Electrophysiological characterizations of pancreatic islet cells
Toshinori Hoshi Laboratory
Abstract
Hormone release from pancreatic cells, for example glucagon from α-cells and insulin from β-cells, is controlled by the cells’ membrane potentials (Vm), which are in turn finely controlled by multiple classes of ion channels, transporters, and pumps. The perforated whole-cell patch-clamp method represents a direct and well-established way to monitor Vm in individual cells and also to investigate ionic currents (Im) that contribute to determination of Vm. Here a step-by-step guide to obtain various patch-clamp measurements from pancreatic cells in isolated intact human islets is provided. The method requires a standard electrophysiological station and β escin is used as the perforating agent. Select exemplar current-clamp Vm results and voltage-clamp Im results are provide. The electrophysiological characteristics may be used to distinguish pancreatic cell types, for example α-cells vs. β-cells in real time.
Steps
Procedure
Sonicate 8 μM escin diluted with the internal solution in the bath sonicator for > 10 min
4 μM escin is sometimes sufficient
Start the perfusion of the recording chamber so that the chamber solution is at 35°C; the perfusion is continuous
Take a culture dish with a coverslip with pancreatic islets ( or pancreatic islet cells) using the dissection microscope
Cut out a small section, with an islet (or cells), using a diamond pen, and transfer the coverslip piece to the recording chamber filled with the desired recording solution (e.g., 5 mM glucose)
Equilibrate the islet (or cells) in the chamber for > 10 min
Fill the tip of a polished, sylgard- or wax-coated patch electrode with the internal recording without escin and back fill with the recording with escin
The input resistance of the electrode should be 3 to 6 Mohms, depending on the types of the data required; lower for voltage-clamp experiments and higher for current-clamp experiments.
Form a seal with a few Gohms in resistance
Change the holding voltage to —70 mV (in the whole-cell mode convention) and apply small short square pulses ( —10 mV in size from the holding voltage and 20 ms in duration) to monitor the resistance and capacitance
Within 5 to 10 min, adequate perforated whole-cell access should be achieved
Apply short small square pulses in the voltage-clamp mode ( —10 mV in size from the holding voltage and 20 ms in duration) to measure the input resistance and capacitance
Compensate the whole-cell capacitance and series resistance
Measure membrane potential (Vm) in the current-clamp mode; change the external solutions if desired
13 Measure membrane potential (Im) in the voltage-clamp mode; change the external solutions if desired
Data analysis
-
Export data to lgorPro (Wavemerics) for data plotting and analysis
-
Data visualization
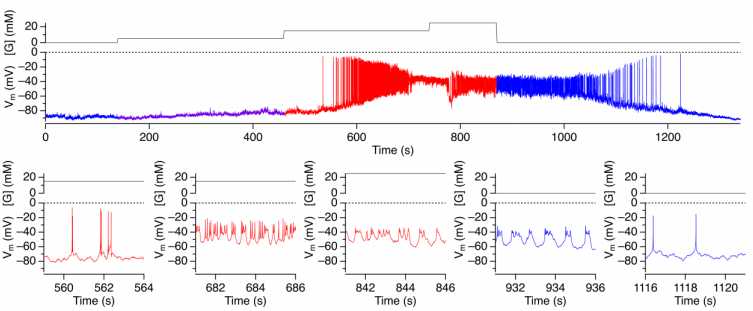
a. Current-clamp Vm measurement example (from a cell in an intact islet)
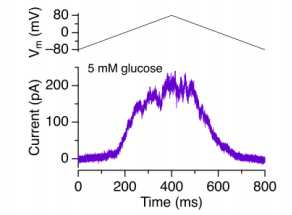
b. Ramp voltage-clamp Im measurement example (from a sorted β cell)
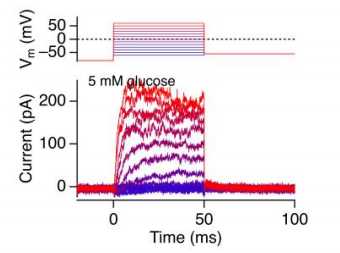
c. Step voltage-clamp Im measurement example (from a sorted β cell)
- Other custom analysis
a. Igor scripts may be written for custom and automated analysis procedures as needed

