Efficient Isolation and Purification of High-Quality Arabidopsis thaliana Trichomes
Jan W. Huebbers, Jan W. Huebbers, Kim Büttgen, Kim Büttgen, Ralph Panstruga, Ralph Panstruga
Abstract
Trichomes are fine outgrowths on the surface of aerial plant organs which play a role in protecting plants against water loss, UV radiation, and herbivore feeding. Throughout the years, trichomes have become a popular paradigm in biological research. For example, trichomes on rosette leaves of the reference plant Arabidopsis thaliana have been used as a model to investigate cell development, cell differentiation, and, more recently, cell wall biogenesis. State of the art -omics studies on specific cell types or tissues often require physical separation, enrichment, and purification. This, of course, also applies to leaf trichomes, and various methods have thus been proposed to separate trichomes and leaf tissue. Though most of these methods are indeed suitable for trichome isolation, they suffer in part from tedious operating procedures, low yield, poor sample purity, and reduced trichome integrity. We have thus revised a previously reported method for trichome isolation, and report here an efficient and scalable procedure for the isolation and gradient centrifugation–based purification of high-quality A. thaliana trichomes. We describe the preparation of plant material and trichome release, which is based on prolonged gentle agitation of plant seedlings in the presence of a cation-chelating agent that weakens trichome-leaf interactions. We also outline the steps for the subsequent recovery and purification of the isolated crude trichome fraction, which is based on the use of discontinuous sucrose gradient centrifugation. In addition to A. thaliana , we have found that this procedure can be applied to release and enrich glandular and non-glandular trichomes from various species, including Solanum lycopersicum and Nicotiana benthamiana. The resulting purified leaf trichomes can be subjected to different types of bioassays, including histochemistry, biochemical quantification of cell wall monosaccharides, and transcriptomics, as well as proteomic profiling. © 2022 The Authors. Current Protocols published by Wiley Periodicals LLC.
Basic Protocol 1 : Preparation of plant material for release and enrichment of A. thaliana trichomes
Basic Protocol 2 : Purification of A. thaliana trichomes by density gradient centrifugation
INTRODUCTION
Plant hairs (trichomes) are fine outgrowths or appendages on the surface of plant organs. The morphology and physiology of trichomes varies drastically across different species and even within the same organism, as trichomes can be uni- or multicellular, alive or dead, glandular or non-glandular, and linear or branched (Payne, 1978). Leaf hairs (hereafter, and for simplicity, referred to as “trichomes”) protect plants against water loss, ultraviolet radiation, and insect feeding (Johnson, 1975). Concerning the latter, the secretion of, for instance, phenolic substances (mainly by glandular trichomes) and the deployment of an initial physical barrier (mainly by non-glandular trichomes) have been identified as pivotal strategies to prevent herbivore attack (Karabourniotis, Liakopoulos, Nikolopoulos, & Bresta, 2020). Moreover, non-glandular trichomes of the model plant Arabidopsis thaliana were recently described as mechanosensors that initiate immune responses upon rainfall, to preempt attacks by rain-dispersed fungal spores or bacteria (Matsumura et al., 2022).
Trichomes have been the subject of extensive research across various topics. A. thaliana trichomes, for example, became a popular model in cell biology due to their exposed position on the leaf surface, their immense size for a single cell, and their dispensable nature (Hülskamp, 2004; Hülskamp, Miséra, & Jürgens, 1994). The latter of these two references featured the study of processes associated with deficiencies in trichome development, and led to the identification of GLABRA and similar transcriptional regulators required for proper morphogenesis (Larkin, Oppenheimer, Lloyd, Paparozzi, & Marks, 1994; Szymanski, Jilk, Pollock, & Marks, 1998). The discovery that these processes are frequently not trichome-specific but apply to many different tissues spurred the role of A. thaliana trichomes as a paradigm for cell developmental processes (Hülskamp, 2004). More recently, A. thaliana trichomes have been proposed as a model to study the biogenesis of the plant cell wall (Fornero, Suo, Zahde, Juveland, & Kirik, 2017; Kulich et al., 2018; Suo, Seifert, & Kirik, 2013). The eminent role of trichomes as a model for various cellular, developmental, and physiological processes in plant biology has made them an attractive target for global profiling approaches.
State-of-the-art profiling approaches, such as RNA sequencing (RNAseq; transcriptomics) and nano-liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS; proteomics), require pure sample materials. Similarly, modern cell wall analytics usually involve HPAEC-PAD (high-performance anion-exchange chromatography with pulsed amperometric detection; Yeats, Vellosillo, Sorek, Ibáñez, & Bauer, 2016) or FTIR (Fourier-transform infrared) spectroscopy (Liu, Renard, Bureau, & Le Bourvellec, 2021), which rely on the use of isolated yet intact trichomes. Thus, various procedures for the separation of trichomes and leaf tissue have been proposed in the past, including (i) the manual clipping of trichomes by forceps (Perazza et al., 1999), (ii) the use of microcapillaries to retrieve the cytosolic content of attached trichomes (Lieckfeldt et al., 2008), and (iii) trichome removal with the aid of a frozen paint brush (Balcke, Bennewitz, Zabel, & Tissier, 2014). All of these methods, however, come with certain drawbacks that either hamper trichome yield, due to an elaborate sampling procedure, or result in poor sample integrity, as trichomes are broken off the leaf surface rather than being cautiously released.
An alternative and elegant approach for the isolation of trichomes was proposed by Marks and colleagues in 2008. Briefly, seedlings of A. thaliana are incubated in a buffer solution containing the cation chelator ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′ -tetraacetic acid (EGTA) and glass beads. Subsequently, the buffer-seedling suspension is mixed for three cycles of 30 s using a generic benchtop vortex mixer, and trichomes are then retrieved from the liquid phase using two consecutive filtration steps. The capture of calcium ions by EGTA is thought to weaken trichome-leaf interactions, as described previously (Zhang & Oppenheimer, 2004), while high-speed mixing in the presence of glass beads provides a mechanical stimulus for trichome release (Marks et al., 2008). Whereas Marks and co-workers successfully used this method for a biochemical analysis of the monosaccharide content in trichome cell walls and transcriptomics (Marks, Wenger, Gilding, Jilk, & Dixon, 2009), they did not provide a detailed proteomic profile of their isolated trichomes. This may be ascribed to the high amounts of sample material required for LC-MS/MS compared to transcriptomics, which benefits from nucleic acid amplification. Based on the procedure by Marks et al. (2008), we have developed an enhanced method for the release of trichomes that takes advantage of prolonged yet gentle agitation of comparably large amounts (up to 36 g) of A. thaliana seedlings (Huebbers et al., 2022). Besides the larger batch size compared to the original protocol, we observed that prolonged agitation by magnetic stirring boosted trichome yield per harvested plant mass by 60% in comparison to high-speed mixing. Furthermore, we introduced a density gradient centrifugation (DGC) step after trichome enrichment, to reduce the burden of crude foliaceous debris and fine particles in the trichome samples. This purification step might be particularly advantageous for nucleic acid research that relies on the amplification of sample material, and for high-resolution techniques such as LC-MS/MS. Additionally, we observed that (i) EGTA can be replaced by the considerably cheaper EDTA (ethylene diamine N,N,N′,N′ -tetraacetic acid), (ii) trichome integrity may benefit from gentle agitation conditions, and (iii) glass beads are not necessary for efficient trichome release (Huebbers et al., 2022).
