Acute Myeloid Leukemia Minimal Residual Disease Detection: The Difference from Normal Approach
Brent L. Wood, Brent L. Wood
Abstract
The identification of residual leukemia following therapy, termed minimal or measurable residual disease (MRD), has emerged as one of the most important prognostic factors for patients with acute leukemia, including acute myeloid leukemia (AML). Flow cytometry is a preferred method for MRD detection due to its general applicability and the rapid results that it makes available. In this article, the basic protocol outlines a simple and efficient method for the labeling of hematopoietic cells from bone marrow or peripheral blood with a panel of monoclonal antibodies designed both to highlight patterns of normal maturation and allow identification of neoplastic hematopoietic progenitor populations with a high degree of sensitivity and specificity. The method was developed in a clinical laboratory setting for the diagnosis of myeloid stem cell disorders and neoplasms, and has been extensively validated both technically and clinically for the detection of MRD in AML. © 2020 The Authors.
Basic Protocol : Staining and flow cytometry for AML minimal residual disease detection
Support Protocol : Analysis and interpretation of data for AML minimal residual disease detection
INTRODUCTION
The detection of residual acute myeloid leukemia (AML) after therapy by flow cytometry is dependent on identifying immunophenotypic differences between leukemic cells and discrete stages of normal hematopoietic maturation. This implies the ability to observe normal stages of hematopoietic maturation in order to make this assessment. It also requires that immunophenotypic abnormalities that are different from normal maturation be present on the leukemic cells. Such differences may take the form of alterations in antigen intensity for antigens normally expressed by cells of a particular lineage and maturational stage, abnormalities in the timing of expression relative to a maturational sequence, or expression of antigens not normally expressed by a particular maturational stage or lineage of cells, e.g., lymphoid antigens expressed on myeloid progenitors. The assay described in this protocol is designed to allow the observation of maturation from hematopoietic stem cells along each of the major hematopoietic cell lineages (neutrophilic, monocytic, erythroid, B cell, plasmacytoid dendritic cell, and basophil) as a reference point for the identification and enumeration of discrete populations of leukemic cells that differ from normal maturation.
The Basic Protocol describes the procedure for labeling bone marrow or peripheral blood cells with a combination of antibodies that allow simultaneous dissection of normal hematopoietic maturation and identification of residual AML. Also included is a Support Protocol discussing the approaches used for interpretation of the resulting flow cytometric data. Reagents and Solutions outlines the preparation of solutions required for the Basic Protocol to be successfully executed. The Commentary discusses critical features of the assay, including troubleshooting, comparison with the leukemia-associated immunophenotype (LAIP) approach, and time requirements.
Basic Protocol: STAINING AND FLOW CYTOMETRY FOR AML MINIMAL RESIDUAL DISEASE DETECTION
The following method was devised to minimize sample manipulation and maximize recovery for all white blood cell populations while providing low fluorescence background and preserving the spectral characteristics of tandem fluorochromes. The addition of a low concentration of fixative to the lysing solution allows for the simultaneous lysing and fixing of the sample and preservation of the light scatter properties of the sample, and provides improved separation of compromised from viable cells. Addition of a subsequent wash step reduces background fluorescence due to unbound fluorochrome and provides more controlled timing of fixation, which enhances the stability of certain tandem fluorochromes. The procedure outlined is generic and can be used for any surface immunophenotyping assay.
Antibody panel
The AML MRD assay consists of three antibody combinations that have been highly optimized to minimize antibody interactions and provide optimal antigen intensities with low background. The antibody combinations may be acquired on any flow cytometer capable of detecting the fluorochromes indicated—in our laboratory a BD LSRII equipped with blue, red, violet, and yellow lasers—or with substitution of the CD38 conjugate, other suitable 10+ color cytometers lacking a yellow laser, e.g., BC Navios. Each lot of antibody must be individually titered for optimal signal-to-noise and binding saturation prior to being put into use. The antibody combinations may be pipetted as individual antibodies, but creation of antibody cocktails is advised to minimize pipetting errors and provide consistent reagent performance.
The M1 reagent combination (Table 1) is designed to observe the early maturation of hematopoietic stem cells (CD34+, CD38low) into each of the major cell lineages. The M2 reagent combination (Table 2) is designed to observe the maturation of early myelomonocytic precursors to later-stage mature forms, and includes identification of plasmacytoid dendritic cells and basophil cell lineages. The M3 reagent combination (Table 3) is designed to identify the abnormal expression of T and NK cell−associated lymphoid antigens on progenitor populations, a common type of abnormality in AML. In particular, the inclusion of CD34 and CD38 in each combination allows the assessment of all antigens on hematopoietic stem cells—a small normal subpopulation commonly showing immunophenotypic abnormality in AML that is of potential therapeutic and prognostic significance. Together, these three tubes form a comprehensive strategy for the identification of residual AML.
| M1 | Fluorochrome | Clone | Vendor | Catalog no. |
|---|---|---|---|---|
| CD13 | PC7 | My7 | BC | Custom |
| CD15 | FITC | MMA | BD | 340703 |
| CD19 | PE-CF594 | HIB19 | BD | 562294 |
| CD33 | PE | P67.6 | BD | 340679 |
| CD34 | APC | 8G12 | BD | 340667 |
| CD38a | A594 | HB-7 | BD | Custom |
| CD45 | APC-H7 | 2D1 | BD | 641408 |
| CD71 | APC-A700 | YDJ1.2.2 | BC | Custom |
| CD117 | PC5 | 104D2D1 | BC | IM2733U |
| HLA-DR | PB | Immu-357 | BC | A74781 |
- a
Substitute CD38 BV510 (Clone HB-7; BioLegend, #356612) for instruments lacking a yellow (594 nm) laser.
| M2 | Fluorochrome | Clone | Vendor | Catalog no. |
|---|---|---|---|---|
| CD4 | ECD | SFCI12T4D11 | BC | 6604727 |
| CD13 | PC7 | My7 | BC | Custom |
| CD14a | PURE | RMO52 | BC | IM0643 |
| CD14 | PC5.5 | RMO52 | BC | A70204 |
| CD16 | APC-A700 | 3G8 | BC | B20023 |
| CD34 | APC | 8G12 | BD | 340667 |
| CD38b | A594 | HB-7 | BD | Custom |
| CD45 | APC-H7 | 2D1 | BD | 641408 |
| CD64 | FITC | 22 | BC | IM1604U |
| CD123 | PE | 7G3 | BD | 554529 |
| HLA-DR | PB | Immu-357 | BC | A74781 |
- a
PURE antibody is used to reduce the intensity of CD14 while maintaining antibody saturation. The desired level of CD14 intensity is about 1 log lower than maximum intensity on the instrument used.
- b
Substitute CD38 BV510 (Clone HB-7; BioLegend, #356612) for instruments lacking a yellow (594 nm) laser.
| M3 | Fluorochrome | Clone | Vendor | Catalog no. |
|---|---|---|---|---|
| CD5 | PC5 | BL1a | BC | IM2637U |
| CD7 | PE | M-T701 | BD | 340656 |
| CD33 | PC7 | P67.6 | BD | 333949 |
| CD34 | APC | 8G12 | BD | 340667 |
| CD38a | A594 | HB-7 | BD | Custom |
| CD45 | APC-H7 | 2D1 | BD | 641408 |
| CD56 | A488 | B159 | BD | 557699 |
| HLA-DR | PB | Immu-357 | BC | A74781 |
- a
Substitute CD38 BV510 (Clone HB-7; BioLegend, #356612) for instruments lacking a yellow (594 nm) laser.
If antibody cocktails are created, a procedure should be used to verify that each antibody has been added to the cocktail and that the performance of each antibody is as expected. This may be easily done by running the cocktail on a normal bone marrow sample and comparing the results to results from a prior sample prepared using another lot of cocktail, or to results obtained with individually pipetted reagents. The detailed procedure for performing the verification is outside the scope of this protocol.
Support Protocol: ANALYSIS AND INTERPRETATION OF DATA FOR AML MINIMAL RESIDUAL DISEASE DETECTION
The analysis of data produced by this assay is complex and requires an experienced interpreter familiar with normal immunophenotypic patterns of hematopoietic maturation as well as common immunophenotypic abnormalities seen in AML. A complete discussion of both topics is beyond the scope of this article, and the reader is referred to published literature (Cherian & Wood, 2012; Cherian, Wood, & Borowitz, 2016; Wood, 2004, 2007). However, there are a few common analytical tasks that can be performed on any sample to provide an improved starting point for interpretation (Wood, 2006). Assessment of the quality of the acquisition and preparation of the sample are important initial tasks.
Acquisition stability
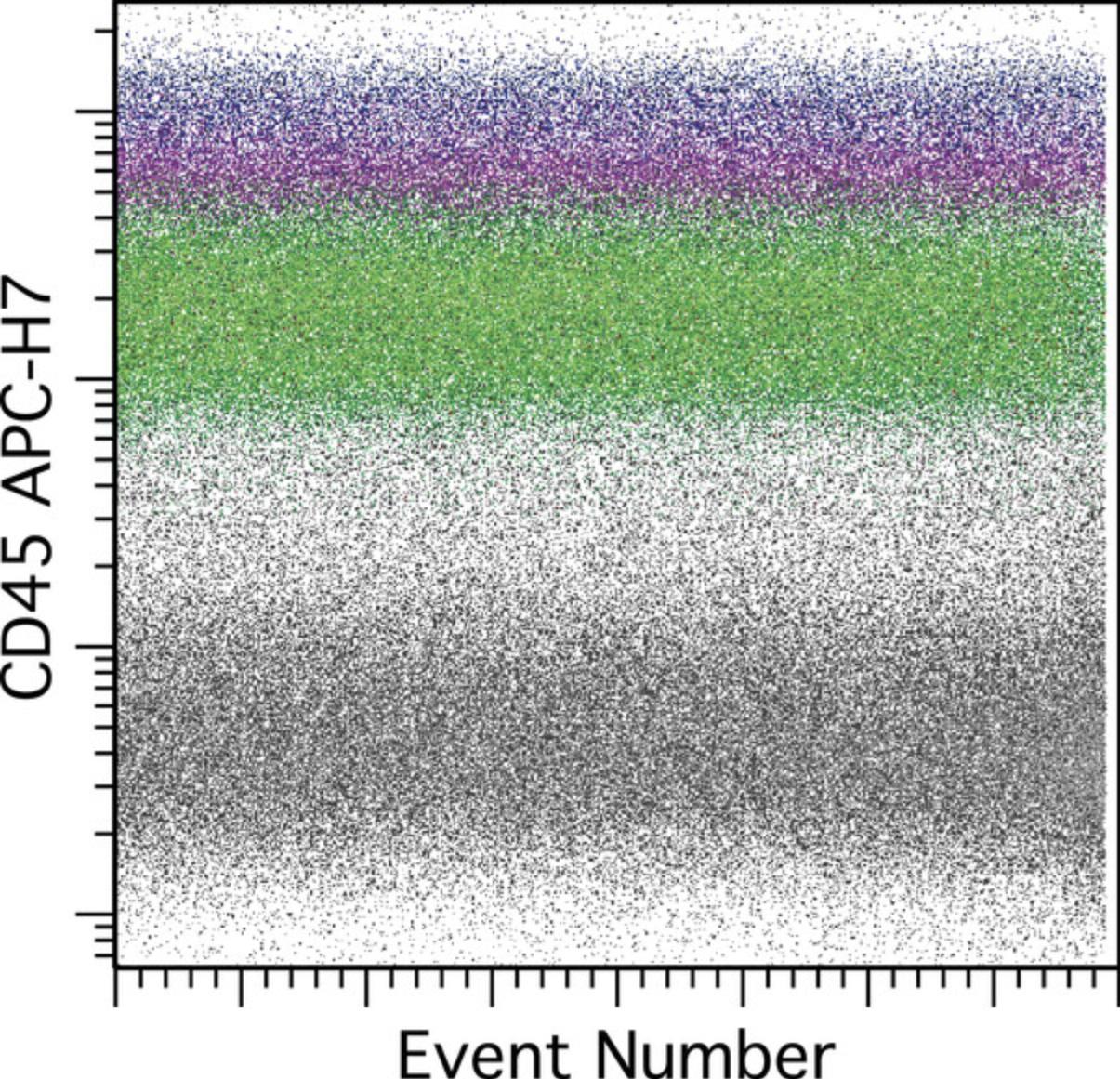
The data from each tube should be displayed versus time or event number to confirm stability of the acquisition. A parameter should be chosen that is sensitive to alterations in fluidic stability, typically a parameter that has a signal of moderate to high intensity and that is on a laser different from that used to trigger data acquisition, e.g., not the 488-nm blue laser. In this assay CD45 APC-H7 is a good choice; see Figure 1.
