Slide Preparation and Transect Counting of Paleoecological Microfossils - SPaTCoPM
Jacopo Niccolo Cerasoni, Megan C. O'Toole, Richa Patel
Abstract
Paleoecology attempts to reconstruct history through geochemical isotopic studies, trace fossil analyses, examination of microbe communities, and the presence or lack of microfossils. Ancient lakes are useful for reconstructing paleoecology because they accumulate sediment through time. Paleobotanical microfossils, including diatoms and phytoliths, allow inference of vegetation, pH, salinity, water chemistry, and environmental temperature, among others. Here, we propose a protocol split into two sections to prepare slides and count diatoms and phytoliths along transects. Researchers can develop an ecological history by analyzing microfossils from different locations and times. As an example, we apply this method to study the palaeobotanical microfossils of an 820 cm deep stratigraphy, composed of 10.3my Miocene diatomite, from a high-elevation desert paleolake in Northern Nevada (Fernley District). The preliminary results hint at a complex climatic and environmental variance. Another application of this method includes the development of environmental interpretations and hypotheses regarding the future of modern vegetation.
Before start
Make a database of all samples and an orderly sample inventory. Include important information such as country, site, feature, level, etc., to help identify the samples. Give each sample a unique label code (using SITE, YEAR, or ID NUMBER). Digitally create and print an appropriate form for logging observations made while samples are prepared in the lab.
Steps
Slide Preparation
Using a needle tool, grind the sample inside the falcon tube to create a fine powder.
Using the needle tool, put about 6mg of the sample onto the slide.

Place a COVER SLIP onto the slide and let it sit for about 30 seconds.

Store slides according to needs.
Microscopy (Brightfield) and Transect Counting
Use a compound microscope, binocular or trinocular, for brightfield viewing (light placed below the sample, viewing from above).
A polarizing lens will help discern shapes and features in phytoliths and diatoms.
A magnification of 40x is good for the initial observation. For individual identification of microfossils and microscope photography, a magnification of 100x will be required.
Up to five transects are recommended. The total number of counted transects depends on microfossil density of the specific sample.
The first transect is placed at 0.5mm distance from the upper and lateral sides of the cover slide. All other transects are placed 5mm below the previous transect.
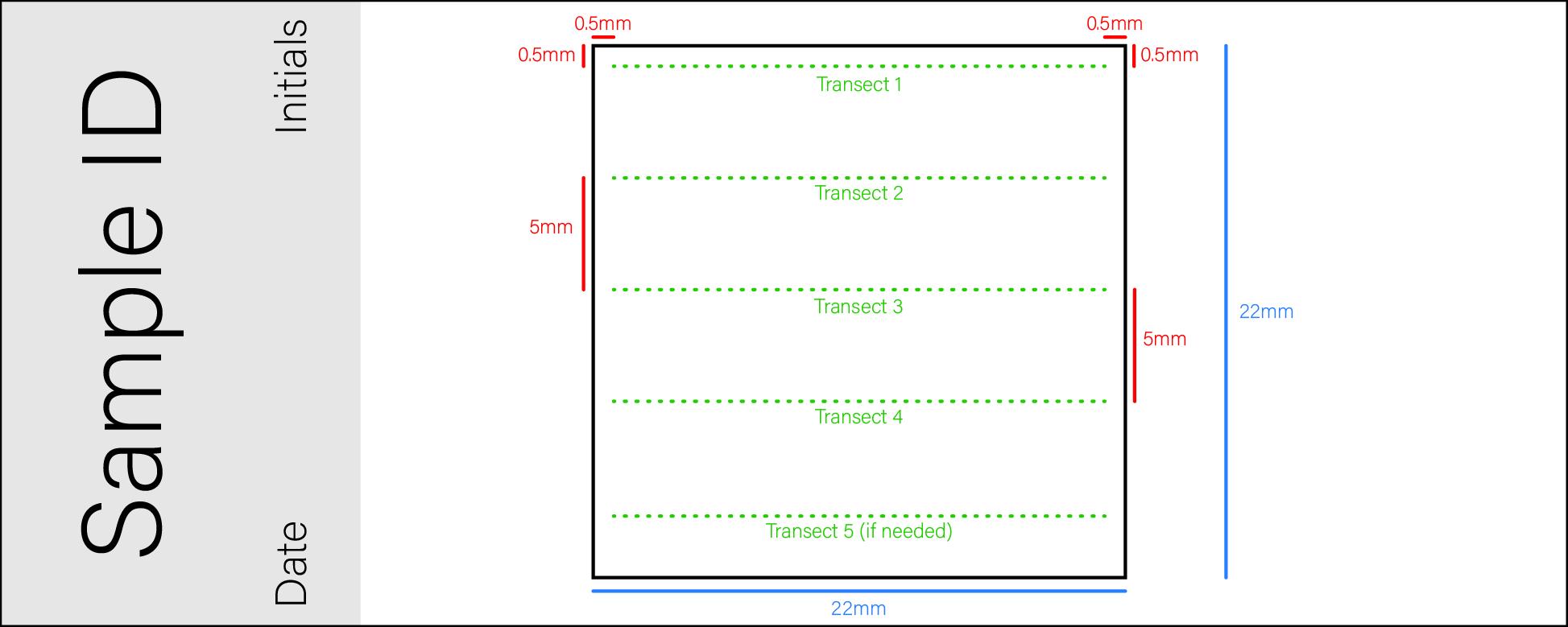
Start the first transect at 0.5 mm down and 0.5 mm to the right of the top left corner of the slide.