Here, we describe our approach for the efficient isolation and purification of A. thaliana trichomes. First, we provide a step-by-step protocol for the gentle release of trichomes by magnetic stirring, and their subsequent enrichment (Basic Protocol 1, Fig. 1). Furthermore, we describe the purification of enriched trichomes by sucrose DGC or by using the alternative density gradient medium Nycodenz® (Basic Protocol 2, Fig. 2). The trichomes provided on the basis of these protocols can be used for histochemical assays, biochemical quantification of monosaccharides and cellulose, as well as transcriptomic and proteomic profiling. Thus, we anticipate that this method will be useful for the detailed analysis of developmental defects in trichomes on A. thaliana mutant plants. Moreover, we suspect that the downstream applications enabled by our procedure will additionally advance our understanding of trichome biology in plant species other than A. thaliana.
STRATEGIC PLANNING
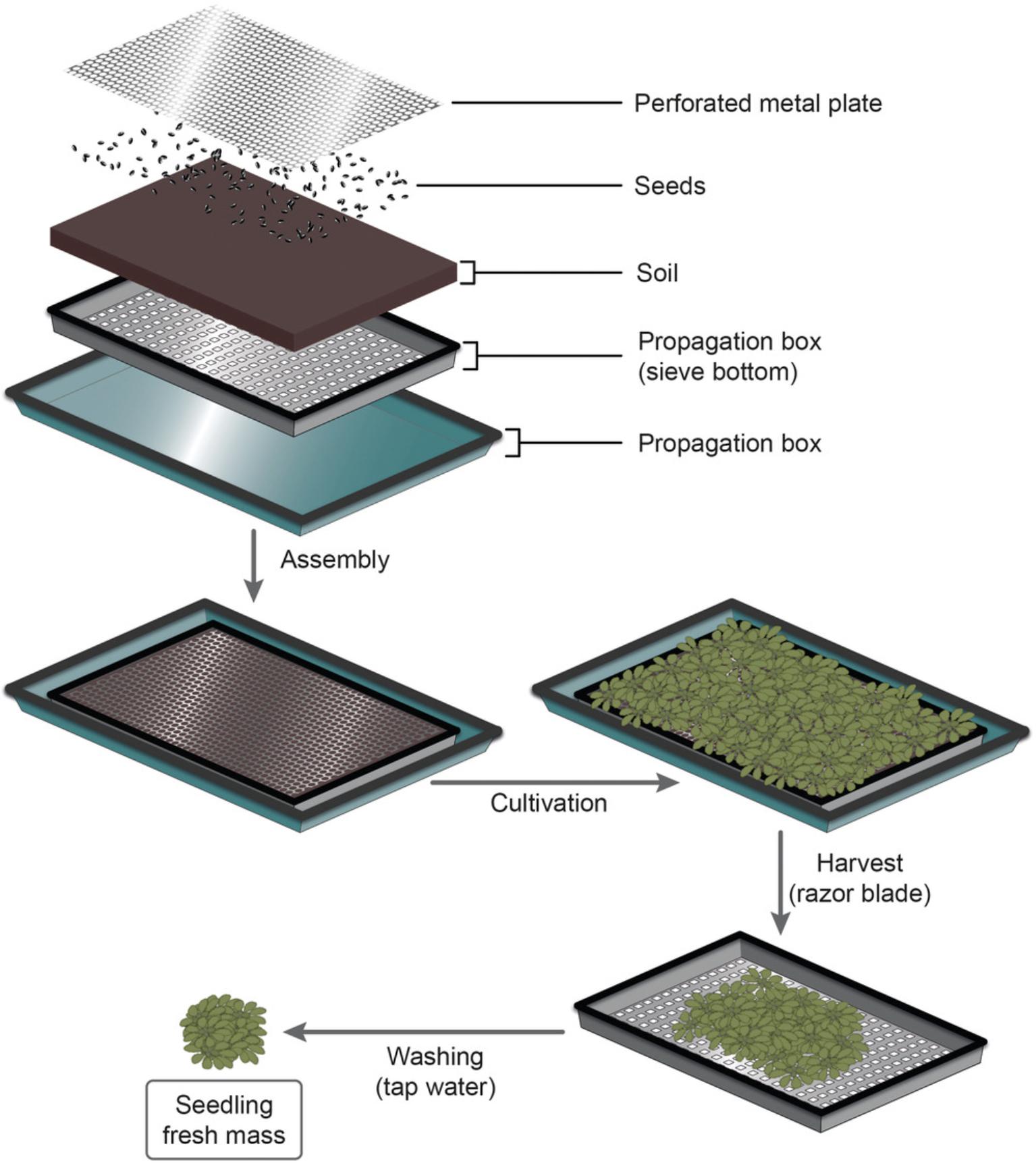
Based on to the desired age of the A. thaliana seedlings for the assay, sufficient time should be scheduled for their growth. Note that plants can be grown in any desired setup, as long as the setting provides the required amount of plant material for the protocol. For A. thaliana , however, we recommend the cultivation system detailed in Figure 1, as this setup facilitates the cultivation and harvest of large amounts of fresh plant mass and, at the same time, reduces the necessity of extensive washing for soil removal. Harvesting and washing of A. thaliana seedlings can be carried out one day before trichome release and enrichment. In this case, overnight storage of seedlings should take place in a covered vessel at 4°C.
EDTA and phosphate-buffered saline (PBS) stock solutions should be prepared in advance. It is advisable to prepare the 10× PBS buffer first, as it needs to be mixed on a magnetic stirrer for about 30 min, until all components are completely dissolved. In the meantime, the 250 mM EDTA stock solution can be prepared. For this purpose, EDTA is dissolved in sterile, distilled water. The resulting solution is milky-cloudy. Users can then adjust the pH to 8.0 with potassium hydroxide while constantly mixing on a magnetic stirrer. Use a pH meter to monitor the pH value during this process. Once the pH has been adjusted to the proper value, the solution should be transparent. The final EDTA stock solution is stored at 4°C until usage. All additional materials can also be prepared the day before.
Either sucrose or Nycodenz® can be used for the purification of trichomes by DGC. Sucrose is readily available in most laboratories and is less expensive than Nycodenz®. However, sucrose remnants in samples of isolated trichomes might distort glucose and fructose values during subsequent (e.g., cell wall and metabolome) analytics. Alternatively, the metabolically inert gradient medium Nycodenz® can be used for DGC (Huebbers et al., 2022). In this protocol, we have focused on a discontinuous sucrose gradient (Fig. 2).
Preparation of a sufficient quantity of cut pipette tips for 200- and 1000-μl micropipettes is suggested, as these are a helpful tool for any transfer of resuspended trichomes. It is also recommended to prepare a procedure sheet, for monitoring plant and trichome masses. A suitable spreadsheet including an example data set is provided in the Supporting Information (Supplementary File 1).
Depending on the desired downstream analyses, specific precautions should be taken to protect the samples during collection. For proteomics, these include, among others, the thorough washing of glass and plastic equipment with ultrapure water to avoid contamination by keratin, polyethylene glycol, or other similarly abundant substances. Moreover, chemicals should be taken from fresh containers, while the use of old buffers should be avoided (Guerra, Truebridge, Eyles, Treffon, & Vierling, 2018). For transcriptomics, a ribonuclease-free workspace should be ensured, to prevent RNA degradation.