Doublet discrimination
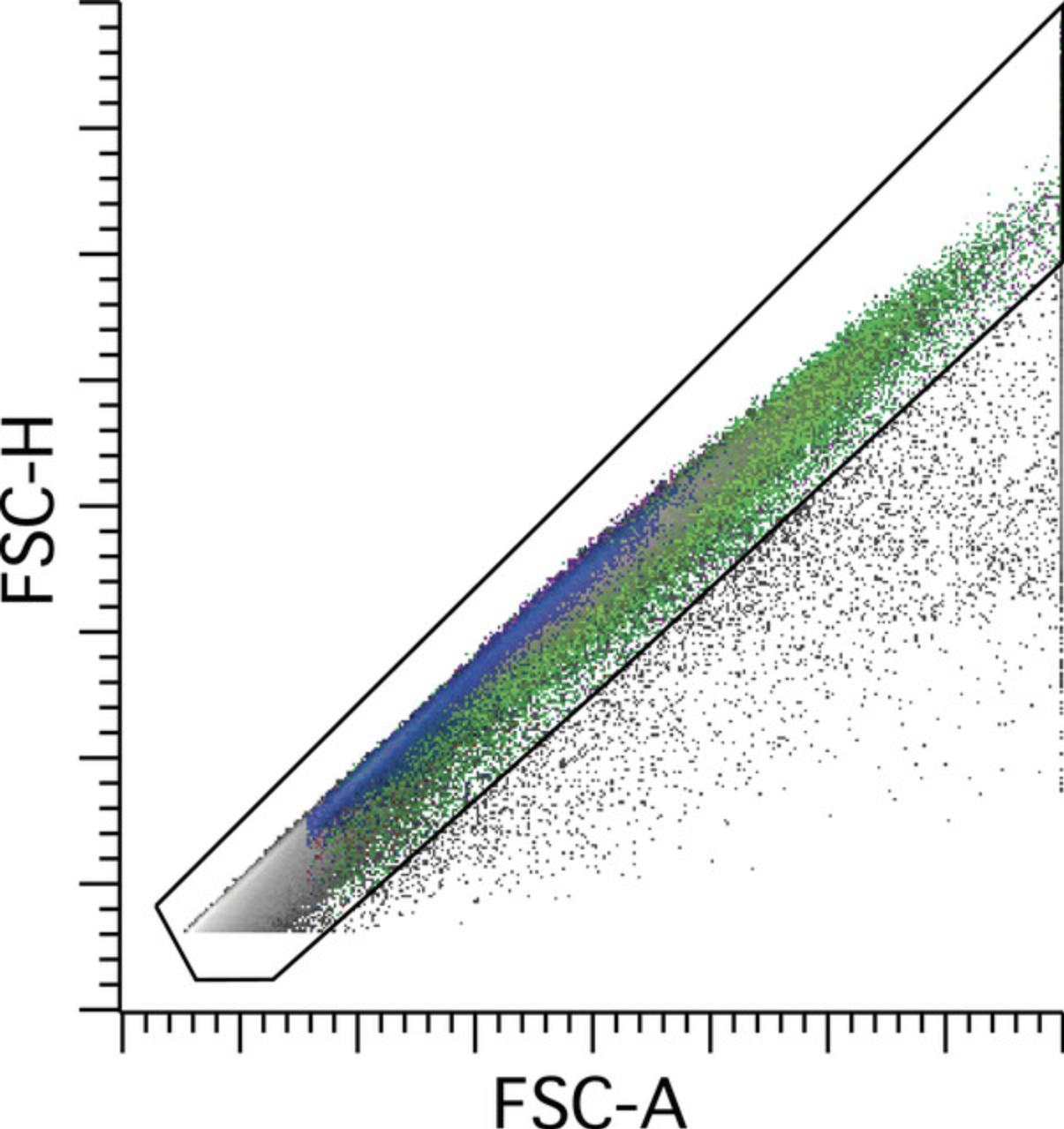
Doublet discrimination should then be performed to exclude coincident events or aggregates of cells and ensure as much as possible that events interpreted are individual cells or singlets. This is typically achieved by plotting forward scatter in a two-parameter dot plot as a combination of area, height, and/or width, followed by gating of the singlet population; see Figure 2.
Viability
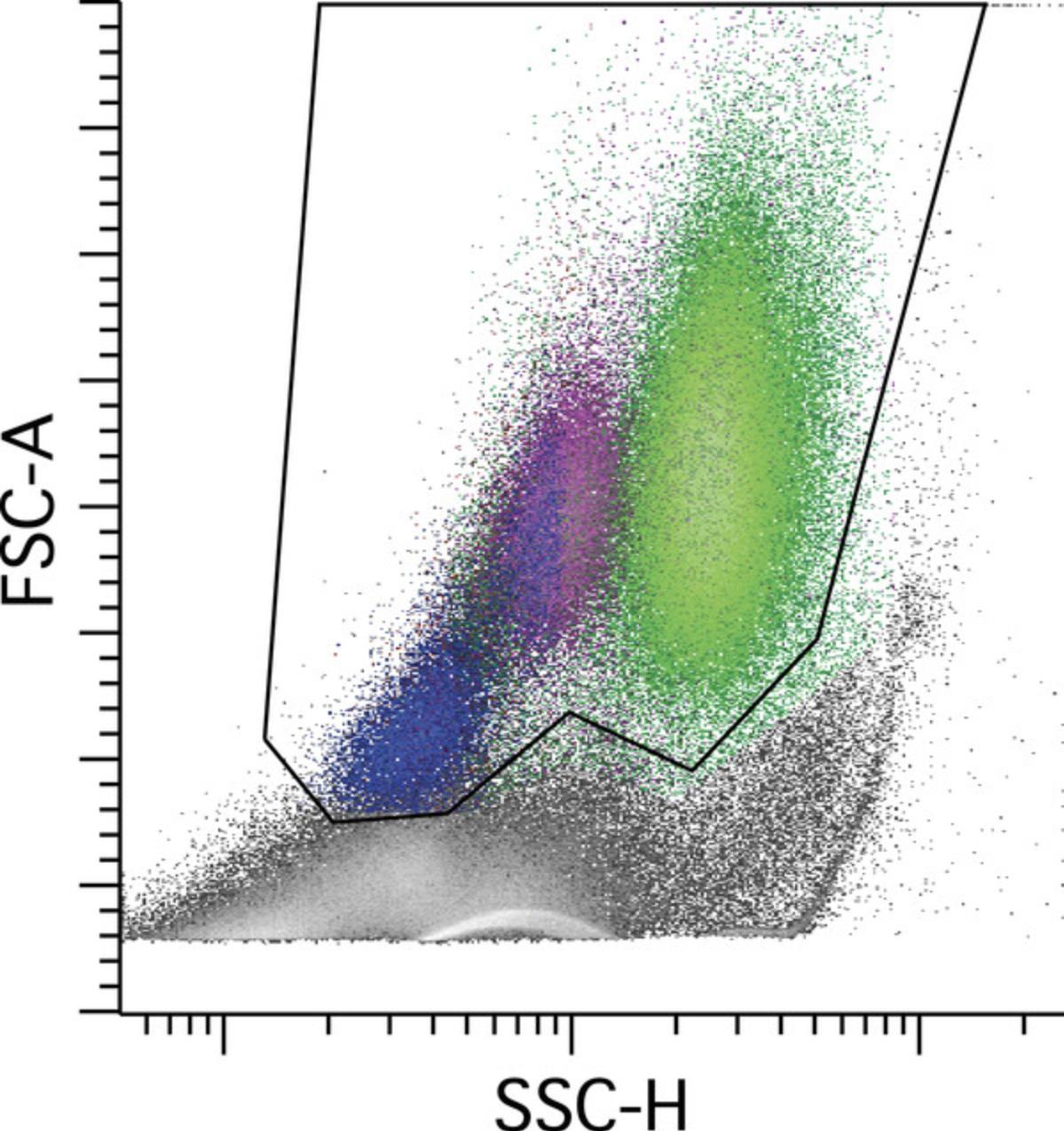
Viability and overall quality of the sample are assessed by examining the light scatter properties of the sample, generally by plotting forward scatter (FS) versus side scatter (SS); see Figure 3. Apoptotic cells will show an initial loss of FS and gain of SS, followed by loss of both FS and SS as the cells further degenerate. The ability to see these changes is highly dependent on the way in which the sample is processed; the method of choice must be optimized to provide consistent light-scatter properties that reflect cell viability. Of note, nucleated erythroid cells show a loss of FS using this method due to the lysing reagent, but are retained, and the FS instrument settings (threshold and FS PMT voltage) must be properly set to ensure inclusion of this population during acquisition. A viability gate is used to reduce background nonspecific antibody binding to compromised cells and stromal material, the intention being to include viable white cells without regard to nucleated erythroid cells. While the viability gate improves the quality and interpretability of the data, it assumes proportional loss of white cells with reduced cell viability, which is generally but not always correct. Relaxation of the gate to include lower-FS events is possible and perhaps desirable under some circumstances, provided that data quality is not significantly impaired. In principle, a viability dye that emits at a long wavelength following excitation from the violet laser could be incorporated, provided that the instrument configuration is suitable, but this would require independent validation.