Go across the slide, horizontally, until reaching the end of the slide. Count diatom and phytolith morphotypes present in the field of view.
Start the second transect 5 mm below the first. This would be at 5.5 mm down the slide, and still 0.5 mm to the right of the top left corner. Continue counting microfossils across the transect using the clicker.
Start the third transect 5 mm below the second, now at 10.5 mm down from the top of the slide, and still 0.5 mm to the right. Continue counting microfossils across the transect using the clicker.
Start the fourth transect 5 mm below the third, now at 15.5 mm down from the top of the slide, and still 0.5 mm to the right. Continue counting microfossils across the transect using the clicker.
If necessary, start the fifth transect 5 mm below the fourth, now at 20.5 mm down from the top of the slide, and still 0.5 mm to the right. Count the microfossils across the transect using the clicker.
Once all microfossils are counted along each transect, ensure no novel morphotypes exist in the rest of the slide. Scan the entire slide for microfossils that have yet to be counted and are of a different morphotype than what has been counted thus far. Identify the new morphotype rather than count it.
Note the number of each morphotype in each sample in an Excel database.
Final Results
A complete spreadsheet will be produced from the application of this method, with microfossil counts represented by type and/or morphotype as separate transects per each sample. Below is an example of a completed count for a single sample.
| A | B | C | D | E | F | G | H |
|---|---|---|---|---|---|---|---|
| Microfossil_Type | Morphotype | Genus_Type | Species | Variety | Sample_ID | Transect | Count |
| Diatom | A | 1 | a | x | ID_0001 | 1 | 7 |
| Diatom | B | 2 | b | x | ID_0002 | 1 | 8 |
| Diatom | C | 3 | c | x | ID_0003 | 1 | 2 |
| Phytolith | D | 4 | d | x | ID_0004 | 1 | 43 |
| Phytolith | E | 5 | e | x | ID_0005 | 1 | 12 |
| Diatom | A | 1 | a | x | ID_0001 | 2 | 3 |
| Diatom | B | 2 | b | x | ID_0002 | 2 | 90 |
| Diatom | C | 3 | c | x | ID_0003 | 2 | 35 |
| Phytolith | D | 4 | d | x | ID_0004 | 2 | 14 |
| Phytolith | E | 5 | e | x | ID_0005 | 2 | 76 |
| Diatom | A | 1 | a | x | ID_0001 | 3 | 35 |
| Diatom | B | 2 | b | x | ID_0002 | 3 | 76 |
| Diatom | C | 3 | c | x | ID_0003 | 3 | 21 |
| Phytolith | D | 4 | d | x | ID_0004 | 3 | 1 |
| Phytolith | E | 5 | e | x | ID_0005 | 3 | 56 |
| Diatom | A | 1 | a | x | ID_0001 | 4 | 1 |
| Diatom | B | 2 | b | x | ID_0002 | 4 | 0 |
| Diatom | C | 3 | c | x | ID_0003 | 4 | 57 |
| Phytolith | D | 4 | d | x | ID_0004 | 4 | 23 |
| Phytolith | E | 5 | e | x | ID_0005 | 4 | 1 |
Example table of final results. The table structure was based on Wickham (2014).Reference: Wickham, H. (2014). Tidy data. Journal of statistical software, 59, 1-23.