Basic Protocol 1: PREPARATION OF PLANT MATERIAL FOR RELEASE AND ENRICHMENT OF A. thaliana TRICHOMES
This protocol describes the procedure to isolate A. thaliana trichomes at high yield, based on our previous publication (Huebbers et al., 2022). This is achieved by extended incubation of A. thaliana seedlings in EDTA-containing buffer, in combination with mild agitation on a magnetic stirrer. The low-cost chelating agent EDTA is thought to weaken the trichome-leaf interaction by complexing calcium ions that are essential for the crosslinking of pectin in the middle lamella. At the same time, agitation creates the necessary mechanical stimulus to gently detach the trichomes from the leaf. Essential in this regard is the size ratio between stirring bar and beaker, to allow efficient mixing while avoiding crushing the seedlings. Although glass beads were used during trichome release in our original protocol (Huebbers et al., 2022), in subsequent experiments we obtained similar yields without glass beads. We, therefore, describe here the procedure without the use of glass beads.
After their release, trichomes are separated from the seedlings by two consecutive filtration steps. Several cycles of release and enrichment are carried out to achieve full trichome yield and prevent a potential loss of cytosolic trichome content, which might be caused by long agitation periods (>1 hr), as described elsewhere (Huebbers et al., 2022). Since the trichomes remain intact and retain their cellular content during this procedure, trichome samples obtained by this protocol can be used for various types of downstream analyses.
Materials
- A. thaliana seeds [here: Col-0 ecotype, e.g., Nottingham Arabidopsis Stock Center (NASC) ID N1093]
- Note that the protocol is likely to work with many different A. thaliana ecotypes and also other plant species.
- SoMi 513 soil (HAWITA, Vechta, Germany, EB/19748)
- 50 mM EDTA-PBS buffer (see recipe) prepared using:
- 10× PBS buffer, pH 7.5 (see recipe)
- 250 mM EDTA stock solution (see recipe)
- Propagation box with sieve bottom (500 × 320 × 60 mm; Wiesauplast, Wiesau, Germany; provider: Hermann Meyer KG, cat. no. 749501 or similar, for cultivation)
- Sieve or a second propagation box with sieve bottom (to capture and wash harvested seedlings)
- Propagation tray (600 × 400 × 60 mm; provider: Hermann Meyer KG, cat. no.: 749114 or similar)
- Cover for propagation tray (600 × 400 × 60 mm; Hermann Meyer KG, cat. no.: 749150 or similar)
- Spray bottle
- Perforated metal plate (470 × 270 × 1 mm, perforation: ISO 7806-1983 Rv 0.5-1.09, custom-made)
- Controlled growth chamber for short-day (10 hr light/14 hr darkness) conditions
- Razor blade
- Four 500-ml beakers (inner diameter: 85 mm; provider: VWR, cat. no.: 213-1126 or similar)
- Paper towels
- Four stirring bars, triangular shape (80 mm)
- Precision balance KERN 572 (Kern & Sohn GmbH, Balingen, Germany, cat. no. 572-33 ODER 572-35 or similar)
- Analytical balance M-Power (Sartorius Stedim Biotech, Göttingen, Germany, cat. no. AZ214 or similar)
- IKAMAG Combimag RCT magnetic stirrer [IKA, Staufen, Germany; this product is no longer available, so an IKA RCT Basic stirrer (cat. no.: 0003810000) or similar devices can be used as an alternative]
- Screen door mesh, nominal pore size of 1.2 × 1.4 mm (FB-Factory, Lübbecke, Germany; provider: Hermann Meyer KG, cat. no.: B01N363O0I)
- Funnel
- Cell strainer, nominal pore size of 100 μm (VWR, Radnor, USA)
- Small spatula (width: 5 mm)
- Harris Uni-Core leaf disc sampler (6.0 mm; Carl Roth GmbH + Co. KG, Karlsruhe, Germany, cat. no.: 6799.1)
- 2.0-ml microcentrifuge tubes (Sarstedt, Nümbrecht, Germany)
- 200 µl UltraPoint® graduated TipOne® pipette tips (Starlab, Hamburg, Germany, cat. no.: S1113-1006).
- Note that these tips were cut at the 10-µl graduation for proper trichome transfer.
- Transmitted light and epifluorescence microscope, and slides
Preparation of A. thaliana seedlings for trichome isolation
1.For the cultivation of A. thaliana plants, place a propagation box with sieve bottom (500 × 320 × 60 mm) in a large propagation tray (600 × 400 × 60 mm; Fig. 1) and fill the propagation box with sieve bottom with soil.
2.Spray the soil thoroughly with tap water. Also, fill the large propagation tray with water so that the soil gets completely soaked.
3.Evenly distribute A. thaliana seeds on the soil surface (Fig. 1). Adjust the number of seeds according to the desired plant age. For 42-day-old seedlings, we recommend about 200 seeds per propagation box.
4.Moisten the seeds by spraying them with tap water.
5.After seeding, place a perforated metal plate (470 × 270 × 1 mm, perforation: ISO 7806-1983 Rv 0.5-1.09) on the soil surface (Fig. 1).
6.Moisten a cover for the propagation tray (provider, Hermann Meyer KG) from the inside and place it over the propagation box.
7.Cultivate the A. thaliana seedlings under short-day conditions until they reach the desired age.
8.When the seedlings are old enough and have grown through the holes in the perforated metal plate, harvest the aboveground part of the seedlings by cutting them with a razor blade (Fig. 1, “Harvest”). Capture the seedlings in a second propagation box with sieve bottom or any suitable sieve, for washing (Fig. 1, “Washing”).
9.Remove soil-derived impurities by rinsing the seedlings in the sieve thoroughly with tap water for at least 30 s.
10.Dry the seedlings thoroughly using paper towels.
11.Place a single triangular stirring bar into each of the required 500-ml beakers and prepare the seedling batches (Fig. 1, “4: Batch preparation”).
Place a 500-ml beaker on a balance, tare the balance, and transfer your desired seedling fresh mass per batch (36 g max. for A. thaliana) to the beaker. Repeat the process for all beakers.