Progenitor population identification
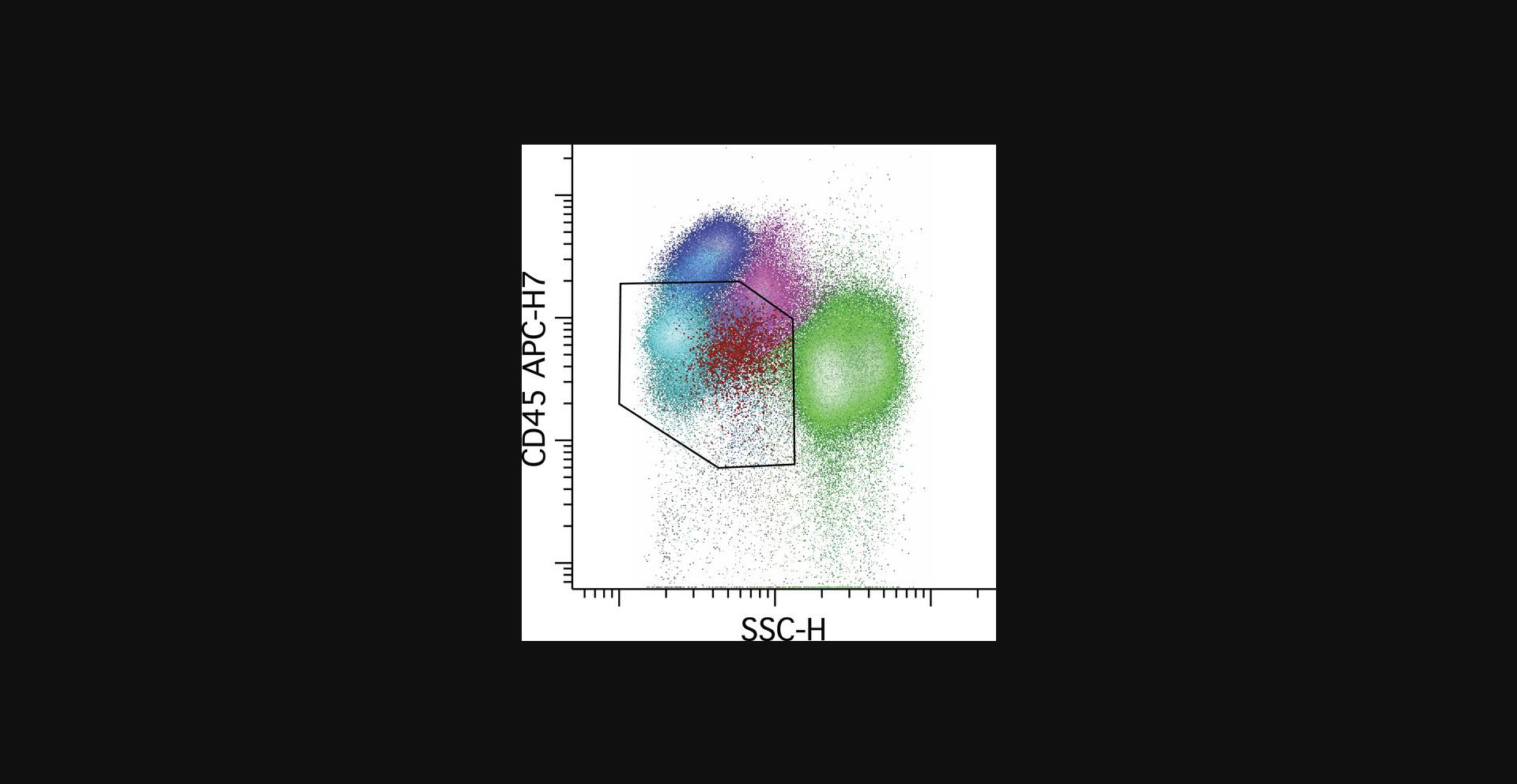
The initial identification of progenitors is readily accomplished using CD45 and SS gating in the progenitor or “blast” area; see Figure 4. Mature lymphocytes are always present in marrow and blood samples, and serve as a reference point for identification of progenitors, having uniform bright CD45 and low SS. The progenitor gate should include all events having CD45 less intense than the mature lymphocyte population. Due to the large number of maturing neutrophilic progenitors usually present, the progenitor gate should exclude as much as possible the high-SS maturing myeloid populations. However, some samples may show variable hypogranularity of the maturing myeloid cells, recognized by a level of SS similar to that of monocytes and approaching that of lymphocytes, in which case it is more important to include all CD34+ progenitors in the progenitor gate at the expense of contamination by maturing myeloid cells. This recognition is facilitated by gating and coloring the CD34+ progenitors so they can be directly visualized on CD45 versus SS plots. CD45 very low to negative events largely consist of nucleated erythroid cells, plasma cells, aggregated platelets, and debris, and thus can be safely excluded, as AML only very rarely shows loss of CD45 expression to that degree.
Leukemic population identification
The identification of residual leukemia requires a detailed knowledge of normal maturational patterns of antigen expression that is beyond the scope of this procedure, and also requires an experienced interpreter. The general strategy is to identify a subset of events that is different from any normal stage of hematopoietic maturation, with a distribution of events distinct from artifact and discrete enough to allow sequential gating of the population in multidimensional space in a manner that excludes artifactual events and normal hematopoiesis and includes all leukemic events. See Figure 5.
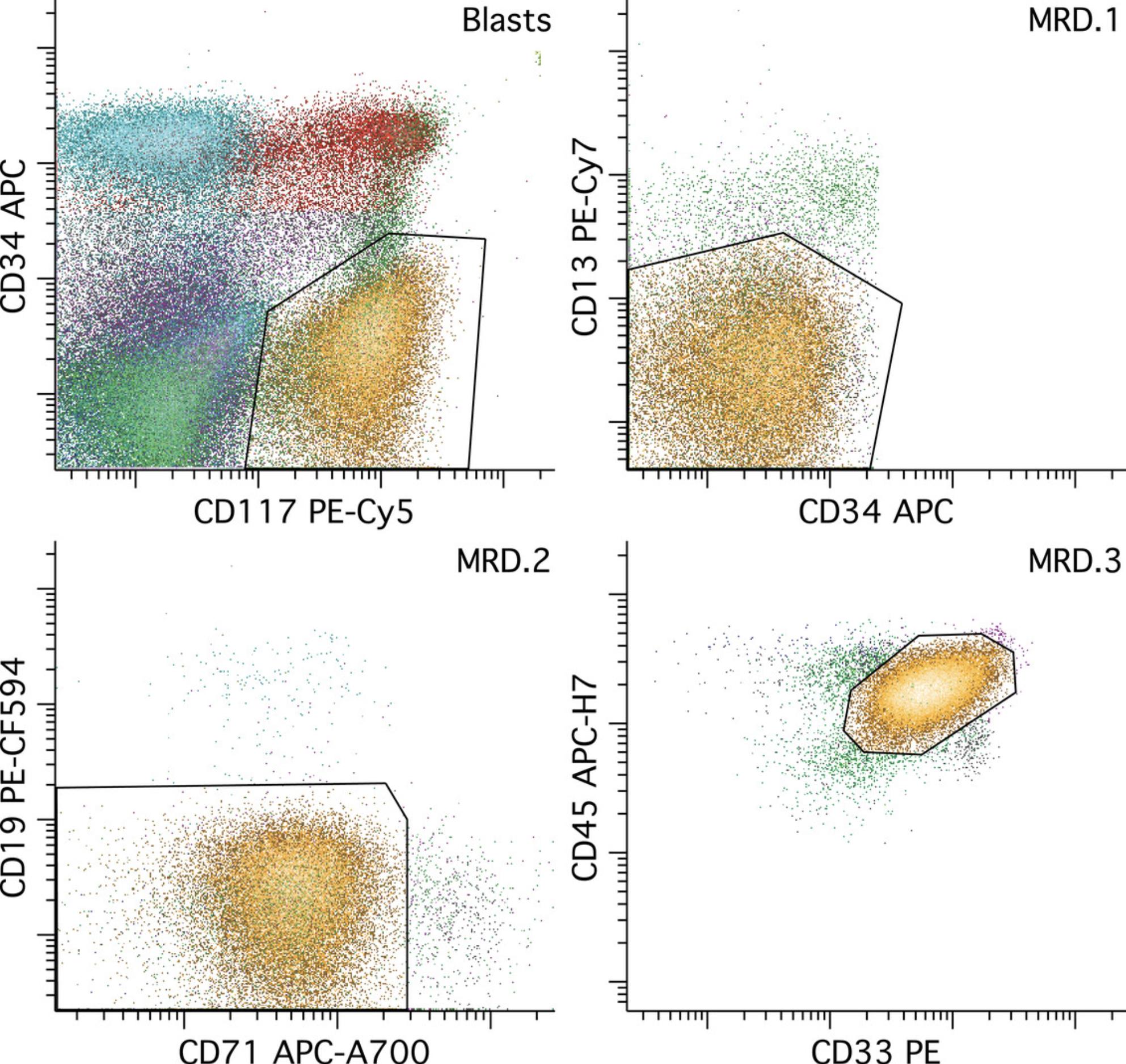
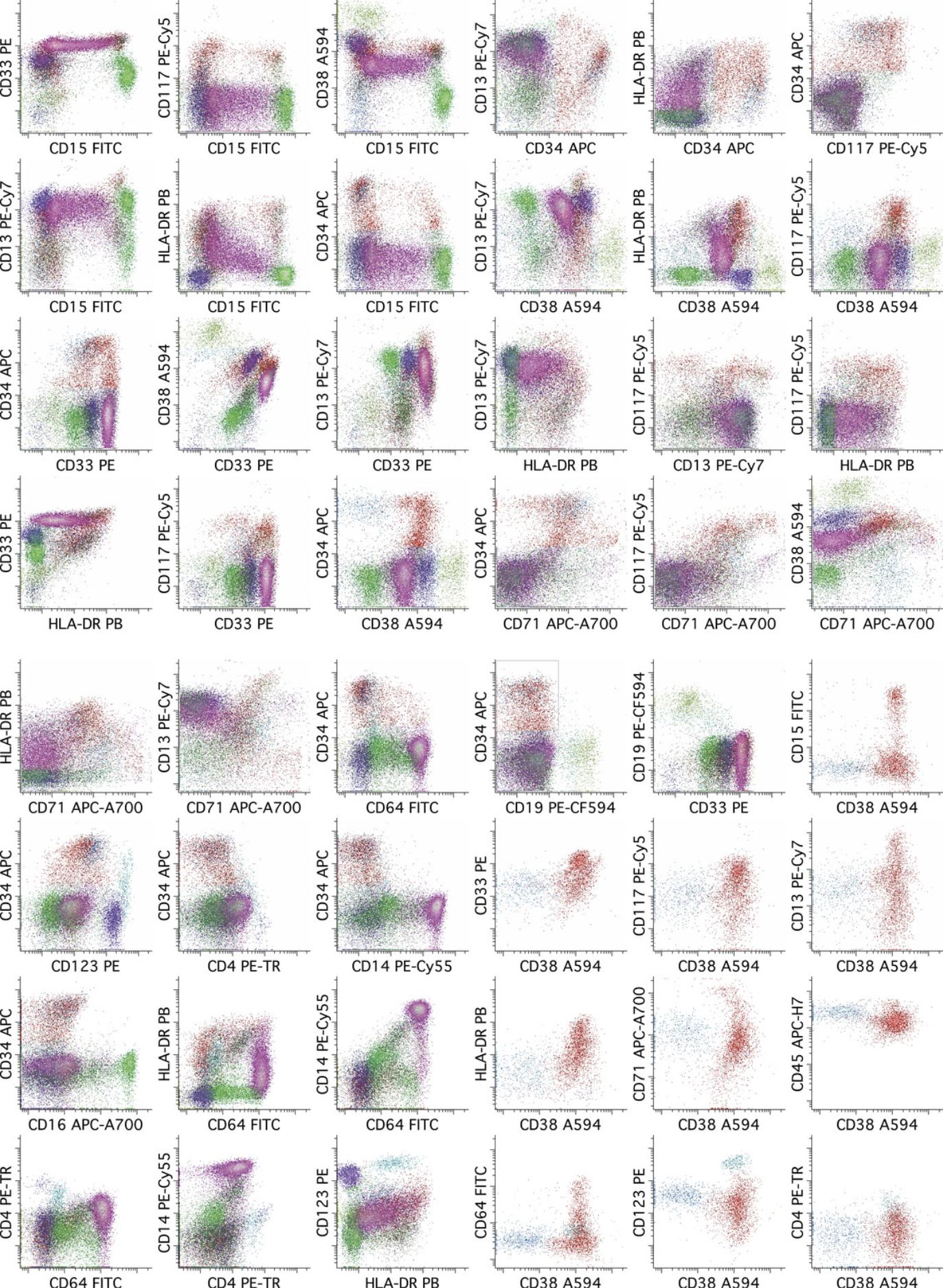
Recognition of normal populations can be facilitated by coloring the progenitors (CD34+ and/or CD117+) and each of the major cell lineages a different color. The interpreter should review all combinations of dot plots that are likely to be informative for this purpose, which need not be all possible dot plots but will generally be a major subset of these. The subset of dot plots that we have found to be most informative with this reagent panel is illustrated in Figure 6. Of particular importance is evaluation of the hematopoietic stem cell population (CD34++/CD38low), which is readily performed by displaying CD34+ events in a series of two-parameter dot plots containing CD38 versus each of the other parameters in the reagent combinations. It is also important to review the full range of maturing monocyte and myeloid populations in order to recognize abnormal immature populations of these lineages—as may be seen in AML having monocytic or myeloid differentiation—as well as to recognize potential aberrant maturational changes in these lineages that might suggest a preexisting myeloid stem cell disorder.
