 16500×g for 5 minutes at 4°C.
The absorbance of the reaction mixture was read at 280 nm using a UV-VIS Spectrophotometer (Shimadzu, Model: UV-1800). Enzyme activity was defined as the release of 1 µg of tyrosine per minute, as per Walter (1984). All measurements were conducted in duplicate.
In vitro digestion of feed ingredients and diets
The in vitro digestion method included two steps: acidic and alkaline hydrolysis which is meant to correspond to the conditions in the stomach and the intestine, respectively.
An appropriate amount of sample, equivalent to approximately 80 mg of protein, was weighted in a round bottom tube (13 mL).
An acidic (0.01N HCl, pH 2) and an alkaline solution (0.01N NaOH, pH 8) were prepared by diluting HCl and a weighted amount of NaOH in Milli-Q water.
After 1 h, a set of samples were stopped after the first step of digestion (gastric simulation, acidic hydrolysis) and kept for further analysis, while another set of samples were processed for the second step of digestion (gastrointestinal simulation, acid hydrolysis followed by alkaline hydrolysis).
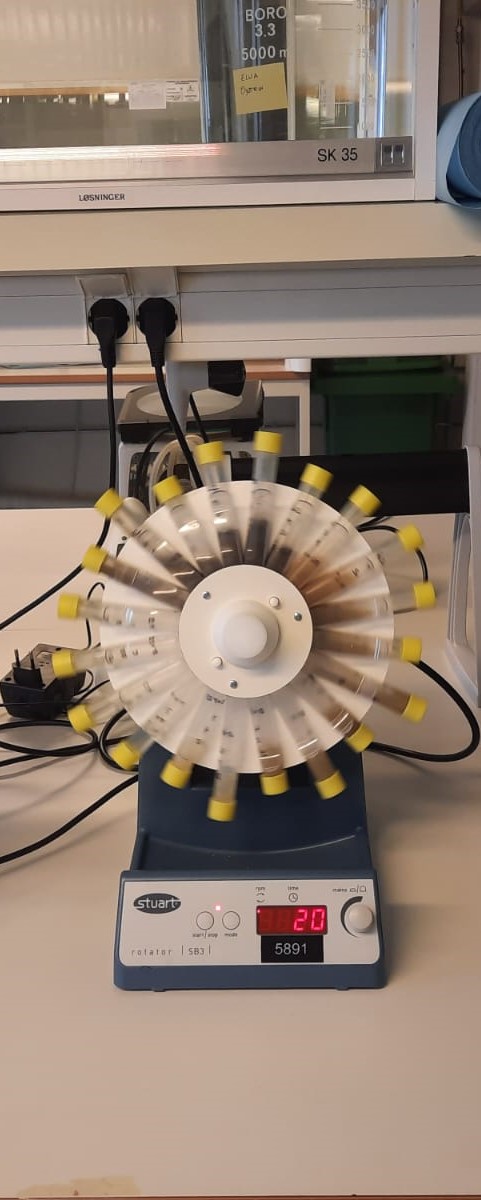
The second step of digestion was started by incubating the samples with the 3.3 mL of intestinal crude enzyme and alkaline solution to make up to a volume of 10 mL. This mixture was again allowed to stand for 1 h at room temperature under continuous rotation (20 rpm) by keeping the ratio of 5U of pepsin or protease per mg of protein.
After removing the samples from the rotator, they were submitted to centrifugation (3000 g, 10 min) and the soluble fractions were transferred to new tubes. All tubes were immediately placed on ice to stop the enzyme activity.
Note: In this experiment, a set of tubes without sample was included (blanks). The purpose of these tubes is to evaluate background inputs from enzymes and working solutions. All samples should be studied at least in duplicates.
For the ingredients and diets, the soluble fractions and non-soluble samples were collected from the gastric simulation phase (acid hydrolysis, GS) and from the gastrointestinal simulation phases (acid hydrolysis followed by alkaline hydrolysis, GIS). Samples were stored at -20°C before analysis.