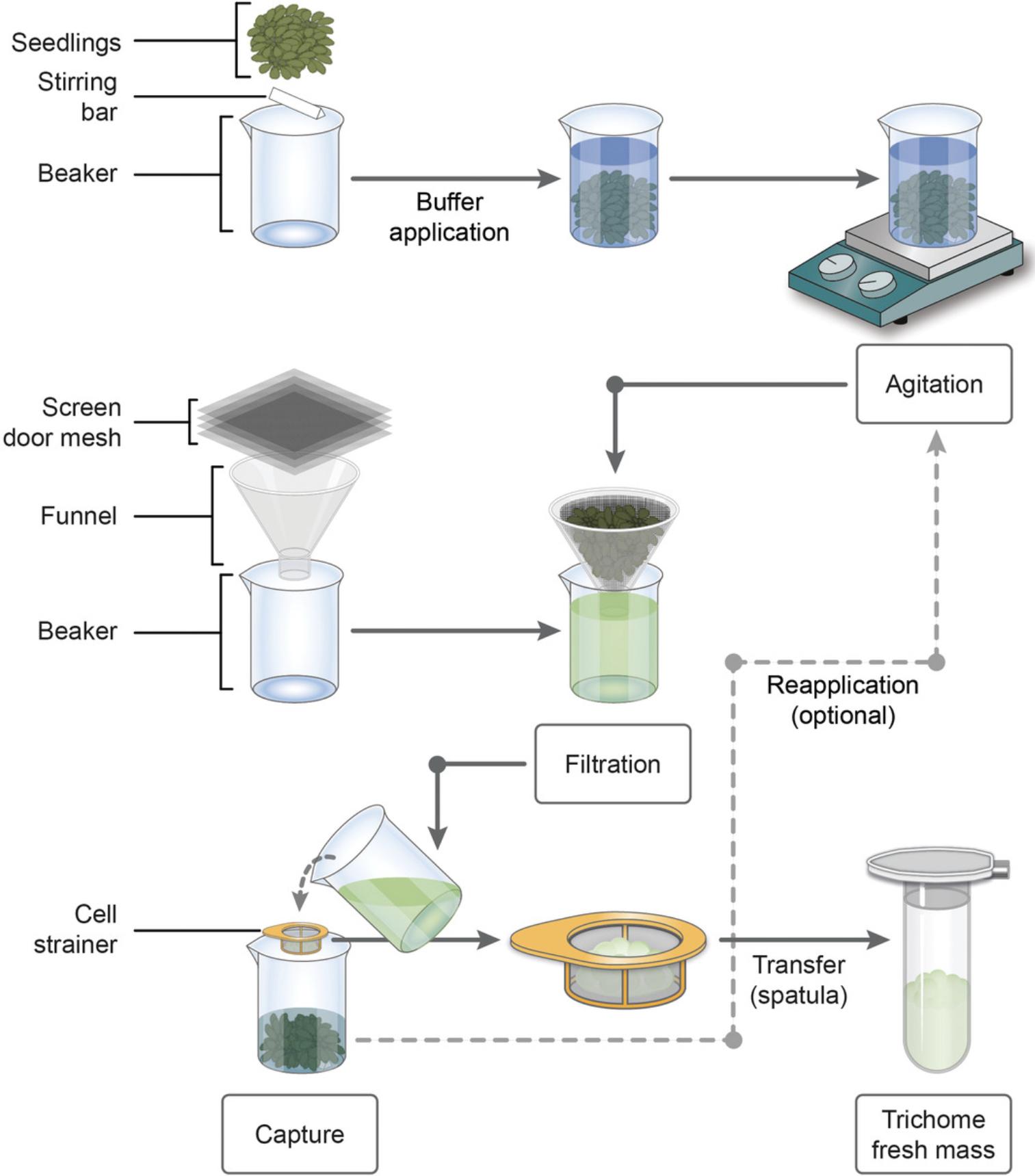
Release of trichomes
12.For each 500-ml beaker, freshly prepare 250 ml of 50 mM EDTA-PBS buffer using the EDTA and PBS stock solutions as described in Reagents and Solutions. Add the buffer to the beakers with the seedlings (Fig. 2, “Buffer application”).
13.Optional : Cover each beaker with aluminum foil.
14.Stir the seedlings in the beaker at room temperature for 30 min and 300 rpm using a magnetic stirrer (Fig. 2, “Agitation”).
Enrichment of released trichomes by filtration
15.Separate trichomes from seedlings by filtration, using four layers of screen door mesh and a funnel. To do this, pour the buffer solution containing the trichomes through mesh and funnel, and collect it in a clean beaker (Fig. 2, “Filtration”). Keep the residual plant mass in its original 500-ml beaker.
16.Pour the buffer-trichome suspension through a cell strainer to capture the trichomes (Fig. 2, “Capture”). Collect the buffer solution in its original 500-ml beaker with the processed seedling mass.
17.Transfer the gathered trichomes from the cell strainer to a 2.0-ml microcentrifuge tube using a small spatula (Fig. 2, “Transfer”).
18.Optional : Repeat steps 14–17 (we recommend two times) using agitation periods of 15 min each to enhance trichome recovery (Fig. 2, “Reapplication”).
19.After enrichment, use the trichomes immediately for purification (see Basic Protocol 2) or store temporarily for future use.
| Organism | FMPlant [g] | FMTrichome [mg] | DMTrichome [mg] | Rel. FMTrichome [mg g-1] | Rel. DMTrichome [mg g-1] |
|---|---|---|---|---|---|
| A. thaliana | ∼36 | ∼300 | ∼20 | 8.33 | 0.56 |
| S. lycopersicum | ∼15 | ∼275 | ∼16 | 18.33 | 1.07 |
| N. benthamiana | ∼15 | ∼285 | ∼17 | 19.00 | 1.13 |
| H. annuus | ∼18 | ∼70 | ∼5 | 3.89 | 0.28 |
-
aTrichome mass was determined before (fresh mass, FM) and after (dry mass, DM) lyophilization. Values for A. thaliana FM and DM represent approximate benchmarks that were met regularly in our hands. Values for plant species other than A. thaliana represent the outcome of a single experiment. Relative (Rel.) trichome amounts indicate trichome yield per harvested plant fresh mass. Data from Huebbers et al. (2022).
20.After isolation, monitor the successful release of trichomes by microscopy. To this end, use a leaf disc sampler to prepare leaf discs (5-10 mm diameter) of the processed plant material and check for remaining trichomes on the leaf surface.
Basic Protocol 2: PURIFICATION OF A. thaliana TRICHOMES BY DENSITY GRADIENT CENTRIFUGATION
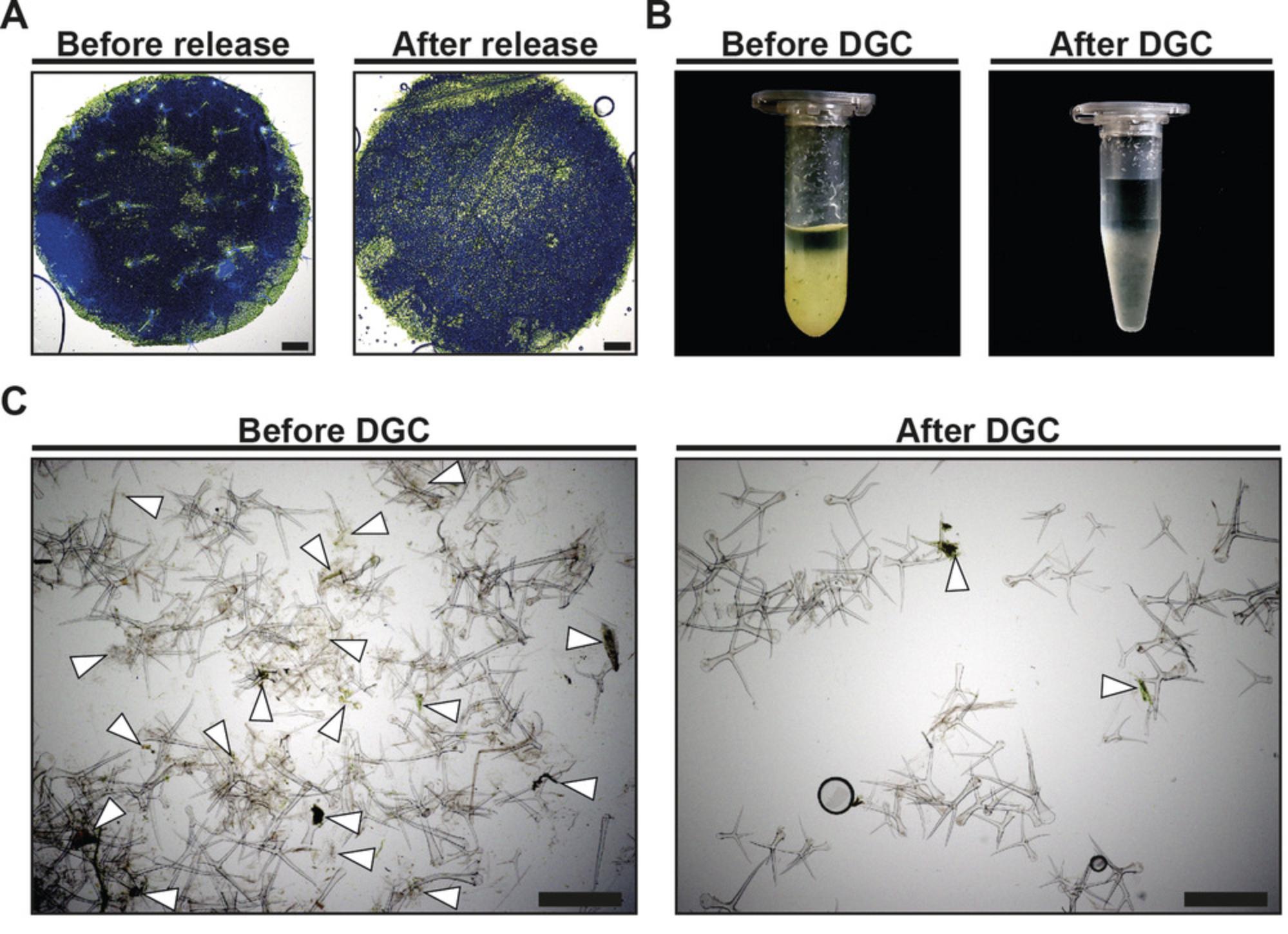
After release and enrichment (Basic Protocol 1), trichome samples may display impurities such as foliaceous debris and fine particles (Fig. 3B and 3C, “Before DGC”). Thus, we developed a procedure based on discontinuous sucrose DGC for the purification of enriched trichomes. While these impurities do not considerably influence the biochemical analysis of cell wall monosaccharides, we have encountered differences during transcript profiling and after LC-MS/MS between trichome samples before DGC and after DGC (Huebbers et al., 2022), so users may want to consider this protocol depending on their downstream application.