Immunophenotypic abnormalities in AML can be grouped into classes having recurring features that often reflect the predominant maturational stage of the leukemia.
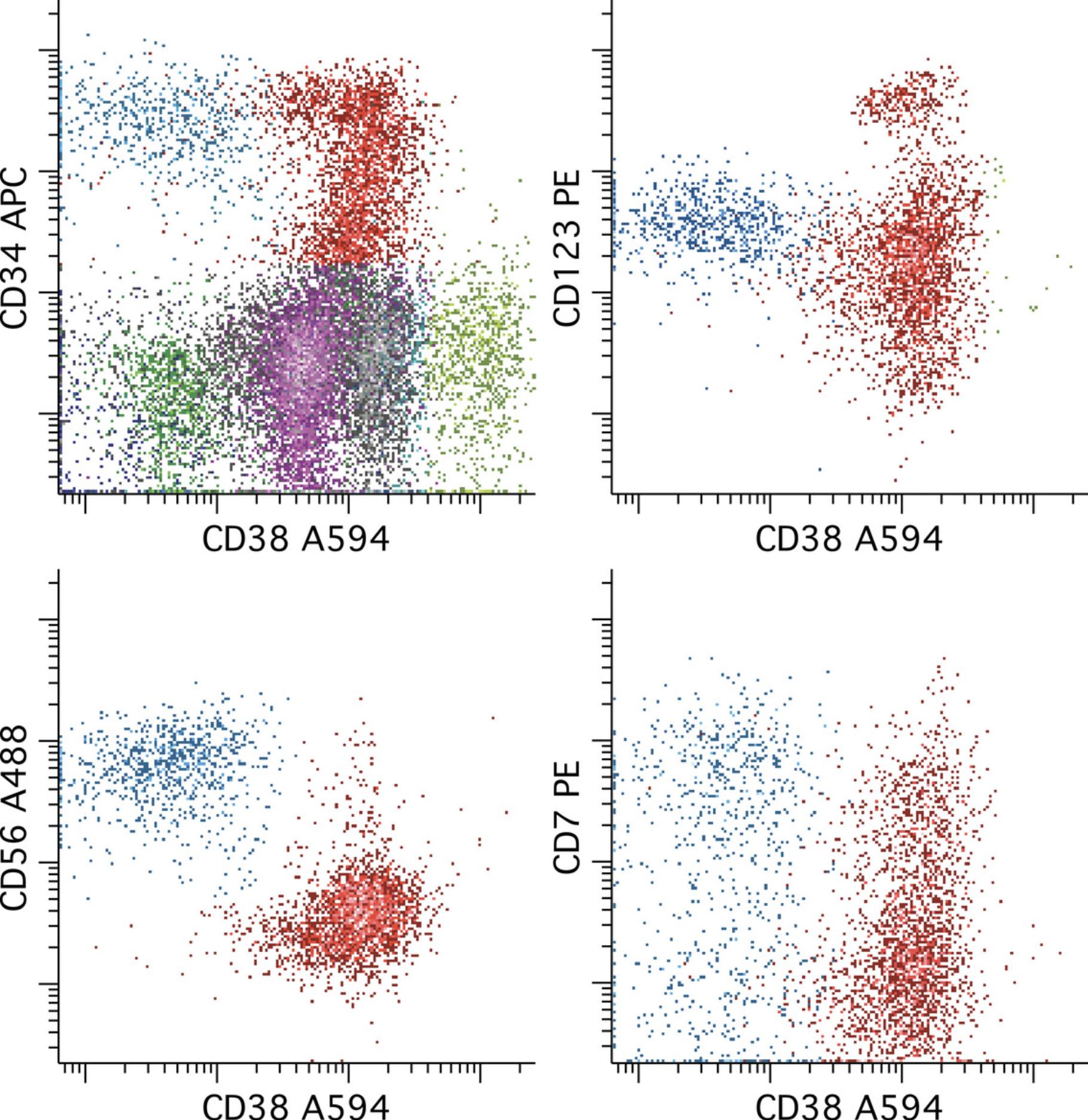
Abnormalities in subsets of leukemic cells having immunophenotypes similar to those of normal hematopoietic stem cells are common in AML and may be associated with properties such as self-renewal and resistance to chemotherapy, and thus are important to evaluate (van Rhenen et al., 2007; Zeijlemaker et al., 2019). It is important to keep in mind that while this component of the leukemia may be relatively easy to recognize, there may be more mature components (CD34+ or CD117+) derived from these more primitive cells that are more challenging or perhaps impossible to define with a particular reagent combination. A judgment must then be made about the remaining CD34+ progenitors as to whether they are of sufficient concern, by also being potentially aberrant, to be included in the MRD enumeration, or whether only the stem cells that can be cleanly gated as aberrant should enumerated. An example of MRD having an immunophenotype similar to hematopoietic stem cells is provided in Figure 7.
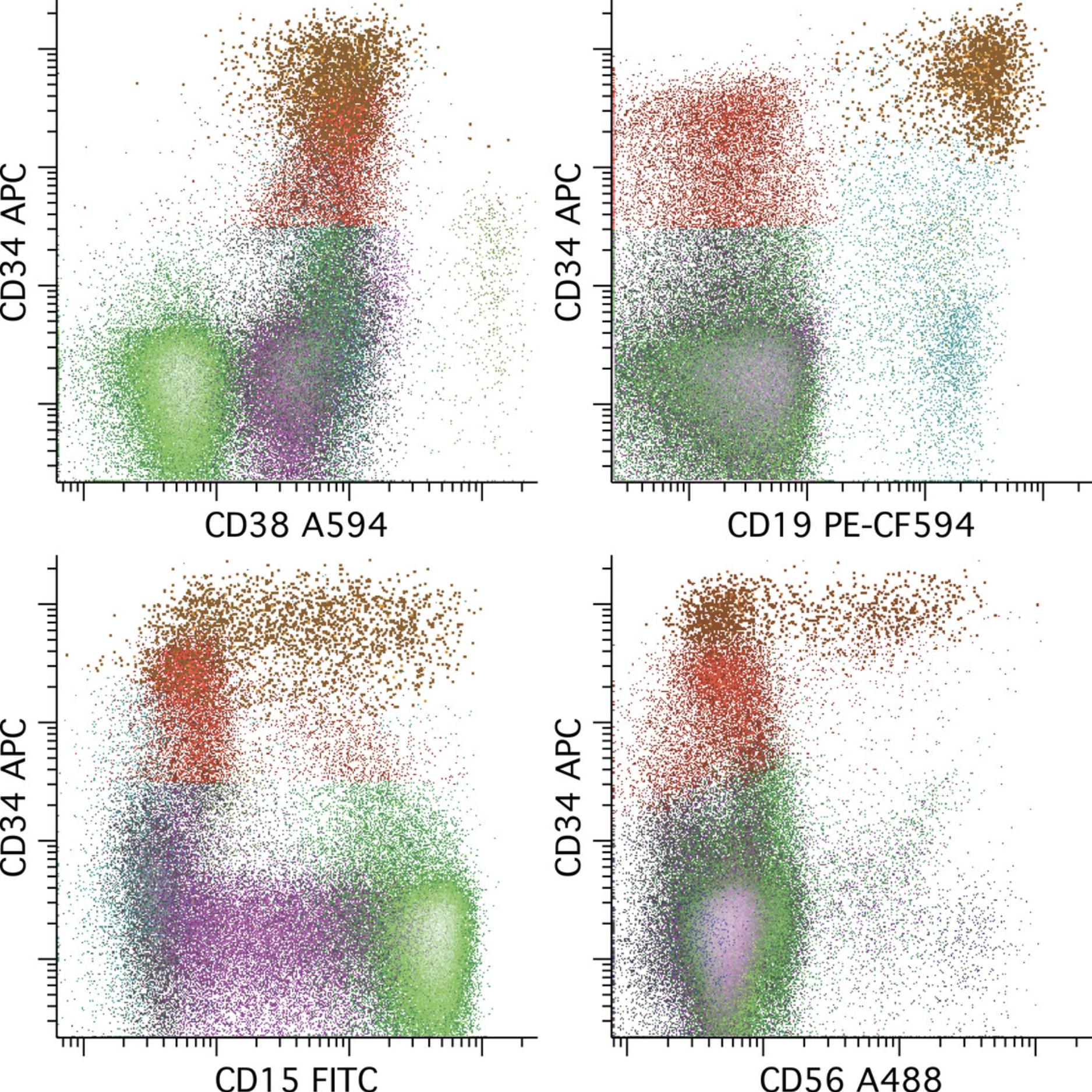
A subset of AML expresses CD34 without a well-defined hematopoietic stem cell−like subpopulation. In these cases, knowledge of normal patterns of early antigen expression with commitment to each of the major lineages (B cell, monocytic, neutrophilic, erythroid, basophilic, plasmacytoid dendritic cell) is critical for MRD recognition. A relatively clear example of this principle is seen in t(8;21) AML due to a characteristic immunophenotype that includes varied aberrant expression of increased CD34, B cell antigens such as CD19, and CD56 (Khoury et al., 2003). An example of MRD having an immunophenotype similar to CD34+ myeloid committed progenitors is provided in Figure 8.
