Immunofluorescent Staining of Mouse Pancreas for Islet Cell Mass Analysis
Islet and Pancreas Analysis Core
Abstract
This SOP defines the assay method used by the Vanderbilt Diabetes Center Islet and Pancreas Analysis (IPA) Core for quantitative determination of the islet cell composition and islet cell mass of mouse pancreas by immunofluorescent staining.
Steps
Reagent preparation
10% Triton X-100 stock (30 mL) – Combine 3 mL Triton-X-100 and 27 mL 1X PBS. Mix on shaker for 0h 30m 0s or until Triton X-100 is completely dissolved. Store at 4°C for up to 1 month.
Permeabilization solution (0.2% Triton, 50 mL) – Combine 1 mL 10% Triton stock and 49 mL 1X PBS.
Blocking buffer (5% Normal Donkey Serum, 4 mL) – Combine 0.2 mL NDS and 3.8 mL 1X PBS.
Antibody buffer (10 mL) – Combine 0.1 g BSA, 0.1 mL 10% Triton stock, and 9.8 mL 1X PBS. Filter solution through a 0.22 μm syringe filter and store at 4°C.
DAPI staining solution (1:25,000, 50 mL) – Combine 2 µl DAPI stock (5mg/mL) and 50 mL 1X PBS. Keep protected from light or prepare right before step 17.
Wash buffer (150 mL) - Combine 148.5 mL 1X PBS and 1.5 mL 10% Triton X-100 stock.
Immunofluorescent staining
Gather reagents for immunostaining, noting that steps 9-11, 15, and 17-18 can be performed in Kartell staining chambers (each holds ~50 mL).
Let the frozen cryosections thaw at room temperature and air-dry for about 0h 30m 0s.
To remove OCT, wash slides 3 times in 1X PBS for 0h 5m 0s, decanting or switching to a new chamber after each wash.
Permeabilize the tissue section with permeabilization solution for 0h 15m 0s at room temperature.
Wash the sections 3 times in 1X PBS for 0h 5m 0s, decanting or switching to a new chamber after each wash.
Draw rectangles around the sections with PAP pen and let them dry for about 0h 5m 0s.
Block the sections with blocking buffer at room temperature for 1h 30m 0s in a humidified chamber. Use a sufficient volume (approximately 200-300 µl) to ensure each section is entirely covered with buffer.
Aspirate the blocking buffer, add primary antibodies diluted in antibody buffer (see Table 1 ), and incubate in a humidified chamber at 4°C. Use a sufficient volume (approximately 200-300 µl) to ensure each section is entirely covered with antibody solution.
Aspirate the primary antibody solution and wash the sections 3 times in wash buffer for 0h 10m 0s, decanting or switching to a new chamber after each wash.
Add secondary antibodies diluted in antibody buffer (see Table 1 ) and incubate for 1h 30m 0s at room temperature in a humidified chamber. Use a sufficient volume (approximately 200-300 µl) to ensure each section is entirely covered with antibody solution.
Aspirate the secondary antibody solution and incubate slides in DAPI solution to counterstain for 0h 10m 0s at room temperature.
Wash slides 3 times in 1x PBS for 0h 15m 0s, decanting or switching to a new chamber after each wash.
Add a sufficient volume of SlowFade Gold mounting medium (approximately 5-10 µl) to each tissue section, ensuring tissue section will be entirely covered after medium spreads. Carefully mount coverslips, making sure to avoid creating air bubbles between the tissue and coverslip.
Blot away any excess mounting medium from slide edges and seal the coverslipped slides with nail polish on all edges. Allow slides to dry completely before imaging.
Imaging and analysis
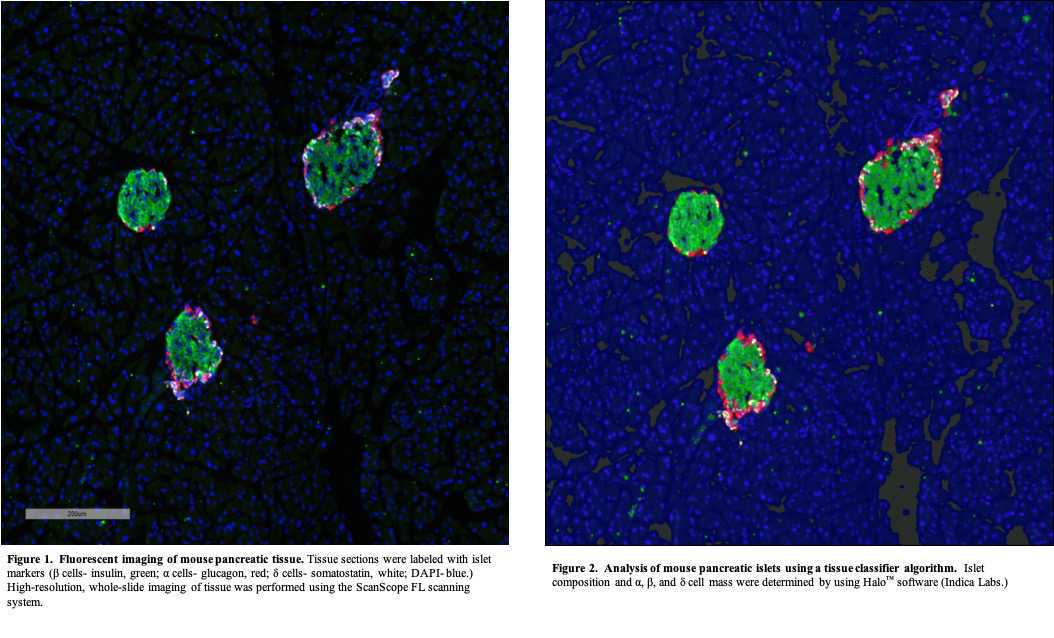
Acquire images of stained pancreas sections using a high-resolution whole slide scanning system (Aperio ScanScope FL, Leica Biosystems). An example field of view is shown below in Figure 1 .
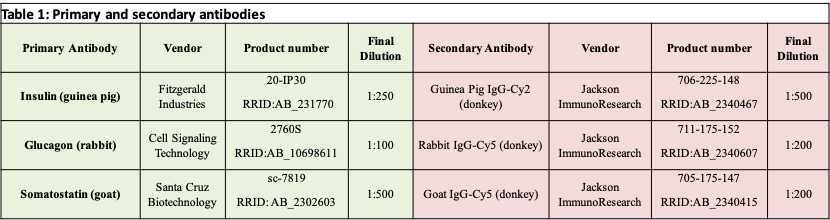
Apply a tissue classifier algorithm (HALO® image analysis platform, Indica Labs) to quantify cross-sectional area of islet alpha (glucagon+), beta (insulin+), and delta (somatostatin+) cells. An example markup image is shown in Figure 2 .