Materials
- 20%, 40%, and 80% (w/v) sucrose in water, or 15%, 30%, and 60% (m/v) Nycodenz® (Progen, Heidelberg, Germany, cat. no.: 18003) in water
- Enriched trichomes (Basic Protocol 1, step 19)
- 1× PBS buffer (see recipe for 10×)
- 15-ml reaction tubes (Sarstedt, Nümbrecht, Germany)
- 1-ml syringe (e.g., Injekt® Luer Solo; Braun, Melsungen, Germany, cat. no.: 916601V or similar)
- Cannula (e.g., Sterican 21G × 2 Braun, Melsungen, Germany, cat. no.: 4657527 or similar)
- Micropipettes and pipette tips
- 1000-µl UltraPoint® graduated TipOne® pipette tips (Starlab, Hamburg, Germany, cat. no.: S1111-6001 or similar).
- Note that these tips were cut with scissors at the 250-µl graduation for proper trichome transfer.
- Centrifuge (Centrifuge 5810 R, Eppendorf) with swing-out rotor (Eppendorf, Hamburg, Germany)
- 2.0-ml microcentrifuge tube (Sarstedt, Nümbrecht, Germany)
- Analytical balance M-Power (Sartorius Stedim Biotech, Göttingen, Germany, cat. no. AZ214 or similar)
Purification of A. thaliana trichomes by density gradient centrifugation
1.Create a discontinuous sucrose gradient. To do this, carefully layer 1 ml each of 0%, 20%, 40%, and 80% (m/v) sucrose in a 15-ml reaction tube (Fig. 4). Prepare one DGC tube per trichome sample and the necessary number of additional tubes for balancing the centrifuge.
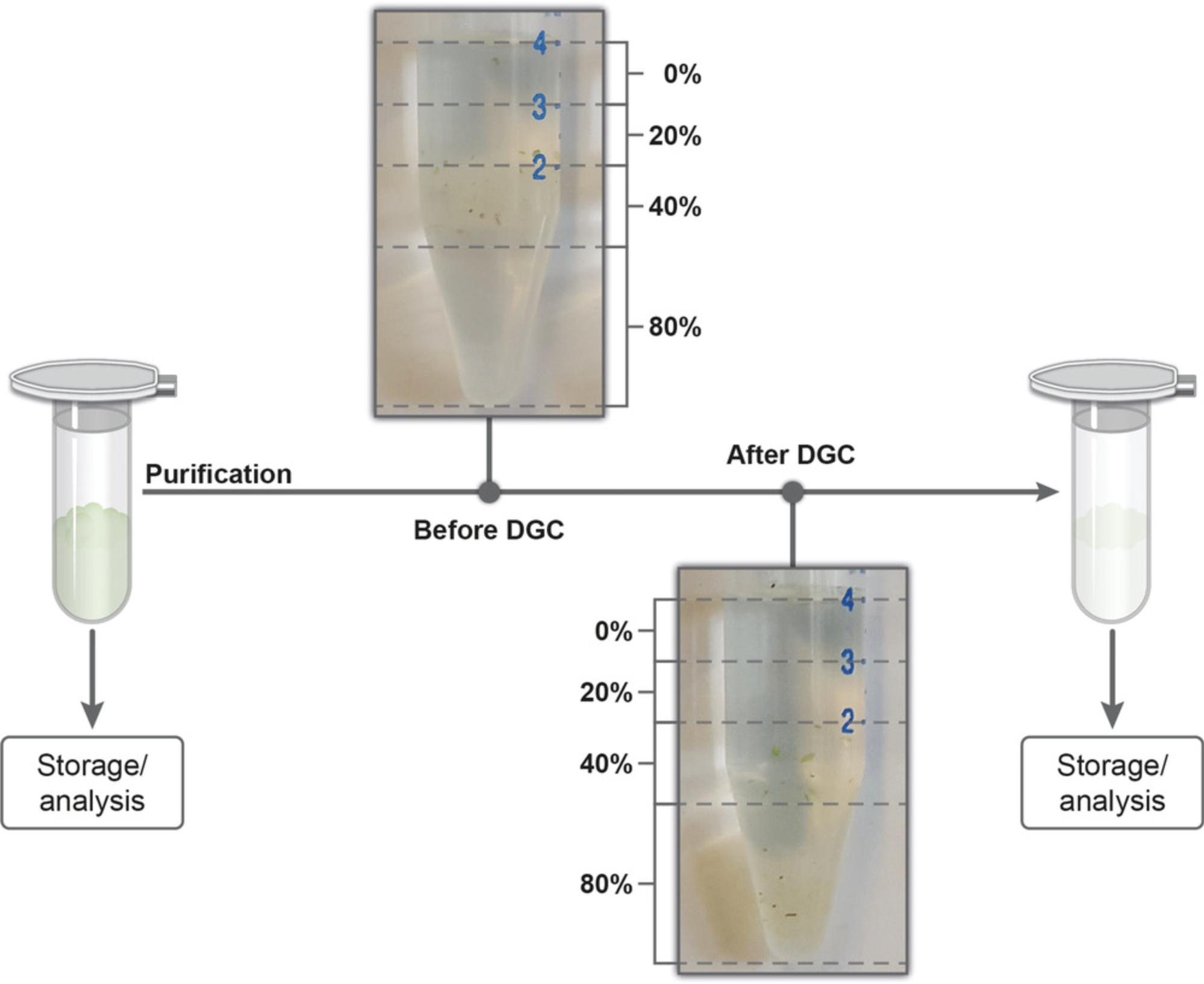
2.Resuspend the enriched trichomes (Basic Protocol 1, step 19) in 1 ml of 1× PBS buffer.
3.Carefully place the trichomes in PBS buffer on top of the density gradient.
4.Centrifuge 10 min at 3000 × g , room temperature, using a swing-out rotor.
5.Carefully remove the 0%, 20%, and 40% sucrose fractions and discard them.
6.Fill the whole 15-ml reaction tube with 1× PBS buffer, and mix its contents by inverting the tube.
7.Centrifuge 3 min at 3000 × g , room temperature.
8.Discard the supernatant and repeat steps 6 and 7.
9.Discard supernatant and carefully remove excess liquid.