Roughly one-third of normal-karyotype AML contain a mutation in the NPM1 gene, and these cases have a relatively distinctive immunophenotype that is important to recognize for MRD detection (Zhou et al., 2019). Characteristic of this type of AML is a more mature immunophenotype with little to no expression of CD34, decreased to absent HLA-DR, and increased CD33, often at a level higher that monocytes, monocytes being the normal population with the highest level of CD33 expression and thus a useful reference point. As a result, a dot plot of CD33 versus HLA-DR gated on progenitors by CD45 versus SS is a useful starting point for evaluation. An example of NPM1+ AML MRD is provided in Figure 9. A similar gating strategy can be used for acute promyelocytic leukemia.
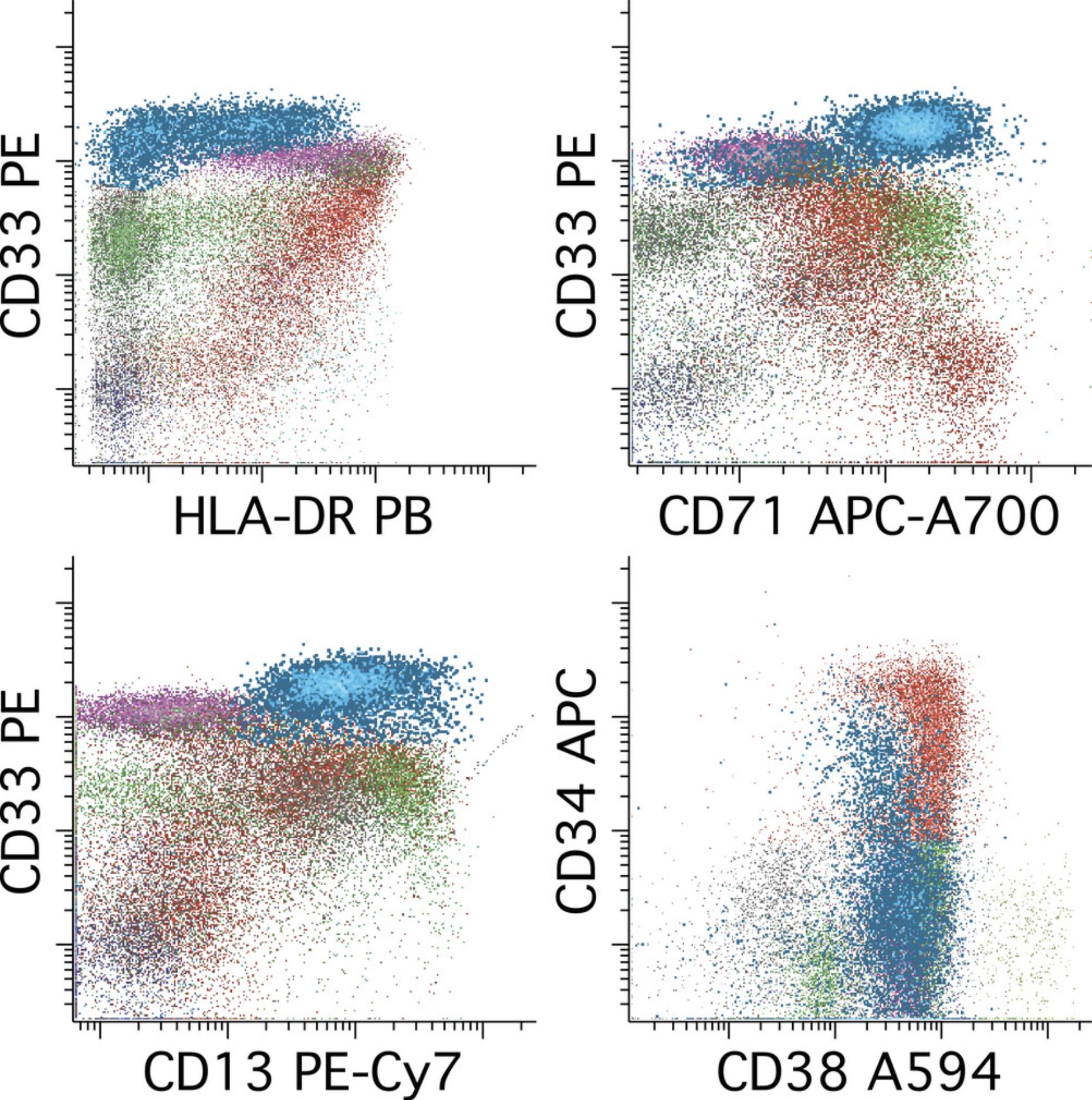
AML with monocytic differentiation can be a particularly difficult subset of AML on which to perform MRD detection using this and other reagent panels. Often the key is to identify a population of immature monocytes (usually CD14 low to negative) having immunophenotypic abnormalities in the expression of CD4, CD14, CD15, CD64, and/or HLA-DR with aberrant CD56 expression at a moderate to high level seen in a significant subset of cases. Relying on expression of high CD64 or HLA-DR to identify monocytes is risky, as these antigens are fairly commonly decreased in this subtype of AML. Rather, a comprehensive evaluation of antigen expression with cross-correlation between reagent combinations is required for confident identification. An example of monocytic AML MRD is provided in Figure 10.
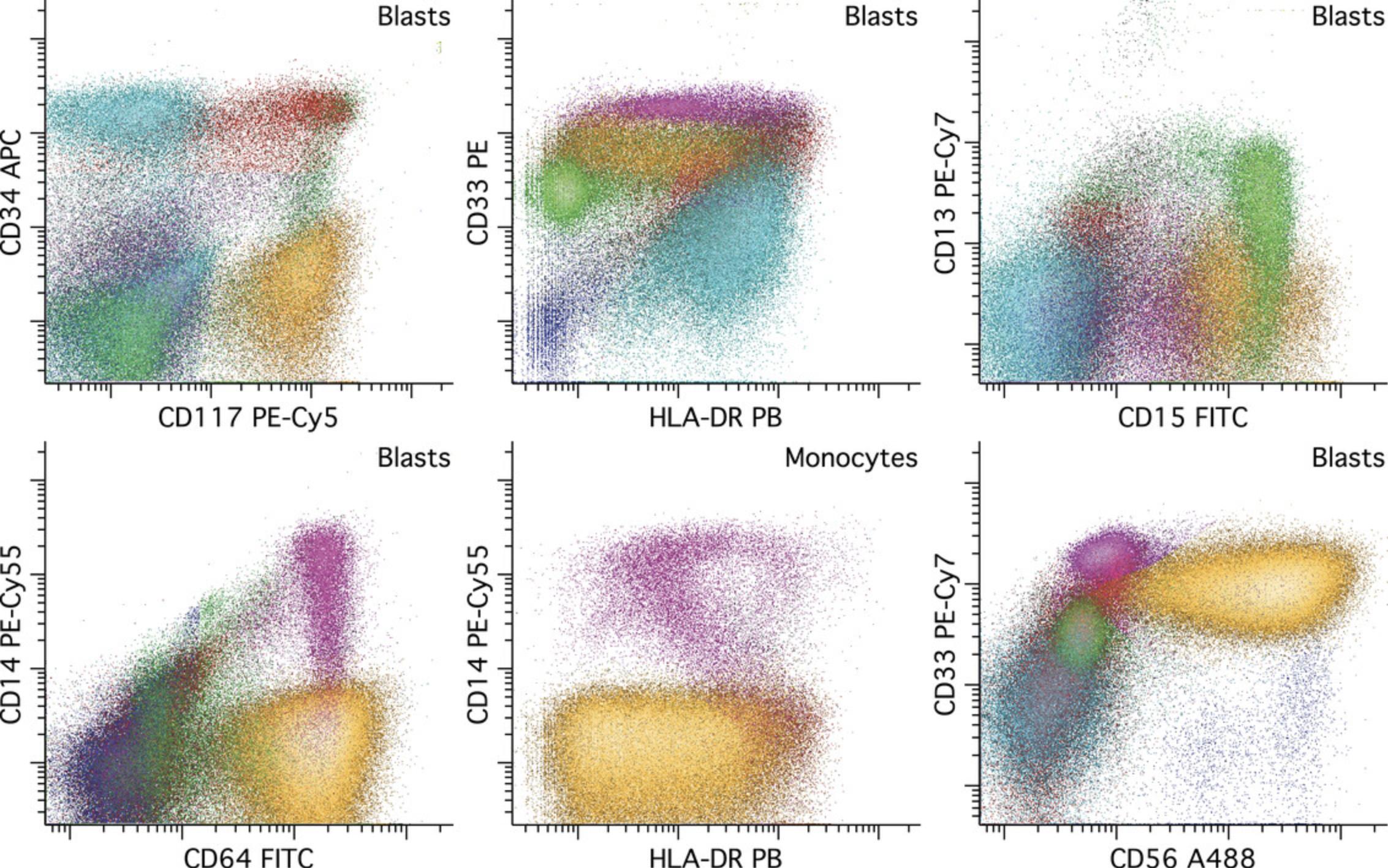
Enumeration
Relatively commonly, the leukemic population is best recognized in one of the three reagent combinations acquired. Every effort should be made to identify a corresponding population in each of the two other reagent combinations, relying on the common reagents to extrapolate between tubes. Finding a similar population at a similar frequency in more than one combination provides assurance that the finding is real and less likely to be due to a technical artifact. Rarely, significant differences in enumeration may be seen between combinations, in which case the interpreter must make a judgement as to which combination is likely most informative and most complete for identifying the leukemic population. That combination should be used for enumeration, and is not always the one with the highest enumeration, due to specificity issues with a particular combination. The denominator for enumeration historically is total CD45+ events, although total nucleated cells may ultimately be preferable, but would require use of a nucleic acid−binding dye and CD45 in a fourth reagent combination.
REAGENTS AND SOLUTIONS
Lyse-fix reagent
Add 80.2 g ammonium chloride (NH4Cl), 8.4 g sodium bicarbonate (NaHCO3), and 3.7 g disodium EDTA to a 1-L volumetric flask. Fill flask to 1 L with reagent-grade deionized water. Mix thoroughly and filter through a 45-μm Millipore filter. This results in a 10× stock that can be stored at 4°C for 3 months. Add 50 ml of 10× stock and 11.25 ml of 10% formaldehyde (methanol-free, Polysciences, #04018) to a 500-ml volumetric flask. Fill flask to 500 ml and mix thoroughly. Prepare lysing solution fresh every day of use.