10.Transfer the purified trichomes to a 2.0-ml microcentrifuge tube.
11.Use the purified trichome samples for further downstream processes, or store temporarily.
12.Visually inspect the trichome sample. After a successful purification, the trichome sample should have no remnants of greenish coloration or crude debris (Fig. 3B, “After DGC”).
REAGENTS AND SOLUTIONS
EDTA stock solution, 250 mM
- Dissolve 250 mM EDTA (Carl Roth GmbH + Co. KG, Karlsruhe, Germany, cat. no. 8040.2) in sterile, distilled water.
- Adjust to pH 8.0 using 10 M potassium hydroxide (KOH).
- The solution should now be transparent
- Store at 4°C
We have successfully used this solution after 3 months of storage at 4°C.
EDTA-PBS buffer, 50 mM
- Dilute 250 mM EDTA stock solution with 1× PBS (see recipe for 10). Prepare freshly immediately before use.
PBS buffer, 10×(pH 7.5)
- 1.37 M sodium chloride (NaCl)
- 27 mM potassium chloride (KCl)
- 100 mM disodium hydrogen phosphate dihydrate (Na2HPO4 2H2O)
- 20 mM potassium dihydrogen phosphate (KH2PO4)
- 5 mM magnesium chloride hexahydrate (MgCl2 6H2O)
- Dissolve all components in sterile, distilled water. First, dissolve the components in a smaller volume than your desired final volume. Mix the buffer on a magnetic stirrer until all components are completely dissolved. Then, fill up to the final volume.
- Store at room temperature
- We have successfully used this solution after 3 months of storage at 4°C.
COMMENTARY
Background Information
Leaf tissue and trichomes were initially separated via the manual clipping of trichomes using forceps (Perazza et al., 1999). The clipping-based procedure was later extended by freezing rosette leaves in liquid nitrogen before trichome detachment. Although the latter method provided initial insights into the proteome of A. thaliana trichomes (Wienkoop et al., 2004), the data set was limited, probably due to the comparably low amount of biological sample material obtained using this method. As an alternative approach, the cell content of trichomes was extracted using microcapillaries (Kryvych, Nikiforova, Herzog, Perazza, & Fisahn, 2008; Lieckfeldt et al., 2008). Coupled to gas chromatography, this single-cell sampling was successfully used for the metabolomic profiling of A. thaliana trichomes (Ebert et al., 2010). The method, however, inherently prevents the acquisition of trichome cell wall material, while the elaborate sampling procedure generally restricts yield.
These drawbacks were circumvented by the biochemical release of trichomes using cation chelators. The method was originally proposed by Zhang and Oppenheimer (2004), who used EGTA to capture calcium ions, thereby mitigating the crosslinking of the pectic matrix between trichomes and the leaf surface. A modified version of this EGTA-based trichome release was published by Marks and colleagues in 2008. The authors omitted an initial fixation step of A. thaliana leaves, and streamlined the conditions for plant cultivation and harvest, as well as those for trichome detachment and enrichment. The protocol outlined here represents an enhanced method of the procedure published by Marks and co-workers. As detailed before (Huebbers et al., 2022), and as mentioned in the introduction, we found that prolonged yet mild agitation of A. thaliana seedlings in an EGTA-containing buffer increases trichome yield by more than 60%. Adding to this, magnetic stirring in beakers allows for the simultaneous processing of comparably large seedling batches and enables a hypothetical process scale-up to fermenter size. Against the backdrop of scalability, we showed that the chelating agent EDTA is as effective for trichome release as the ∼50-times more expensive EGTA (Huebbers et al., 2022). In general, EGTA/EDTA-based trichome isolation may preserve trichome integrity better than methods that rely on forceful mechanical separation, such as the clipping or brushing of frozen trichomes (Balcke et al., 2014; Perazza et al., 1999). In this context, our method may be particularly beneficial due to the mild agitation conditions deployed during trichome release. Furthermore, we found that glass beads, which were initially used to provide an additional mechanical stimulus for trichome detachment (Huebbers et al., 2022; Marks et al., 2008), can be omitted. As considerable amounts of foliaceous debris and fine particles may co-purify during trichome enrichment, we introduced a subsequent DGC step to enhance the purity of trichome samples even more. We reviewed the success of trichome purification by visual inspection of microcentrifuge tubes and via microscopy of trichome samples before and after DGC. Upon visual inspection, we found a considerable reduction of crude foliaceous debris and greenish appearance in our trichome samples after DGC (Fig. 3B). Moreover, microscopy revealed a lower burden of fine particles in trichome samples after DGC (Fig. 3C).
Altogether, this enhanced method for trichome release, enrichment, and purification enables the convenient isolation of comparably large amounts of A. thaliana rosette leaf trichomes. The retrieved trichomes are characterized by a high integrity and purity, and can be used for various downstream applications. Indeed, we have already demonstrated that these trichome samples are suitable for histochemical and biochemical cell wall analyses and transcriptomic studies (Huebbers et al., 2022). Moreover, the large amount of pure trichomes provided by our adapted method have enabled profound insights into the A. thaliana proteome. Metabolomic profiling, however, requires an altered buffer composition during trichome release, as sample preparation for metabolomics requires the quenching of metabolic processes, for instance, by cold methanol (de Jonge, Douma, Heijnen, & van Gulik, 2012). Lastly, we note that the EDTA-based trichome release protocol described here is applicable to plant species other than A. thaliana , including N. benthamiana (tobacco) and S. lycopersicum (tomato) (Huebbers et al., 2022). However, the exact amount of deployed plant mass and the time frame for agitation in EDTA-PBS buffer requires optimization for specific plant species. Helianthus annuus (sunflower), for instance, provided a comparably low yield of 3.89 mg g-1 trichome fresh mass per harvested leaf mass (Table 1), and microscopy of processed leaves revealed that a considerable amount of trichomes remained attached to the leaf surface during the release step (Huebbers et al., 2022).
Critical Parameters
Preparation of A. thaliana seedlings for trichome isolation
Due to the cultivation of multiple A. thaliana plants in a single propagation box, puny growth may occur because of (too) close proximity between the individual seedlings. As the proximity between the seedlings will depend on the ultimate plant size desired, sowing density should be adjusted according to the favored plant age. For extended cultivation periods (>5 weeks), we recommend an especially thrifty application of seeds.
After harvesting, the thorough rinsing of seedlings with tap water ensures a reduction of soil-associated impurities.
Release of trichomes
The release of trichomes by agitation of seedlings in EDTA-PBS buffer is a critical step during trichome isolation. In this context, free floating of the seedlings ensures proper trichome detachment and prevents damaging of the seedlings by the stirring bar, which may affect trichome integrity or increase impurities such as foliaceous debris. Thus, we recommend a maximum seedling fresh mass of 36 g in 250 ml of EDTA-PBS buffer. Moreover, prolonged seedling agitation (>1 hr) may cause a sluice of cytosolic trichome material, as indicated by a reduction in trichome density (Huebbers et al., 2022). Accordingly, an agitation time of 30 min before trichome enrichment should not be exceeded. Lower trichome yields due to the reduced stirring period can be compensated for by a circulatory system of agitation and filtration at 15-min intervals, as detailed in Basic Protocol 1.
Enrichment of released trichomes by filtration
During trichome enrichment, two consecutive filtration steps are used to separate seedlings and buffer, as well as trichomes and bulk liquid. Concerning the latter, the excess buffer may be captured and reapplied to the processed seedlings for an additional cycle of trichome release.
Special care should be taken during trichome collection from the cell strainer with a small spatula, to preserve trichome integrity.
Purification of A. thaliana trichomes by density-gradient centrifugation
Trichome purification by DGC benefits from a sharp discrimination of the gradient layers. Hence, gradient assembly should be carried out carefully. Distortion of the gradient during loading can be avoided by applying the sample with a cut pipette tip to the wall of the centrifuge tube. Likewise, layer separation after DGC should be carried out with a cut pipette tip, to ensure maximum trichome yield.
Troubleshooting
Table 2 lists some common problems with the protocols, their causes, and potential solutions. Below, we comment on some specific elements.
| Problem | Possible cause | Solution |
|---|---|---|
| Poor plant growth | • Tight sowing | • Follow thrifty seed application |
| • Low nutrient concentration in soil | • Use nutrient-dense soil or extra fertilizer | |
| Low trichome yield | • Poor plant quality | • See “Poor plant growth” |
| • Too low seedling age or leaf age | • Use older seedlings or leaves | |
| • Precipitates in EDTA or PBS stock solutions | • Prepare fresh stock solutions and double check buffer storage conditions | |
| • Overloading of beakers with seedlings or leaves during release | • Ensure free rotation of seedlings or leaves during agitation | |
| • Insufficient stirring times | • Use enhanced stirring times or multiple cycles of release and enrichment | |
| Impaired trichome integrity or sample purity | • Poor plant quality | • See “Poor plant growth” |
| • Overloading of beakers with seedlings or leaves during release | • Ensure free rotation of seedlings or leaves during agitation | |
| • High agitation velocity | • Use a maximum velocity of 300 rpm (may depend on the device) | |
| • Damaging of trichomes during collection | • Take extra care while collecting trichomes with the spatula | |
| • High burden of impurities | • Use DGC for trichome purification | |
| No trichome sedimentation during DGC | • Reduction of trichome density by elevated agitation periods of seedlings or leaves | • Do not exceed certain agitation times (e.g., 30 min for A. thaliana) |
Why do my plants look unhealthy?
The crowded cultivation of plants in a single propagation tray may cause an extra burden on plant health. In case plants show leaf senescence, extensive accumulation of anthocyanins, or puny growth, we recommend a thriftier application of seeds during sowing. In addition, nutrient-dense soil or the application of extra fertilizer may help to overcome poor plant growth (Table 2).
Why do I not meet the described benchmark for trichome yield?
In our hands, the described method has been highly reproducible, especially concerning trichome yield. In case you encounter unusually low trichome amounts, this may be due to (i) poor plant health (see above), (ii) the use of too young seedlings (<28 days for A. thaliana) or leaves, (iii) inadequate buffer preparation or quality, (iv) overloading of beakers during trichome release, and (v) insufficient stirring times. Note that the optimal time for agitation in EDTA-PBS buffer may vary for different plant species (Table 2).
Why is my trichome quality impaired?
After enrichment, we recommend the inspection of trichome quality in terms of integrity and purity by microscopy. In case you observe damaged trichomes or a high burden of impurities, this may have various reasons. Just like trichome quantity, trichome quality depends on the condition of the harvested seedlings or leaves. Necrotic tissue may increase impurities, while physiological properties (e.g., cell wall composition, transcriptome, and proteome) of trichomes detached from unhealthy plants are likely altered. Furthermore, the overloading of beakers and elevated agitation velocities may damage trichomes, but also foliaceous tissue, and hence interfere with trichome integrity and sample purity. The eventual collection of trichomes with a spatula may damage trichomes, if not carried out carefully. Whereas trichome damage inevitably reduces sample quality, purity can be restored by DGC. However, for certain downstream analyses, DGC is not required if trichome isolation is carried out properly (Table 2).
Why do my trichomes not sediment during DGC?
As described elsewhere (Huebbers et al., 2022), elevated periods of agitation during the release step may interfere with trichome density. In case an unusually high amount of trichomes does not pass the 80% sucrose or 60% Nycodenz® layer, this is probably due to a sluice of cytosolic material during stirring. Thus, we recommend a maximum agitation time of 30 min for A. thaliana seedlings (Table 2).
Understanding Results
As published elsewhere (Huebbers et al., 2022), our method provides an average number of intact trichomes per harvested A. thaliana fresh mass of about 3000 g-1 before DGC and 2700 g-1 after DGC. These numbers correspond to average trichome fresh masses of about 300 mg before DGC and about 270 mg after DGC. We furthermore have measured the trichome fresh mass retrieved from plant species other than A. thaliana after trichome enrichment as well as the respective dry mass of these trichomes after lyophilization using a precision balance (Table 1). Microscopy of processed leaf material after trichome release revealed a near-complete removal of trichomes from A. thaliana leaves (Fig. 3A). Similarly, microscopy of isolated trichomes showed that the vast majority of trichomes were not damaged during the release and enrichment procedure (Fig. 3C). Visual inspection and microscopy of purified trichomes demonstrated a drastic reduction of crude debris and fine particles when implementing the discontinuous sucrose DGC (Fig. 3B and 3C).
Time Considerations
We usually prepare two propagation boxes for trichome isolation, which enables us to process at least eight batches of seedlings (equivalent to eight beakers with a maximum of 36 g A. thaliana fresh mass each). For the preparation of two propagation trays and sowing, users should plan for 1 hr. The required cultivation time depends on the desired plant age. Though the full procedure of trichome isolation (including buffer preparation and DGC) can be carried out in 1 day, we recommend the preparation of EDTA and PBS stock solutions in advance (∼1.5 hr, see Strategic Planning). Usually, these stock solutions are prepared in volumes that are sufficient for multiple trichome isolation procedures.
Plant harvesting and batch preparation can be carried out either the day before trichome isolation (cover seedlings and store at 4°C) or on the same day, and require about 1 hr. Depending on the available number of magnetic stirrers, multiple batches can be processed in parallel. We recommend the simultaneous processing of four batches, for which we estimate 1.5 hr, including buffer preparation, an initial release for 30 min, filtration, and capture, as well as two recycles of 15 min each. Accordingly, eight batches would require about 3 hr. In case subsequent purification by DGC is desired (e.g., for transcriptomic or proteomic approaches), an additional 2 hr for gradient layering, centrifugation, layer separation, and washing should be considered. Sucrose or Nycodenz® stock solutions should be prepared in advance (∼1 hr).
Acknowledgments
This project was funded by a grant of the Deutsche Forschungsgemeinschaft (DFG; German Research Foundation) to R.P. (Grant PA 861/20-1; project number 411779037).
Open access funding enabled and organized by Projekt DEAL.
Author Contribution
Jan W. Huebbers : conceptualization, data curation, formal analysis, investigation, methodology, project administration, supervision, validation, visualization, writing original draft, writing review and editing; Kim Büttgen : investigation, methodology, validation, writing original draft; Ralph Panstruga : conceptualization, funding acquisition, project administration, supervision, writing review and editing.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article, as no new data were created or analyzed in this study.