PBS/0.3% BSA
Add one packet of phosphate-buffered saline (PBS) mix (Gibco, #21600-010) and 10 ml of 30% bovine serum albumin (BSA; Sigma-Aldrich, #A7284) to a 1-L volumetric flask. Fill flask to 1 L with reagent-grade deionized water. Mix thoroughly and filter through a 45-μm Millipore filter.
RPMI 1640 medium
Add 1 packet of RPMI 1640 medium (Gibco, #31800-002) and 2 g NaHCO3 to a 1-L volumetric flask. Add about 800 ml of reagent-grade deionized water and mix well, at least 15 min. While mixing, add just enough 1 N HCl to cause the magenta color to turn burnt orange. Fill to 1 L with deionized water and discard 120 ml of the RPMI. Add 10 ml of 100× pen-strep (Invitrogen, #15140-122) and 10 ml of 100× minimal essential medium (MEM; Invitrogen, #1120-052) to the RPMI and adjust pH to 7.1 using 1 N HCl and 1 N NaOH. After mixing 10 min, filter through 45-μm Millipore filter, and then add 100 ml of newborn calf serum (Invitrogen, #20610-074).
COMMENTARY
Background Information
The method outlined above was developed for the definition of normal pathways of hematopoietic maturation and the efficient identification of neoplastic hematopoietic cell populations in a clinical laboratory setting. The assay relies on both the ability of the reagent panel to subdivide hematopoietic progenitors into many discrete and defined subpopulations of cells and the specificity of the reagents to identify immunophenotypic abnormalities on leukemic cells that lie outside the range of normal patterns of expression seen on similar normal precursors. Consequently, the same assay can be used for the diagnosis of a variety of myeloid neoplasms including AML, myelodysplastic syndromes, and myeloproliferative neoplasms.
Extension of the diagnostic assay to the identification of smaller residual populations of leukemic progenitor characteristic of AML largely requires the labeling and acquisition of a larger number of cells to achieve a desired statistical target for sensitivity and reproducibility. Experience with this assay indicates that immunophenotypic abnormalities suitable for detection of residual disease to the level of 0.1% of white cells are seen on at least 90% of AML. A substantial number of AML (∼40%) exhibit more significant immunophenotypic abnormalities that allow detection of residual disease to a level of 0.01% of total white cells. If the desired reproducibility for enumeration is taken to be a CV of 10%, a total of 1 million cells need to be acquired to reach that statistical target for reproducibility at a sensitivity of 0.01%. The assay has been designed to reach that target for marrow samples having relatively normal cellularity, which are most post-treatment samples from end of induction onward. If the sample is less cellular, so that fewer total cells are acquired, the sensitivity for detection is unlikely to be impaired, but the enumeration will exhibit a greater degree of variability while remaining acceptable for most applications.
Critical Parameters
Leukemia-associated immunophenotype versus difference from normal
Leukemic blasts in AML invariably demonstrate immunophenotypic differences from normal hematopoietic precursors, the number and magnitude being dependent on the reagents used and the manner in which the panel is constructed. Immunophenotypic differences that lie outside of normal maturation at diagnosis have been termed the leukemia-associated immunophenotype (LAIP), and can be represented by one or more sequential gates defining a multiparametric space that contains few to no cells in normal bone marrow or peripheral blood.
Once defined at diagnosis, a LAIP may be used to interrogate samples after therapy for the presence of events that meet the definition and hence may represent residual leukemia. While attractive as an objective and relatively simple way to define residual disease, this has a few limitations. (1) It requires a pre-treatment sample to allow definition of the LAIP, and therefore is difficult to apply in environments where only post-therapy samples are obtained, e.g., tertiary referral centers or reference labs. (2) It is dependent on the reference samples used to represent normal hematopoiesis. It is important to use reference samples from a similar time point post-therapy as the time point that will be tested for residual disease, since the background and immunophenotypes of normal hematopoietic cells in regenerating post-therapy samples may be different from those of resting bone marrow. (3) If any event that appears in the regions defined as the LAIP are taken to be residual disease without regard to distribution or shape, nonspecific binding events or noise will impair the specificity of the assay and lead to potential false positive results. (4) It assumes stability of the immunophenotype post-therapy, which is known to be not entirely true for AML, and so may give rise to under-enumeration of residual disease including false negative results. The use of more than one LAIP per leukemia is advocated to minimize this risk. (5) It may be difficult to reconcile the enumeration obtained from multiple LAIPs on the same sample, especially when incomplete or partial populations define the LAIP. Typically, it is advocated that the highest value of all the LAIPs assessed be used for enumeration.
A more general approach for the detection of residual disease in post-therapy samples is to apply a similar process for defining LAIPs as that used for pretreatment samples, but incorporating the shape and distribution of events in multiparametric space to identify discrete abnormal populations that differ from normal hematopoiesis, and to exclude noise. This analytical approach has been termed Difference-from-Normal (DfN), and is a superset of the LAIP approach. Pretreatment LAIPs may be used as the starting point for DfN analysis, if available, but are not required; therefore, the approach may be used without a pre-treatment immunophenotype. Rather than assuming stability of immunophenotypes post-therapy, the analysis may be extended to encompass any abnormal population observed, and thus can take into account even major shifts in immunophenotype. While the DfN approach effectively improves on most of the limitations of a strict LAIP approach, it requires a more detailed knowledge of normal and regenerating hematopoiesis by the interpreter and introduces some subjectivity in the definition of populations and in the recognition and exclusion of noise. Consequently, a higher degree of experience and training is needed for implementation, and standardization between interpreters is more difficult to achieve.
Troubleshooting
A subset of samples may show evidence of nonspecific binding with one or more reagents, often as diagonal or highly correlated linear relationships between parameters. Some antibody conjugates are more prone to this phenomenon than others. While the reagent panel described has been optimized to reduce the likelihood of this problem, it may still occur in a small subset of samples. Generally, this problem is mediated by plasma components such as complement (Wood & Levin, 2006), antibodies, or other plasma proteins. Washing the sample twice with RPMI or PBS/BSA will eliminate the artifact at the expense of increased cell loss and activation of some cellular subpopulations.
Anticipated Results
Samples without identifiable residual disease represent roughly 75% of post-therapy or pre-transplant samples using this assay.
Time Considerations
Using the Basic Protocol, it takes roughly 45 min to process a single sample.
Literature Cited
- Cherian, S., & Wood, B. L. (2012). Flow cytometry in evaluation of hematopoietic neoplasms: A case based approach. Northfield, IL: CAP Press.
- Cherian, S., Wood, B. L., & Borowitz, M. (2016). Flow Cytometry. Clinical Diagnosis and Management by Laboratory Methods (Henry). Philadelphia: W.B. Saunders.
- Khoury, H., Dalal, B. I., Nevill, T. J., Horsman, D. E., Barnett, M. J., Shepherd, J. D., … Nantel, S. H. (2003). Acute myelogenous leukemia with t(8;21)—Identification of a specific immunophenotype. Leukemia & Lymphoma, 44(10), 1713–1718.
- van Rhenen, A., Moshaver, B., Kelder, A., Feller, N., Nieuwint, A. W., Zweegman, S., … Schuurhuis, G. J. (2007). Aberrant marker expression patterns on the CD34+CD38− stem cell compartment in acute myeloid leukemia allows to distinguish the malignant from the normal stem cell compartment both at diagnosis and in remission. Leukemia , 21(8), 1700–1707. doi: 10.1038/sj.leu.2404754.
- Wood, B. L. (2004). Multicolor immunophenotyping: Human immune system hematopoiesis. Methods in Cell Biology , 75, 559–576. doi: 10.1016/S0091-679X(04)75023-2.
- Wood, B. L. (2006). 9 and 10 color flow cytometry in the clinical laboratory. Archives of Pathology & Laboratory Medicine, 130, 680–690.
- Wood, B., & Levin, G. (2006). Interactions between mouse IgG2 antibodies are common and mediated by plasma C1q. Cytometry Part B: Clinical Cytometry , 70B, 321–328. doi: 10.1002/cyto.b.20138.
- Wood, B. L. (2007). Myeloid neoplasms: Myelodysplasia, myeloproliferative disorders, and acute myeloid leukemia. Clinics in Laboratory Medicine , 27, 551–575. doi: 10.1016/j.cll.2007.05.006.
- Zeijlemaker, W., Grob, T., Meijer, R., Hanekamp, D., Kelder, A., Carbaat-Ham, J. C., … Schuurhuis, G. J. (2019). CD34+CD38− leukemic stem cell frequency to predict outcome in acute myeloid leukemia. Leukemia , 33(5), 1102–1112. doi: 10.1038/s41375-018-0326-3.
- Zhou, Y., Moon, A., Hoyle, E., Fromm, J. R., Chen, X., Soma, L., … Wu, D. (2019). Pattern associated leukemia immunophenotypes and measurable disease detection in acute myeloid leukemia or myelodysplastic syndrome with mutated NPM1. Cytometry Part B: Clinical Cytometry , 96(1), 67–72. doi: 10.1002/cyto.b.21744.
Citing Literature
Number of times cited according to CrossRef: 54
- Mathias Chea, Lucie Rigolot, Alban Canali, Francois Vergez, Minimal Residual Disease in Acute Myeloid Leukemia: Old and New Concepts, International Journal of Molecular Sciences, 10.3390/ijms25042150, 25 , 4, (2150), (2024).
- Paul Sackstein, Alexis Williams, Rachel Zemel, Jennifer A. Marks, Anne S. Renteria, Gustavo Rivero, Transplant Eligible and Ineligible Elderly Patients with AML—A Genomic Approach and Next Generation Questions, Biomedicines, 10.3390/biomedicines12050975, 12 , 5, (975), (2024).
- Jennifer Moritz, Antonia Schwab, Andreas Reinisch, Armin Zebisch, Heinz Sill, Albert Wölfler, Measurable Residual Disease Detection in Acute Myeloid Leukemia: Current Challenges and Future Directions, Biomedicines, 10.3390/biomedicines12030599, 12 , 3, (599), (2024).
- Weihao Chen, Jingtao Huang, Yeqian Zhao, Luo Huang, Zhiyang Yuan, Miner Gu, Xiaojun Xu, Jimin Shi, Yi Luo, Jian Yu, Xiaoyu Lai, Lizhen Liu, Huarui Fu, Chenhui Bao, Xin Huang, Zhongzheng Zheng, He Huang, Xiaoxia Hu, Yanmin Zhao, Measurable residual disease monitoring by ddPCR in the early posttransplant period complements the traditional MFC method to predict relapse after HSCT in AML/MDS: a multicenter retrospective study, Journal of Translational Medicine, 10.1186/s12967-024-05114-w, 22 , 1, (2024).
- Benjamin Lebecque, Joevin Besombes, Louis‐Thomas Dannus, Marie De Antonio, Victoria Cacheux, Victoria Grèze, Valentin Montagnon, Lauren Veronese, Andrei Tchirkov, Olivier Tournilhac, Marc G. Berger, Richard Veyrat‐Masson, Faster clinical decisions in B‐cell acute lymphoblastic leukaemia: A single flow cytometric 12‐colour tube improves diagnosis and minimal residual disease follow‐up, British Journal of Haematology, 10.1111/bjh.19390, 204 , 5, (1872-1881), (2024).