Supporting Information
| Filename | Description |
|---|---|
| cpz1541-sup-0001-SuppMat.docx15.3 KB | Supplementary Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
Literature Cited
- Balcke, G. U., Bennewitz, S., Zabel, S., & Tissier, A. (2014). Isoprenoid and metabolite profiling of plant trichomes. Methods in Molecular Biology , 1153, 189–202. doi: 10.1007/978-1-4939-0606-2_13
- Ebert, B., Zöller, D., Erban, A., Fehrle, I., Hartmann, J., Niehl, A., … Fisahn, J. (2010). Metabolic profiling of Arabidopsis thaliana epidermal cells. Journal of Experimental Botany , 61, 1321–1335. doi: 10.1093/jxb/erq002
- Fornero, C., Suo, B., Zahde, M., Juveland, K., & Kirik, V. (2017). Papillae formation on trichome cell walls requires the function of the mediator complex subunit Med25. Plant Molecular Biology , 95, 389–398. doi: 10.1007/s11103-017-0657-x
- Guerra, D., Truebridge, I., Eyles, S. J., Treffon, P., & Vierling, E. (2018). Direct measurement of S-nitrosothiols with an Orbitrap Fusion mass spectrometer: S-nitrosoglutathione reductase as a model protein. Methods in Molecular Biology , 1747, 143–160. doi: 10.1007/978-1-4939-7695-9_12
- Huebbers, J. W., Büttgen, K., Leissing, F., Mantz, M., Pauly, M., Huesgen, P. F., & Panstruga, R. (2022). An advanced method for the release, enrichment and purification of high-quality Arabidopsis thaliana rosette leaf trichomes enables profound insights into the trichome proteome. Plant Methods , 18, 12. doi: 10.1186/s13007-021-00836-0
- Hülskamp, M. (2004). Plant trichomes: A model for cell differentiation. Nature Reviews Molecular Cell Biology , 5, 471–480. doi: 10.1038/nrm1404
- Hülskamp, M., Miséra, S., & Jürgens, G. (1994). Genetic dissection of trichome cell development in Arabidopsis. Cell , 76, 555–566. doi: 10.1016/0092-8674(94)90118-X
- Johnson, H. B. (1975). Plant pubescence: An ecological perspective. Botanical Review , 41, 233–258. doi: 10.1007/BF02860838
- de Jonge, L. P., Douma, R. D., Heijnen, J. J., & van Gulik, W. M. (2012). Optimization of cold methanol quenching for quantitative metabolomics of Penicillium chrysogenum. Metabolomics , 8, 727–735.
- Karabourniotis, G., Liakopoulos, G., Nikolopoulos, D., & Bresta, P. (2020). Protective and defensive roles of non-glandular trichomes against multiple stresses: Structure–function coordination. Journal of Forestry Research , 31, 1–12. doi: 10.1007/s11676-019-01034-4
- Kryvych, S., Nikiforova, V., Herzog, M., Perazza, D., & Fisahn, J. (2008). Gene expression profiling of the different stages of Arabidopsis thaliana trichome development on the single cell level. Plant Physiology and Biochemistry , 46, 160–173. doi: 10.1016/j.plaphy.2007.11.001
- Kulich, I., Vojtíková, Z., Sabol, P., Ortmannová, J., Neděla, V., Tihlaříková, E., & Žárský, V. (2018). Exocyst subunit EXO70H4 has a specific role in callose synthase secretion and silica accumulation. Plant Physiology , 176, 2040–2051. doi: 10.1104/pp.17.01693
- Larkin, J. C., Oppenheimer, D. G., Lloyd, A. M., Paparozzi, E. T., & Marks, M. D. (1994). Roles of the GLABROUS1 and TRANSPARENT TESTA GLABRA genes in Arabidopsis trichome development. Plant Cell , 6, 1065–1076. doi: 10.2307/3869885
- Lieckfeldt, E., Simon-Rosin, U., Kose, F., Zoeller, D., Schliep, M., & Fisahn, J. (2008). Gene expression profiling of single epidermal, basal and trichome cells of Arabidopsis thaliana. Journal of Plant Physiology , 165, 1530–1544. doi: 10.1016/j.jplph.2007.06.017
- Liu, X., Renard, C. M. G. C., Bureau, S., & le Bourvellec, C. (2021). Revisiting the contribution of ATR-FTIR spectroscopy to characterize plant cell wall polysaccharides. Carbohydrate Polymers , 262, 117935. doi: 10.1016/j.carbpol.2021.117935
- Marks, M. D., Betancur, L., Gilding, E., Chen, F., Bauer, S., Wenger, J. P., … Haigler, C. H. (2008). A new method for isolating large quantities of Arabidopsis trichomes for transcriptome, cell wall and other types of analyses. Plant Journal , 56, 483–492. doi: 10.1111/j.1365-313X.2008.03611.x
- Marks, M. D., Wenger, J. P., Gilding, E., Jilk, R., & Dixon, R. A. (2009). Transcriptome analysis of Arabidopsis wild-type and gl3–sst sim trichomes identifies four additional genes required for trichome development. Molecular Plant , 2, 803–822. doi: 10.1093/mp/ssp037
- Matsumura, M., Nomoto, M., Itaya, T., Aratani, Y., Iwamoto, M., Matsuura, T., … Tada, Y. (2022). Mechanosensory trichome cells evoke a mechanical stimuli-induced immune response in Arabidopsis thaliana. Nature Communication , 13, 1216. doi: 10.1038/s41467-022-28813-8
- Payne, W. W. (1978). A glossary of plant hair terminology. Brittonia , 30, 239. doi: 10.2307/2806659
- Perazza, D., Herzog, M., Hülskamp, M., Brown, S., Dorne, A.-M., & Bonneville, J.-M. (1999). Trichome cell growth in Arabidopsis thaliana can be derepressed by mutations in at least five genes. Genetics , 152, 461–476. doi: 10.1093/genetics/152.1.461
- Suo, B., Seifert, S., & Kirik, V. (2013). Arabidopsis GLASSY HAIR genes promote trichome papillae development. Journal of Experimental Botany , 64, 4981–4991. doi: 10.1093/jxb/ert287
- Szymanski, D. B., Jilk, R. A., Pollock, S. M., & Marks, M. D. (1998). Control of GL2 expression in Arabidopsis leaves and trichomes. Development , 125, 1161–1171. doi: 10.1242/dev.125.7.1161
- Wienkoop, S., Zoeller, D., Ebert, B., Simon-Rosin, U., Fisahn, J., Glinski, M., & Weckwerth, W. (2004). Cell-specific protein profiling in Arabidopsis thaliana trichomes: Identification of trichome-located proteins involved in sulfur metabolism and detoxification. Phytochemistry , 65, 1641–1649. doi: 10.1016/j.phytochem.2004.03.026
- Yeats, T., Vellosillo, T., Sorek, N., Ibáñez, A. B., & Bauer, S. (2016). Rapid determination of cellulose, neutral sugars, and uronic acids from plant cell walls by one-step two-step hydrolysis and HPAEC-PAD. Bio-Protocol , 6, e1978–e1978. doi: 10.21769/BioProtoc.1978
- Zhang, X., & Oppenheimer, D. G. (2004). A simple and efficient method for isolating trichomes for downstream analyses. Plant & Cell Physiology, 45, 221–224.
Citing Literature
Number of times cited according to CrossRef: 3
- Qi Guo, Shayan Sarkar, Tracy Punshon, Ryan Tappero, Bronwyn J Barkla, Kendal D Hirschi, Proteomic Insights into Trichome Responses to Elevated Elemental Stress in Cation Exchanger (CAX) Mutants, Plant And Cell Physiology, 10.1093/pcp/pcae097, (2024).
- Shoujuan Yuan, Qian Li, Heng Shen, Wenqian Wang, Taotao Wang, Zhibiao Ye, Changxian Yang, Advances in the regulatory mechanisms of multicellular trichome formation and its secondary metabolite synthesis in vegetable crops, Vegetable Research, 10.48130/VR-2023-0024, 3 , 1, (0-0), (2023).
- Jan W. Huebbers, Melissa Mantz, Ralph Panstruga, Pitter F. Huesgen, Proteomics dataset on detached and purified Arabidopsis thaliana rosette leaf trichomes, Data in Brief, 10.1016/j.dib.2023.108897, 46 , (108897), (2023).