- N. Nalini, D Ferlin Deva Shahila, Ashwini A, Vaishnavi T, Rosi A, Detection of Tongue-Based Myeloid Sarcoma Using Novel Cat Swarm Optimization and Comparison with Particle Swarm Optimization Algorithm, 2024 7th International Conference on Circuit Power and Computing Technologies (ICCPCT), 10.1109/ICCPCT61902.2024.10673123, (347-352), (2024).
- Jesse M. Tettero, Maaike E. Heidinga, Tim R. Mocking, Glenn Fransen, Angèle Kelder, Willemijn J. Scholten, Alexander N. Snel, Lok Lam Ngai, Costa Bachas, Arjan A. van de Loosdrecht, Gert J. Ossenkoppele, David C. de Leeuw, Jacqueline Cloos, Jeroen J. W. M. Janssen, Impact of hemodilution on flow cytometry based measurable residual disease assessment in acute myeloid leukemia, Leukemia, 10.1038/s41375-024-02158-1, 38 , 3, (630-639), (2024).
- Oluwatobi Odetola, Yasmin Abaza, Prognostic Role and Clinical Utility of Measurable Residual Disease in Acute Myeloid Leukemia, Advances in Oncology, 10.1016/j.yao.2024.02.004, (2024).
- Maxine Revoltar, Riana van der Linde, Deborah Cromer, Prudence N. Gatt, Sandy Smith, Marian A. Fernandez, Lachlin Vaughan, Emily Blyth, Jennifer Curnow, Elizabeth Tegg, David A. Brown, Sarah C. Sasson, Indeterminate measurable residual disease by multiparameter flow cytometry is associated with an intermediate risk of clinical relapse in adult patients with acute leukaemia, Pathology, 10.1016/j.pathol.2024.04.009, (2024).
- Connor M. Hartzell, Aaron C. Shaver, Emily F. Mason, Flow Cytometric Assessment of Malignant Hematologic Disorders, Clinics in Laboratory Medicine, 10.1016/j.cll.2024.04.008, 44 , 3, (465-477), (2024).
- Wolfgang Kern, Paul Wallace, Ryan Brinkman, Issue highlights—July 2024, Cytometry Part B: Clinical Cytometry, 10.1002/cyto.b.22199, 106 , 4, (223-227), (2024).
- Kevin Shopsowitz, Jack Lofroth, Geoffrey Chan, Jubin Kim, Makhan Rana, Ryan Brinkman, Andrew Weng, Nadia Medvedev, Xuehai Wang, MAGIC‐DR: An interpretable machine‐learning guided approach for acute myeloid leukemia measurable residual disease analysis, Cytometry Part B: Clinical Cytometry, 10.1002/cyto.b.22168, 106 , 4, (239-251), (2024).
- Corentin Orvain, Naveed Ali, Megan Othus, Eduardo Rodríguez‐Arbolí, Filippo Milano, Calvin M. Le, Brenda M. Sandmaier, Bart L. Scott, Frederick R. Appelbaum, Roland B. Walter, Relative prognostic value of flow cytometric measurable residual disease before allogeneic hematopoietic cell transplantation for adults with MDS/AML or AML, American Journal of Hematology, 10.1002/ajh.27259, 99 , 5, (862-870), (2024).
- Elisa Meddi, Arianna Savi, Federico Moretti, Flavia Mallegni, Raffaele Palmieri, Giovangiacinto Paterno, Elisa Buzzatti, Maria Ilaria Del Principe, Francesco Buccisano, Adriano Venditti, Luca Maurillo, Measurable Residual Disease (MRD) as a Surrogate Efficacy-Response Biomarker in AML, International Journal of Molecular Sciences, 10.3390/ijms24043062, 24 , 4, (3062), (2023).
- Francesca Guijarro, Marta Garrote, Neus Villamor, Dolors Colomer, Jordi Esteve, Mónica López-Guerra, Novel Tools for Diagnosis and Monitoring of AML, Current Oncology, 10.3390/curroncol30060395, 30 , 6, (5201-5213), (2023).
- Riana van der Linde, Prudence N. Gatt, Sandy Smith, Marian A. Fernandez, Lachlin Vaughan, Emily Blyth, Jennifer Curnow, David A. Brown, Elizabeth Tegg, Sarah C. Sasson, Measurable Residual Disease (MRD) by Flow Cytometry in Adult B-Acute Lymphoblastic Leukaemia (B-ALL) and Acute Myeloid Leukaemia (AML): Correlation with Molecular MRD Testing and Clinical Outcome at One Year, Cancers, 10.3390/cancers15205064, 15 , 20, (5064), (2023).
- Olisaemeka Ogbue, Serhan Unlu, Gogo-Ogute Ibodeng, Abhay Singh, Arda Durmaz, Valeria Visconte, John C. Molina, Single-Cell Next-Generation Sequencing to Monitor Hematopoietic Stem-Cell Transplantation: Current Applications and Future Perspectives, Cancers, 10.3390/cancers15092477, 15 , 9, (2477), (2023).
- Corentin Orvain, Eduardo Rodríguez-Arbolí, Megan Othus, Brenda M. Sandmaier, H. Joachim Deeg, Frederick R. Appelbaum, Roland B. Walter, Association between Prior Cytotoxic Therapy, Antecedent Hematologic Disorder, and Outcome after Allogeneic Hematopoietic Cell Transplantation in Adult Acute Myeloid Leukemia, Cancers, 10.3390/cancers15020352, 15 , 2, (352), (2023).
- Jacqueline Cloos, Lok Lam Ngai, Michael Heuser, Understanding differential technologies for detection of MRD and how to incorporate into clinical practice, Hematology, 10.1182/hematology.2023000454, 2023 , 1, (682-690), (2023).
- Anna B. Halpern, Eduardo Rodríguez-Arbolí, Megan Othus, Kelsey-Leigh A. Garcia, Mary-Elizabeth M. Percival, Ryan D. Cassaday, Vivian G. Oehler, Pamela S. Becker, Jacob S. Appelbaum, Janis L. Abkowitz, Johnnie J. Orozco, Siobán B. Keel, Paul C. Hendrie, Bart L. Scott, M. Cristina Ghiuzeli, Elihu H. Estey, Roland B. Walter, Phase 1/2 study of sorafenib added to cladribine, high-dose cytarabine, G-CSF, and mitoxantrone in untreated AML, Blood Advances, 10.1182/bloodadvances.2023010392, 7 , 17, (4950-4961), (2023).
- Ahmed Aribi, Amandeep Salhotra, Michelle Afkhami, Anamaria Munteanu, Haris Ali, Ibrahim Aldoss, Salman Otoukesh, Monzr M. Al Malki, Karamjeet S. Sandhu, Paul Koller, Shukaib Arslan, Forrest Stewart, Andrew Artz, Peter Curtin, Brian Ball, James O’Hearn, Ricardo Spielberger, Eileen Smith, Elizabeth Budde, Ryotaro Nakamura, Anthony Stein, Stephen Forman, Guido Marcucci, Pamela S. Becker, Vinod Pullarkat, WT1 -mutated acute myeloid leukemia is sensitive to fludarabine-based chemotherapy and conditioning regimens , Leukemia & Lymphoma, 10.1080/10428194.2023.2241096, 64 , 11, (1811-1821), (2023).
- Prashant Ramesh Tembhare, Monitoring Measurable/Minimal Residual Disease in Acute Myeloid Leukemia: Multiparametric Flow Cytometry-Based Approach, Indian Journal of Medical and Paediatric Oncology, 10.1055/s-0043-1772203, 44 , 06, (554-565), (2023).
- Katherine Knorr, Jahan Rahman, Caroline Erickson, Eric Wang, Mara Monetti, Zhuoning Li, Juliana Ortiz-Pacheco, Andrew Jones, Sydney X. Lu, Robert F. Stanley, Maria Baez, Nina Fox, Cynthia Castro, Alessandra E. Marino, Caroline Jiang, Alex Penson, Simon J. Hogg, Xiaoli Mi, Hideaki Nakajima, Hiroyoshi Kunimoto, Koutarou Nishimura, Daichi Inoue, Benjamin Greenbaum, David Knorr, Jeffrey Ravetch, Omar Abdel-Wahab, Systematic evaluation of AML-associated antigens identifies anti-U5 SNRNP200 therapeutic antibodies for the treatment of acute myeloid leukemia, Nature Cancer, 10.1038/s43018-023-00656-2, 4 , 12, (1675-1692), (2023).
- Riana van der Linde, Sandy Smith, David A. Brown, Sarah C. Sasson, Elizabeth Tegg, Measurable residual disease in adult acute myeloid leukaemia: evaluation of a multidimensional ‘radar’ flow cytometric plot analysis method, Pathology, 10.1016/j.pathol.2022.10.007, 55 , 3, (383-390), (2023).
- Fabienne Lucas, Christopher B. Hergott, Advances in Acute Myeloid Leukemia Classification, Prognostication and Monitoring by Flow Cytometry, Clinics in Laboratory Medicine, 10.1016/j.cll.2023.04.005, 43 , 3, (377-398), (2023).
- Alberto Quattrocchi, Luca Vincenzo Cappelli, Giovanna De Simone, Elisabetta De Marinis, Martina Gentile, Tecla Gasperi, Alessandro Pulsoni, Paolo Ascenzi, Clara Nervi, Biomarkers in acute myeloid leukemia: From state of the art in risk classification to future challenges of RNA editing as disease predictor and therapy target, Aspects of Molecular Medicine, 10.1016/j.amolm.2023.100023, 2 , (100023), (2023).
- Melissa G. Ooi, Pak Ling Lui, Te Chih Liu, Shir Ying Lee, Flow Cytometric Techniques in the Diagnosis and Monitoring of Acute Leukaemias, Pathogenesis and Treatment of Leukemia, 10.1007/978-981-99-3810-0_4, (47-59), (2023).
- Deborah K. Glencross, Leanne Swart, Melanie Pretorius, Denise Lawrie, Evaluation of fixed-panel, multicolour ClearLLab 10C at an academic flow cytometry laboratory in Johannesburg, South Africa, African Journal of Laboratory Medicine, 10.4102/ajlm.v11i1.1458, 11 , 1, (2022).
- Weijie Li, Measurable Residual Disease Testing in Acute Leukemia: Technology and Clinical Significance, Leukemia, 10.36255/exon-publications-leukemia-measurable-residual-disease, (79-100), (2022).
- Ilias Pessach, Theodoros Spyropoulos, Eleftheria Lamprianidou, Ioannis Kotsianidis, MRD Monitoring by Multiparametric Flow Cytometry in AML: Is It Time to Incorporate Immune Parameters?, Cancers, 10.3390/cancers14174294, 14 , 17, (4294), (2022).
- Colin Godwin, Eduardo Rodríguez-Arbolí, Megan Othus, Anna Halpern, Jacob Appelbaum, Mary-Elizabeth Percival, Paul Hendrie, Vivian Oehler, Siobán Keel, Janis Abkowitz, Jason Cooper, Ryan Cassaday, Elihu Estey, Roland Walter, Phase 1/2 Trial of CLAG-M with Dose-Escalated Mitoxantrone in Combination with Fractionated-Dose Gemtuzumab Ozogamicin for Newly Diagnosed Acute Myeloid Leukemia and Other High-Grade Myeloid Neoplasms, Cancers, 10.3390/cancers14122934, 14 , 12, (2934), (2022).
- Paola Pacelli, Donatella Raspadori, Elena Bestoso, Alessandro Gozzetti, Monica Bocchia, “Friends and foes” of multiple myeloma measurable/minimal residual disease evaluation by next generation flow, Frontiers in Oncology, 10.3389/fonc.2022.1057713, 12 , (2022).
- James S. Blachly, Roland B. Walter, Christopher S. Hourigan, The present and future of measurable residual disease testing in acute myeloid leukemia, Haematologica, 10.3324/haematol.2022.282034, 107 , 12, (2810-2822), (2022).
- Corentin Orvain, Jacob A. Wilson, Min Fang, Brenda M. Sandmaier, Eduardo Rodríguez-Arbolí, Brent L. Wood, Megan Othus, Frederick R. Appelbaum, Roland B. Walter, Relative impact of residual cytogenetic abnormalities and flow cytometric measurable residual disease on outcome after allogeneic hematopoietic cell transplantation in adult acute myeloid leukemia, Haematologica, 10.3324/haematol.2022.281585, 108 , 2, (420-432), (2022).
- Francesco Buccisano, Raffaele Palmieri, Alfonso Piciocchi, Valentina Arena, Luca Maurillo, Maria-Ilaria Del Principe, Giovangiacinto Paterno, Maria-Antonietta Irno-Consalvo, Tiziana Ottone, Mariadomenica Divona, Consuelo Conti, Daniela Fraboni, Serena Lavorgna, William Arcese, Maria Teresa Voso, Adriano Venditti, Clinical relevance of an objective flow cytometry approach based on limit of detection and limit of quantification for measurable residual disease assessment in acute myeloid leukemia. A post-hoc analysis of the GIMEMA AML1310 trial, Haematologica, 10.3324/haematol.2021.279777, 107 , 12, (2823-2833), (2022).
- Gabrielle Paras, Linde M. Morsink, Megan Othus, Filippo Milano, Brenda M. Sandmaier, Lucas C. Zarling, Raffaele Palmieri, Gary Schoch, Chris Davis, Marie Bleakley, Mary E. D. Flowers, H. Joachim Deeg, Frederick R. Appelbaum, Rainer Storb, Roland B. Walter, Conditioning intensity and peritransplant flow cytometric MRD dynamics in adult AML, Blood, 10.1182/blood.2021014804, 139 , 11, (1694-1706), (2022).
- Gege Gui, Christopher S. Hourigan, Measurable Residual Disease Assessment as a Surrogate Marker in New Drug Development in Acute Myeloid Leukemia, The Cancer Journal, 10.1097/PPO.0000000000000572, 28 , 1, (73-77), (2022).
- Roland B. Walter, Brenda M. Sandmaier, Megan Othus, Corentin Orvain, Eduardo Rodríguez-Arbolí, Masumi U. Oshima, Gary Schoch, Chris Davis, H. Joachim Deeg, Rainer Storb, Comparison of reduced intensity and nonmyeloablative conditioning for adults with acute myeloid leukemia undergoing allogeneic hematopoietic cell transplantation in first or second remission, Bone Marrow Transplantation, 10.1038/s41409-022-01909-x, 58 , 4, (377-385), (2022).
- Maximilian A. Röhnert, Michael Kramer, Jonas Schadt, Philipp Ensel, Christian Thiede, Stefan W. Krause, Veit Bücklein, Jörg Hoffmann, Sonia Jaramillo, Richard F. Schlenk, Christoph Röllig, Martin Bornhäuser, Nicholas McCarthy, Sylvie Freeman, Uta Oelschlägel, Malte von Bonin, Reproducible measurable residual disease detection by multiparametric flow cytometry in acute myeloid leukemia, Leukemia, 10.1038/s41375-022-01647-5, 36 , 9, (2208-2217), (2022).
- Lucas C. Zarling, Megan Othus, Brenda M. Sandmaier, Filippo Milano, Gary Schoch, Chris Davis, Marie Bleakley, H. Joachim Deeg, Frederick R. Appelbaum, Rainer Storb, Roland B. Walter, Utility of the Treatment-Related Mortality (TRM) score to predict outcomes of adults with acute myeloid leukemia undergoing allogeneic hematopoietic cell transplantation, Leukemia, 10.1038/s41375-022-01574-5, 36 , 6, (1563-1574), (2022).
- Laura G. Rico, Jorge Bardina, Àngel Bistué‐Rovira, Roser Salvia, Mike D. Ward, Jolene A. Bradford, Jordi Petriz, Accurate identification of cell doublet profiles: Comparison of light scattering with fluorescence measurement techniques, Cytometry Part A, 10.1002/cyto.a.24690, 103 , 5, (447-454), (2022).
- Kah Teong Soh, Alexis Conway, Xiaojun Liu, Paul K. Wallace, Development of a 27‐color panel for the detection of measurable residual disease in patients diagnosed with acute myeloid leukemia, Cytometry Part A, 10.1002/cyto.a.24667, 101 , 11, (970-983), (2022).
- Michael J. Borowitz, Brent L. Wood, Michael Keeney, Benjamin D. Hedley, Measurable Residual Disease Detection in B‐Acute Lymphoblastic Leukemia: The Children's Oncology Group (COG) Method, Current Protocols, 10.1002/cpz1.383, 2 , 3, (2022).
- Christian M. Vonk, Adil S. A. Al Hinai, Diana Hanekamp, Peter J. M. Valk, Molecular Minimal Residual Disease Detection in Acute Myeloid Leukemia, Cancers, 10.3390/cancers13215431, 13 , 21, (5431), (2021).
- Diana Hanekamp, Jesse M. Tettero, Gert J. Ossenkoppele, Angèle Kelder, Jacqueline Cloos, Gerrit Jan Schuurhuis, AML/Normal Progenitor Balance Instead of Total Tumor Load (MRD) Accounts for Prognostic Impact of Flowcytometric Residual Disease in AML, Cancers, 10.3390/cancers13112597, 13 , 11, (2597), (2021).
- Lok Lam Ngai, Angèle Kelder, Jeroen J. W. M. Janssen, Gert J. Ossenkoppele, Jacqueline Cloos, MRD Tailored Therapy in AML: What We Have Learned So Far, Frontiers in Oncology, 10.3389/fonc.2020.603636, 10 , (2021).
- I.V. Galtseva, Yuliya Olegovna Davydova, N.M. Kapranov, K.A. Nikiforova, E.N. Parovichnikova, Technical Aspects of Minimal Residual Disease Detection by Multicolor Flow Cytometry in Acute Myeloid Leukemia Patients, Clinical oncohematology, 10.21320/2500-2139-2021-14-4-503-512, 14 , 4, (503-512), (2021).
- Michael Heuser, Sylvie D. Freeman, Gert J. Ossenkoppele, Francesco Buccisano, Christopher S. Hourigan, Lok Lam Ngai, Jesse M. Tettero, Costa Bachas, Constance Baer, Marie-Christine Béné, Veit Bücklein, Anna Czyz, Barbara Denys, Richard Dillon, Michaela Feuring-Buske, Monica L. Guzman, Torsten Haferlach, Lina Han, Julia K. Herzig, Jeffrey L. Jorgensen, Wolfgang Kern, Marina Y. Konopleva, Francis Lacombe, Marta Libura, Agata Majchrzak, Luca Maurillo, Yishai Ofran, Jan Philippe, Adriana Plesa, Claude Preudhomme, Farhad Ravandi, Christophe Roumier, Marion Subklewe, Felicitas Thol, Arjan A. van de Loosdrecht, Bert A. van der Reijden, Adriano Venditti, Agnieszka Wierzbowska, Peter J. M. Valk, Brent L. Wood, Roland B. Walter, Christian Thiede, Konstanze Döhner, Gail J. Roboz, Jacqueline Cloos, 2021 Update on MRD in acute myeloid leukemia: a consensus document from the European LeukemiaNet MRD Working Party, Blood, 10.1182/blood.2021013626, 138 , 26, (2753-2767), (2021).
- Colin D. Godwin, Yi Zhou, Megan Othus, Mallette M. Asmuth, Carole M. Shaw, Kelda M. Gardner, Brent L. Wood, Roland B. Walter, Elihu H. Estey, Acute myeloid leukemia measurable residual disease detection by flow cytometry in peripheral blood vs bone marrow, Blood, 10.1182/blood.2020006219, 137 , 4, (569-572), (2021).
- Anne E. Austin, Michael Byrne, Detecting and preventing post-hematopoietic cell transplant relapse in AML, Current Opinion in Hematology, 10.1097/MOH.0000000000000686, 28 , 6, (380-388), (2021).
- Jesse M. Tettero, Sylvie Freeman, Veit Buecklein, Adriano Venditti, Luca Maurillo, Wolfgang Kern, Roland B. Walter, Brent L. Wood, Christophe Roumier, Jan Philippé, Barbara Denys, Jeffrey L. Jorgensen, Marie C. Bene, Francis Lacombe, Adriana Plesa, Monica L. Guzman, Agnieszka Wierzbowska, Anna Czyz, Lok Lam Ngai, Adrian Schwarzer, Costa Bachas, Jacqueline Cloos, Marion Subklewe, Michaela Fuering‐Buske, Francesco Buccisano, Technical Aspects of Flow Cytometry‐based Measurable Residual Disease Quantification in Acute Myeloid Leukemia: Experience of the European LeukemiaNet MRD Working Party, HemaSphere, 10.1097/HS9.0000000000000676, 6 , 1, (2021).
- Caroline Dix, Tsun-Ho Lo, Georgina Clark, Edward Abadir, Measurable Residual Disease in Acute Myeloid Leukemia Using Flow Cytometry: A Review of Where We Are and Where We Are Going, Journal of Clinical Medicine, 10.3390/jcm9061714, 9 , 6, (1714), (2020).
- Linde M. Morsink, Brenda M. Sandmaier, Megan Othus, Raffaele Palmieri, Noa Granot, Evandro D. Bezerra, Brent L. Wood, Marco Mielcarek, Gary Schoch, Chris Davis, Mary E. D. Flowers, H. Joachim Deeg, Frederick R. Appelbaum, Rainer Storb, Roland B. Walter, Conditioning Intensity, Pre-Transplant Flow Cytometric Measurable Residual Disease, and Outcome in Adults with Acute Myeloid Leukemia Undergoing Allogeneic Hematopoietic Cell Transplantation, Cancers, 10.3390/cancers12092339, 12 , 9, (2339), (2020).
- Raffaele Palmieri, Francesco Buccisano, Luca Maurillo, Maria I. Del Principe, Giovangiacinto Paterno, Adriano Venditti, Giovanni Martinelli, Claudio Cerchione, Current strategies for detection and approach to measurable residual disease in acute myeloid leukemia, Minerva Medica, 10.23736/S0026-4806.20.07016-0, 111 , 5, (2020).

